Exotic Polychaetes of a Sewage Pollution Influenced Lagoon (Çardak Lagoon, Turkish Straits)
Abstract
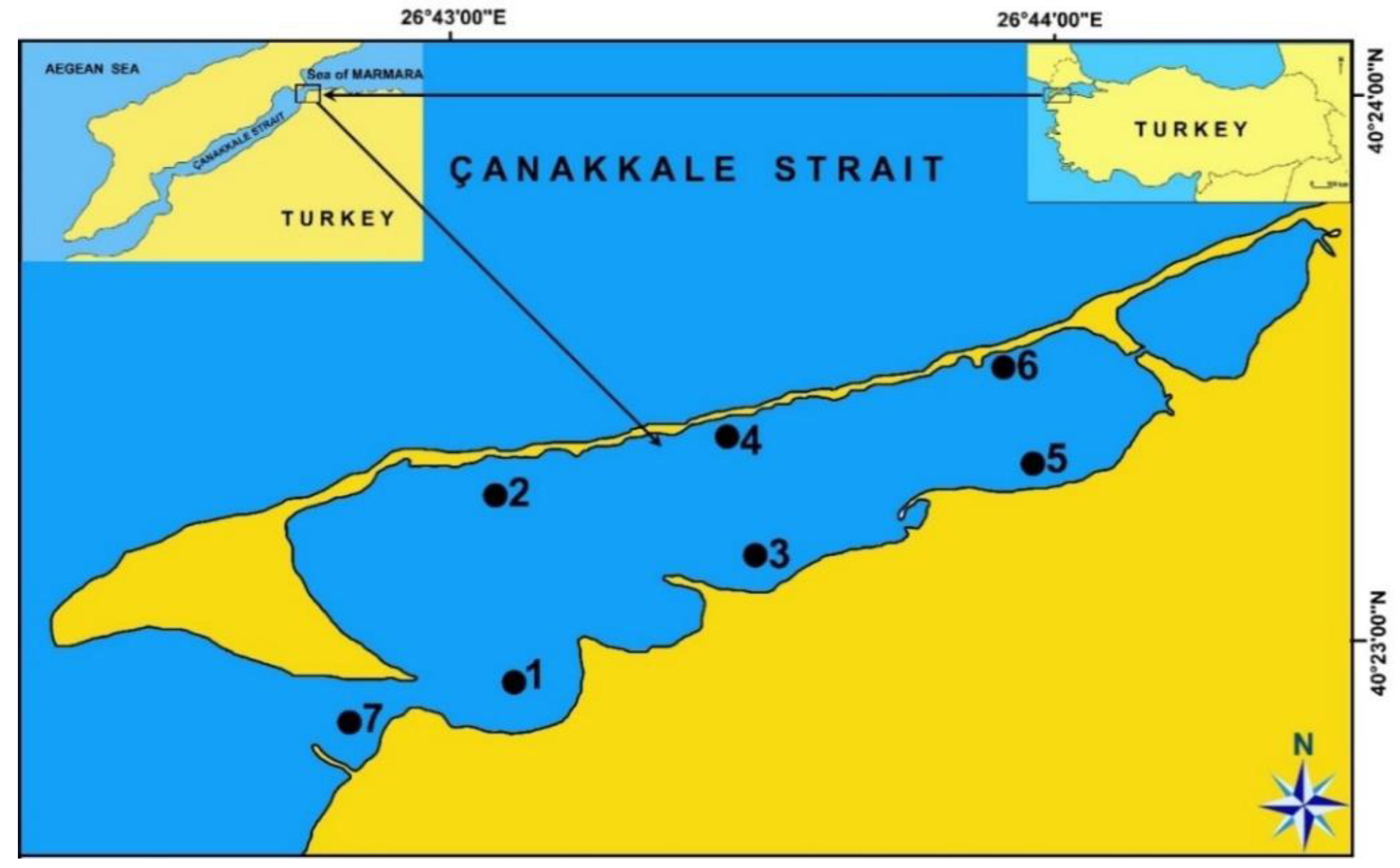
:1. Introduction
2. Material and Methods
2.1. Sediment Samplings
2.2. Faunistic Analyses
2.3. Statistical Analysis
2.4. Water Quality Measurements
2.5. Analyses of Organic Matter and Particle Size in Sediment
3. Results and Discussion
3.1. Faunistic Data of Polydora cornuta Bosc, 1802
| Genus Polydora Bosc, 1802 |
| Polydora cornuta Bosc, 1802 |
| (Figures 2 and 5B) |
3.1.1. Material Examined
3.1.2. Description

3.1.3. Remarks
3.1.4. Distribution
3.2. Faunistic Data of Pseudopolydora paucibranchiata (Okuda, 1937)
| Genus Pseudopolydora Czerniavsky, 1881 |
| Pseudopolydora paucibranchiata (Okuda, 1937) |
| (Figures 3 and 5C) |
3.2.1. Material Examined
3.2.2. Description

3.2.3. Remarks
3.2.4. Distribution
3.2.5. The Population Density
Habitat
3.3. Faunistic Data of Hydroides dianthus (Verrill, 1873)
| Genus Hydroides Gunnerus, 1768. |
| Hydroides dianthus (Verrill, 1873) |
| (Figure 4 and Figure 5A) |
3.3.1. Material Examined
3.3.2. Description

3.3.3. Remarks
3.3.4. Distribution
3.3.5. The Population Density
3.3.6. Habitat

3.4. Physico–Chemical Data
3.5. Correlations between Environmental Variables and the Polychaeta Abundance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pranovi, F.; Da Ponte, F.; Torricelli, P. Historical changes in the structure and functioning of the benthic community in the lagoon of Venice. Estuar. Coast. Shelf Sci. 2008, 76, 753–764. [Google Scholar] [CrossRef]
- Acquavita, A.; Aleffi, I.F.; Benci, C.; Bettoso, N.; Crevatin, E.; Milani, L.; Tamberlich, F.; Toniatti, L.; Barbieri, P.; Licen, S.; et al. Annual characterization of the nutrients and trophic state in a Mediterranean coastal lagoon: The Marano and Grado Lagoon (northern Adriatic Sea). Reg. Stud. Mar. Sci. 2015, 2, 132–144. [Google Scholar] [CrossRef]
- Carvalho, S.; Moura, A.; Gaspar, M.B.; Pereira, P.; da Fonseca, L.C.; Falcão, M.; Drago, T.; Leitão, F.; Regala, J. Spatial and inter-annual variability of the macrobenthic communities within a coastal lagoon (Óbidos lagoon) and its relationship with environmental parameters. Acta Oecol. 2005, 27, 143–159. [Google Scholar] [CrossRef]
- Kress, N.; Herut, B.; Galil, B.S. Sewage sludge impact on sediment quality and benthic assemblages off the Mediterranean coast of Israel—A long term study. Mar. Environ. Res. 2004, 57, 213–233. [Google Scholar] [CrossRef]
- Surugiu, V.; Feunteun, M. The structure and distribution of polychaete populations influenced by sewage from the Romanian coast of the Black Sea. An. Științifice Univ. Al. I. Cuza Iași Sect. Biol. Anim. 2008, 1, 177–184. [Google Scholar]
- Pearson, T.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 1978, 16, 229–311. [Google Scholar]
- Çinar, M.E.; Katağan, T.; Öztürk, B.; Egemen, Ö.; Ergen, Z.; Kocataş, A.; Önen, M.; Kırkım, F.; Bakır, K.; Kurt, G.; et al. Temporal changes of soft–bottom zoobenthic communities in and around Alsancak Harbor (İzmir Bay, Aegean Sea), with special attention to the autecology of exotic species. Mar. Ecol. 2006, 27, 229–246. [Google Scholar] [CrossRef]
- Surugiu, V. The use of Polychaetes as indicators of eutrophication and organic enrichment of coastal waters: A study case—Romanıan Black Sea Coast. An. Științifice Univ. Al. I. Cuza Iași Sect. Biol. Anim. 2005, 51, 55–62. [Google Scholar]
- Çinar, M.E.; Katagan, T.; Koçak, F.; Öztürk, B.; Ergen, Z.; Kocatas, A.; Önen, M.; Kirkim, F.; Bakir, K.; Kurt, G.; et al. Faunal assemblages of the mussel Mytilus galloprovincialis in and around Alsancak Harbour (İzmir Bay, eastern Mediterranean) with special emphasis on alien species. J. Mar. Syst. 2008, 71, 1–17. [Google Scholar] [CrossRef]
- Ceccherelli, V.U.; Ferrari, I.; Viaroli, P. Ecological research on the animal communities of the Po River Delta lagoons. Boll. Zool. 1994, 61, 425–436. [Google Scholar] [CrossRef]
- Bachelet, G.; de Montaudouin, X.; Auby, I.; Labourg, P.-J. Seasonal changes in macrophyte and macrozoobenthos assemblages in three coastal lagoons under varying degrees of eutrophication. ICES J. Mar. Sci. 2000, 57, 1495–1506. [Google Scholar] [CrossRef]
- Çinar, M.E.; Balkıs, H.; Albayrak, S.; Dagli, E.; Karhan, Ü.S. Distribution of polychaete species (Annelida: Polychaeta) on the polluted soft substrate of the Golden Horn estuary with special emphasis on the alien species. Cah. Biol. Mar. 2009, 50, 11–17. [Google Scholar]
- Dagli, E.; Çinar, M.E. Invasion of polluted soft substrate of İzmir Bay (Aegean Sea, eastern Mediterranean) by the Spionid polychaete worm, Pseudopolydora paucibranchiata (Polychaeta: Spionidae). Cah. Biol. Mar. 2008, 49, 87–96. [Google Scholar]
- Dagli, E.; Çinar, M.E. Species of the subgenus Minuspio (Polychaeta: Spionidae: Prionospio) from the southern coast of Turkey (Levantine Sea, eastern Mediterranean), with the description of two new species. Zootaxa 2011, 3043, 35–53. [Google Scholar] [CrossRef]
- Vural, P.; Acarlı, S. Assessment of Çardak Lagoon Fisheries and Aquaculture Production. In Proceedings of the International Ecology 2018 Symposium, Kastamonu, Türkiye, 19–23 June 2018; p. 1051. [Google Scholar]
- Okuda, S. Spioniform polychaetes from Japan. J. Fac. Sci. Hokkaido Univ. Ser. 6 Zool. 1937, 5, 217–254. [Google Scholar]
- Blake, J.A.; Maciolek, N.J.A. redescription of Polydora cornuta Bosc (Polychaeta: Spionidae) and desgnation of a neotype. Bull. Biol. Soc. Wash. 1987, 7, 11–15. [Google Scholar]
- Bastida-Zavala, J.R.; ten Hove, H.A. Revision of Hydroides Gunnerus, 1768 (Polychaeta: Serpulidae) from the Western Atlantic Region. Beaufortia 2002, 52, 103–178. [Google Scholar]
- Bastida-Zavala, J.R.; McCann, L.D.; Keppel, E.; Ruiz, G.M. The fouling serpulids (Polychaeta: Serpulidae) from United States coastal waters: An overview. Eur. J. Taxon. 2017, 344, 1–76. [Google Scholar] [CrossRef]
- Radashevsky, V.I. On adult and larval morphology of Polydora cornuta Bosc, 1802 (Annelida: Spionidae). Zootaxa 2005, 1064, 1–24. [Google Scholar] [CrossRef]
- Dağlı, E.; Ergen, Z. First record of Polydora cornuta Bosc, 1802 (Polychaeta: Spionidae) from the Sea of Marmara, Turkey basin. Aquat. Invasions 2008, 3, 231–233. [Google Scholar] [CrossRef]
- Blake, J.A.; Maciolek, N.J.; Meißner, K. Spionidae Grube, 1850. In Handbook of Zoology: Annelida, Pleistoannelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2020; Volume 2, pp. 1–103. [Google Scholar]
- Radashevsky, V.I. Pseudopolydora (Annelida: Spionidae) from European and adjacent waters with a key to identification and description of a new species. Mar. Biodivers. 2021, 51, 31. [Google Scholar] [CrossRef]
- Bogantes, V.E.; Boyle, M.J.; Halanych, K.M. New reports on Pseudopolydora (Annelida: Spionidae) from the East Coast of Florida, including the non–native species P. paucibranchiata. BioInvasions Rec. 2021, 10, 577–588. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972; Volume 167, p. 310. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1988. [Google Scholar]
- Allen, J.R.L. Subfossil mammalian tracks (Flandrian) in the Severn Estuary, S. W. Britain: Mechanics of formation, preservation and distribution. Philos. Trans. R. Soc. Lond. B 1997, 352, 481–518. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; McGraw-Hill, Inc.: Boston, MA, USA, 2003; p. 1758. [Google Scholar]
- Tena, J.; Capaccioni-Azzati, R.; Porras, R.; Torres-Gavilá, F.J. Cuatro especies de poliquetos nuevas para las costas mediterráneas españolas en los sedimentos del antepuerto de Valencia. Misc. Zool. 1991, 15, 29–41. [Google Scholar]
- Radashevsky, V.I.; Hsieh, H.L. Polydora (Polychaeta: Spionidae) species from Taiwan. Zool. Stud. 2000, 39, 203–217. [Google Scholar]
- Çinar, M.E.; Ergen, Z.; Dagli, E.; Petersen, M.E. Alien species of spionid polychaetes (Streblospio gynobranchiata and Polydora cornuta) in İzmir Bay, the eastern Mediterranean. J. Mar. Biol. Assoc. U. K. 2005, 85, 821–827. [Google Scholar] [CrossRef]
- Surugiu, V. Systematics and ecology of species of the Polydora-complex (Polychaeta: Spionidae) of the Black Sea. Zootaxa 2012, 3518, 45–65. [Google Scholar] [CrossRef]
- Radashevsky, V.I.; Selifonova, Z.P. Records of Polydora cornuta and Streblospio gynobranchiata (Annelida, Spionidae) from the Black Sea. Mediterr. Mar. Sci. 2013, 14, 261–269. [Google Scholar] [CrossRef]
- Bertasi, F. The occurrence of the alien species Polydora cornuta Bosc. 1802 (Polychaeta: Spionidae) in North Adriatic lagoons: An overlooked presence. Ital. J. Zool. 2016, 83, 77–88. [Google Scholar] [CrossRef]
- Webster, H.E. Annelida Chætopoda of New Jersey. In Annual Reports of the New York State Museum of Natural History 1879; Regents of the University of the State of New York: Albany, NY, USA, 1879; Volume 32, pp. 101–128. [Google Scholar]
- Light, W.J. Spionidae, Annelida, Polychaeta (Invertebrates of the San Francisco Bay Estuary System); California Academy of Sciences Pacific Grove: San Francisco, CA, USA, 1978; 211p. [Google Scholar]
- Blake, J.A. Revision of the genus Polydora from the East coast of North America (Polychaeta: Spionidae). Smithson. Contrib. Zool. 1971, 75, 1–32. [Google Scholar] [CrossRef]
- Bosc, L.A.G. Histoire Naturelle des Vers, Contenant Leur Déscription et Leurs Mœurs, Avec Fgures Dessinées D’après Nature; Tome 1; Deterville: Paris, France, 1802; 324p. [Google Scholar]
- Çinar, M.E.; Katagan, T.; Öztürk, B.; Dagli, E.; Açik, S.; Bitlis, B.; Bakir, K.; Dogan, A. Spatio–temporal distributions of zoobenthos in Mersin Bay (Levantine Sea, eastern Mediterranean) and the importance of alien species in benthic communities. Mar. Biol. Res. 2012, 8, 954–968. [Google Scholar] [CrossRef]
- Kurt-Sahin, G.; Çinar, M.E.; Dagli, E. New records of polychaetes (Annelida) from the Black Sea. Cah. Biol. Mar. 2019, 60, 153–165. [Google Scholar]
- Karhan, S.U.; Kalkan, E.; Simboura, N.; Mutlu, E.; Bekolet, M. On the occurrence and established populations of the alien polychaete Polydora cornuta Bosc, 1802 (Polychaeta: Spionidae) in the Sea of Marmara and the Bosphorus Strait (Turkey). Mediterr. Mar. Sci. 2008, 9, 5–19. [Google Scholar] [CrossRef]
- Dagli, E.; Çinar, M.E.; Ergen, Z. Spionidae (Annelida: Polychaeta) from the Aegean Sea (eastern Mediterranean). Ital. J. Zool. 2011, 78, 49–64. [Google Scholar] [CrossRef]
- Çinar, M.E.; Dagli, E.; Açik, S. Annelids (Polychaeta and Oligochaeta) from the Sea of Marmara, with descriptions of five new species. J. Nat. Hist. 2011, 45, 2105–2143. [Google Scholar] [CrossRef]
- Blake, J.A.; Woodwick, A.; Keith, H. Reproduction and larval development of Pseudopolydora paucibranchiata (Okuda) and Pseudopolydora kempi (Southern) (Polychaeta: Spionidae). Biol. Bull. 1975, 149, 109–127. [Google Scholar] [CrossRef]
- Ramberg, J.P.; Schram, T.A. A systematic review of the Oslofjord species of Polydora Bosc and Pseudopolydora Czerniavsky, with some new biological and ecological data (Polychaeta: Spionidae). Sarsia 1982, 68, 233–247. [Google Scholar] [CrossRef]
- Hutchings, P.A.; Turvey, S.P. The Spionidae of South Australia (Annelida: Polychaeta). Trans. R. Soc. S. Aust. 1984, 108, 1–20. [Google Scholar]
- Radashevsky, V.I. Revision of the genus Polydora and related genera from the North West Pacific (Polychaeta: Spionidae). Publ. Seto Mar. Biol. Lab. 1993, 36, 1–60. [Google Scholar] [CrossRef]
- Radashevsky, V.I.; Malyar, V.V.; Pankova, V.V.; Gambi, M.C.; Giangrande, A.; Keppel, E.; Nygren, A.; Al–Kandari, M.; Carlton, J.T. Disentangling invasions in the sea: Molecular analysis of a global polychaete species complex (Annelida: Spionidae: Pseudopolydora paucibranchiata). Biol. Invasions 2020, 22, 3621–3644. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bakir, K.; Öztürk, B.; Katağan, T.; Doğan, A.; Açik, Ş.; Kurt-Sahin, G.; Özcan, T.; Dağli, E.; Bitlis-Bakir, B.; et al. Macrobenthic fauna associated with the invasive alien species Brachidontes pharaonis (Mollusca: Bivalvia) in the Levantine Sea (Turkey). J. Mar. Biol. Assoc. U. K. 2017, 97, 613–628. [Google Scholar] [CrossRef]
- Benedict, J.E. Descriptions of ten species and one new genus of annelids from the dredgings of the U. S. Fish Commission steamer. Albatross. Proc. United States Natl. Mus. 1887, 9, 547–553. [Google Scholar] [CrossRef]
- Zibrowius, H. Les espèces Méditerranéennes du genre Hydroides (Polychaeta Serpulidae). Remarques sur le pretendu polymorphisme de Hydroides uncinata. Tethys 1971, 2, 691–746. Available online: http://paleopolis.rediris.es/benthos/REF/som/T-pdf/1970_2-3-691.pdf (accessed on 21 February 2023).
- Bastida-Zavala, J.R.; Salazar-Vallejo, S.I. Serpúlidos (Polychaeta: Serpulidae) del Caribe noroccidental: Hydroides y Serpula. Rev. Biol. Trop. 2000, 48, 841–858. [Google Scholar] [PubMed]
- Link, H.; Nishi, E.; Tanaka, K.; Bastida-Zavala, R.; Kupriyanova, E.K.; Yamakita, T. Hydroides dianthus (Polychaeta: Serpulidae), an alien species introduced into Tokyo Bay, Japan. Mar. Biodivers. Rec. 2009, 2, E87. [Google Scholar] [CrossRef]
- Zibrowius, H. Ongoing modification of the Mediterranean marine fauna and flora by the establishment of exotic species. Mesogee 1992, 51, 83–107. [Google Scholar]
- Verrill, A.E. Report upon the Invertebrate Animals of Vineyard Sound and the Adjacent Waters, with an Account of the Physical Characters of the Region; Government Printing Office: Washington, DC, USA, 1853; pp. 295–778.
- Read, G.B.; ten Hove, H.A.; Sun, Y.; Kupriyanova, E.K. Hydroides Gunnerus, 1768 (Annelida, Serpulidae) is feminine: A nomenclatural checklist of updated names. ZooKeys 2017, 642, 1–52. [Google Scholar] [CrossRef]
- De Quatrefages, M.A. Histoire Naturelle des Annelides Marins et D’eau Douce. Annelides et Gephyriens. 2. Sedentaira (Paris); Emedturaeg-L1584; Librairie Encyclopédique de Roret: Paris, France, 1865; p. 562. [Google Scholar]
- Ergen, Z. İzmir Körfezi ve Civarı Poliketlerinin Ekolojik ve Taksonomik Özellikleri. Ege Üniversitesi Fen Fakültesi İlmi Rap. Serisi 1976, 209, 73. [Google Scholar]
- Koçak, F.; Ergen, Z.; Çinar, M.E. Fouling Organism and their development in a polluted and an unpolluted marina in the Aegean Sea (Turkey). Ophelia 1999, 50, 1–20. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bakır, K.; Öztürk, B.; Katağan, T.; Dagli, E.; Açık, Ş.; Doğan, A.; Bitlis Bakır, B. TUBI (Turkish Benthic Index): A new biotic index for assessing impacts of organic pollution on benthic communitie. J. Black Sea Mediterr. Environ. 2015, 21, 135–168. [Google Scholar]
- Bat, L.; Akbulut, M.; Sezgin, M.; Çulha, M. Effects of Sewage Pollution on the Structure of the Community of Ulva lactuca, Enteremorpha linza and Rocky Macrofauna in Dışliman of Sinop. Turk. J. Biol. 2001, 25, 93–102. [Google Scholar]
- De-la-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y.; Giménez-Casalduero, F.; Sánchez-Lizaso, J.L.; Dauvin, J.-C. Sensitivity of amphipods to sewage pollution. Estuar. Coast. Shelf Sci. 2012, 96, 129–138. [Google Scholar] [CrossRef]
- Otani, M.; Yamanishi, R. Distribution of the alien species Hydroides dianthus (Verrill, 1873) (Polychaeta: Serpulidae) in Osaka Bay, Japan, with comments on the factors limiting its invasion. Plankton Benthos Res. 2010, 5, 62–68. [Google Scholar] [CrossRef]
- Fofonoff, P.W.; Ruiz, G.M.; Steves, B.; Simkanin, C.; Carlton, J.T. National Exotic Marine and Estuarine Species Information System. Available online: http://invasions.si.edu/nemesis/2018 (accessed on 22 February 2022).
- Boltacheva, N.A.; Lisitskaya, E.V. About species of Polydora (Polychaeta: Spionidae) from the Balaklava bay (the Black Sea). Mar. Ecol. J. 2007, 6, 33–35. (In Russian) [Google Scholar]
- Surugiu, V. The influence of sewage pollution on polychaetes associated with mussel beds of the Southern Romanian Black Sea coast. Geo-Eco-Marina 2009, 15, 77–87. [Google Scholar] [CrossRef]
- Takata, N.; Takahashi, H.; Ukita, S.; Yamasaki, K.; Awakihara, H. Ecology of Polydora cornuta Bosc, 1802 (Spionidae: Polychaeta) in the Eutrophic Port of Fukuyama, with Special Reference to Life Cycle, Distribution, and Feeding Type. J. Water Environ. Technol. 2011, 9, 259–275. [Google Scholar] [CrossRef]
- Simboura, N.; Zenetos, A. Benthic indicators to use in Ecological Quality classification of Mediterranean soft bottom marine ecosystems. Including a new Biotic Index. Mediterr. Mar. Sci. 2002, 3, 77–111. [Google Scholar] [CrossRef]
- Kjerve, B. Coastal lagoon processes. In Coastal Lagoon Processes; Elsevier Oceanography Series; Kjerve, B., Ed.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 60, pp. 1–8. [Google Scholar]
- Ünalan, D. Sustainable Tourism Development and Environment. Master’s Thesis, Institute of Social Sciences, Istanbul University, Istanbul, Türkiye, 1970. [Google Scholar]
- Reise, K.; Olenin, S.; Thieltges, D.W. Are aliens threatening European aquatic coastal ecosystems? Helg. Mar. Res. 2006, 60, 77–83. [Google Scholar] [CrossRef]
- Galil, B. A sea under siegealien species in the Mediterranean. Biol. Invasions 2000, 2, 177–186. [Google Scholar] [CrossRef]

| Sampling Period | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 October | 19 February | 19 April | 19 June | ||||||||||||||||
| Sampling Points | |||||||||||||||||||
| Species | 2 | 4 | 6 | 7 | 2 | 4 | 6 | 7 | 2 | 4 | 6 | 7 | 2 | 4 | 6 | 7 | ∑ | f% | D |
| Hydroides dianthus | 1 | 1 | 6.25 | 2.22 | |||||||||||||||
| Polydora cornuta | 3 | 1 | 4 | 12.50 | 8.88 | ||||||||||||||
| Pseudopolydora paucibranchiata | 3 | 1 | 11 | 5 | 2 | 2 | 5 | 1 | 3 | 3 | 4 | 40 | 68.75 | 88.88 | |||||
| Sampling Points | Temperature (°C) | Salinity (‰) | pH | O2 (mg L−1) | OM% | Clay + Silt % | Sand % | ∑ |
|---|---|---|---|---|---|---|---|---|
| Stn. 1 | 15.59 ± 6.77 | 21.91 ± 1.69 | 7.97 ± 0.19 | 8.18 ± 1.49 | 10.65 ± 1.24 | 14.16 ± 4.30 | 61.73 ± 4.31 | 0 |
| Stn. 2 | 16.74 ± 8.07 | 22.01 ± 1.37 | 8.29 ± 0.08 | 7.76 ± 1.19 | 3.03 ± 0.84 | 3.17 ± 1.02 | 78.67 ± 7.32 | 6 |
| Stn. 3 | 15.61 ± 7.21 | 21.79 ± 1.20 | 8.18 ± 0.09 | 7.21 ± 1.08 | 15.52 ± 1.36 | 13.24 ± 2.95 | 55.14 ± 5.37 | 0 |
| Stn. 4 | 15.53 ± 6.61 | 22.04 ± 1.37 | 8.32 ± 0.09 | 7.07 ± 1.01 | 3.49 ± 0.75 | 5.27 ± 2.57 | 86.19 ± 5.56 | 7 |
| Stn. 5 | 15.89 ± 7.85 | 22.04 ± 0.88 | 8.25 ± 0.15 | 7.18 ± 1.12 | 7.91 ± 1.04 | 16.71 ± 0.76 | 57.61 ± 5.06 | 0 |
| Stn. 6 | 16.33 ± 7.88 | 21.87 ± 0.84 | 8.34 ± 0.20 | 7.08 ± 0.81 | 2.75 ± 0.11 | 3.29 ± 1.92 | 92.74 ± 2.73 | 25 |
| Stn. 7 (Ref.) | 16.19 ± 7.03 | 22.04 ± 1.93 | 8.25 ± 0.09 | 7.76 ± 0.37 | 1.73 ± 0.25 | 1.49 ± 1.86 | 69.09 ± 17.41 | 7 |
| Sampling Points | PO4–P (mg L−1) | TP (mg L−1) | NO2 + NO3 (mg L−1) | NH4 (mg L−1) | TN (mg L−1) | SiO2 (mg L−1) | TSS (mg L−1) |
|---|---|---|---|---|---|---|---|
| Stn. 1 | 0.02 ± 0.012 | 0.048 ± 0.026 | 0.083 ± 0.04 | 0.01 ± 0.00 | 0.480 ± 0.206 | 0.367 ± 0.241 | 11.00 ± 4.62 |
| Stn.2 | 0.015 ± 0.006 | 0.029 ± 0.013 | 0.071 ± 0.059 | 0.01 ± 0.00 | 0.230 ± 0.126 | 0.563 ± 0.442 | 8.07 ± 2.74 |
| Stn. 3 | 0.013 ± 0.005 | 0.026 ± 0.009 | 0.071 ± 0.059 | 0.01 ± 0.00 | 0.427 ± 0.170 | 0.292 ± 0.225 | 22.1 ± 22.5 |
| Stn. 4 | 0.015 ± 0.006 | 0.035 ± 0.018 | 0.036 ± 0.02 | 0.01 ± 0.00 | 0.162 ± 0.047 | 0.575 ± 0.561 | 9.35 ± 4.85 |
| Stn. 5 | 0.01 ± 0.00 | 0.051 ± 0.066 | 0.088 ± 0.074 | 0.01 ± 0.00 | 0.498 ± 0.379 | 0.555 ± 0.632 | 19.1 ± 24.8 |
| Stn. 6 | 0.015 ± 0.01 | 0.065 ± 0.073 | 0.088 ± 0.063 | 0.01 ± 0.00 | 0.498 ± 0.379 | 0.186 ± 0.156 | 28.4 ± 29.7 |
| Stn. 7 (Ref.) | 0.01 ± 0.00 | 0.052 ± 0.072 | 0.094 ± 0.041 | 0.01 ± 0.00 | 0.262 ± 0.075 | 0.487 ± 0.477 | 15.98 ± 8.36 |
| Abundance (ind. 0.09 m−2) | Temperature (°C) | Salinty (‰) | pH | O2 (mg L−1) | OM (%) | Clay + silt (%) | Sand (%) | PO4–P (mg L−1) | TP (mg L−1) | NO2 + NO3 (mg L−1) | NH4 (mg L−1) | TN (mg L−1) | SiO2 (mg L−1) | TSS (mg L−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson Correlation | 1 | 0.44 | −0.14 | 0.58 | −0.39 | −0.59 | −0.64 | 0.83 * | –0.43 | 0.61 | 0.12 | .a | 0.07 | −0.51 | 0.53 |
| Sig. (2-tailed) | 0.33 | 0.77 | 0.17 | 0.40 | 0.16 | 0.13 | 0.02 | 0.34 | 0.15 | 0.80 | 0.90 | 0.25 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dağlı, E.; Ateş, A.S.; Acar, S.; Büyükateş, Y.; Doğan, A.; Bakır, A.K. Exotic Polychaetes of a Sewage Pollution Influenced Lagoon (Çardak Lagoon, Turkish Straits). Sustainability 2023, 15, 8946. https://doi.org/10.3390/su15118946
Dağlı E, Ateş AS, Acar S, Büyükateş Y, Doğan A, Bakır AK. Exotic Polychaetes of a Sewage Pollution Influenced Lagoon (Çardak Lagoon, Turkish Straits). Sustainability. 2023; 15(11):8946. https://doi.org/10.3390/su15118946
Chicago/Turabian StyleDağlı, Ertan, Abdullah Suat Ateş, Seçil Acar, Yeşim Büyükateş, Alper Doğan, and Ahmet Kerem Bakır. 2023. "Exotic Polychaetes of a Sewage Pollution Influenced Lagoon (Çardak Lagoon, Turkish Straits)" Sustainability 15, no. 11: 8946. https://doi.org/10.3390/su15118946





