Application of the PDMS Passive Sampling Method to Assess Bioavailability and Health Risks Associated with PAH-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Analysis
2.1.1. Sample Collection and Processing
2.1.2. Total PAHs and Soil Properties
2.1.3. PAH Bioavailability
2.2. Human Health Risk Assessment
3. Results and Discussion
3.1. Total PAHs in Soil
3.2. PAH Bioavailability in Soil
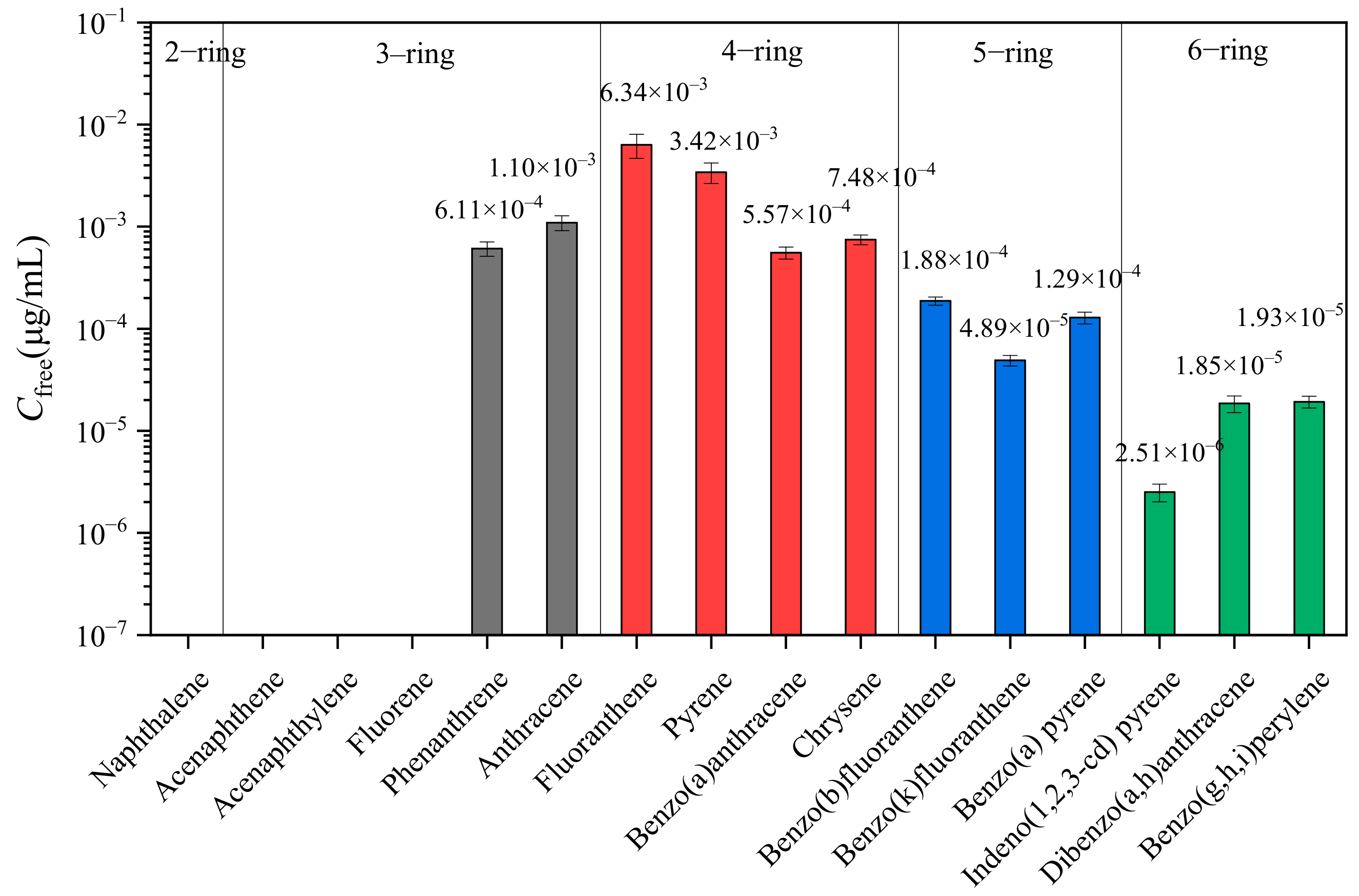
3.2.1. Cfree in PDMS Fiber
3.2.2. Comparison of Cfree and Cw
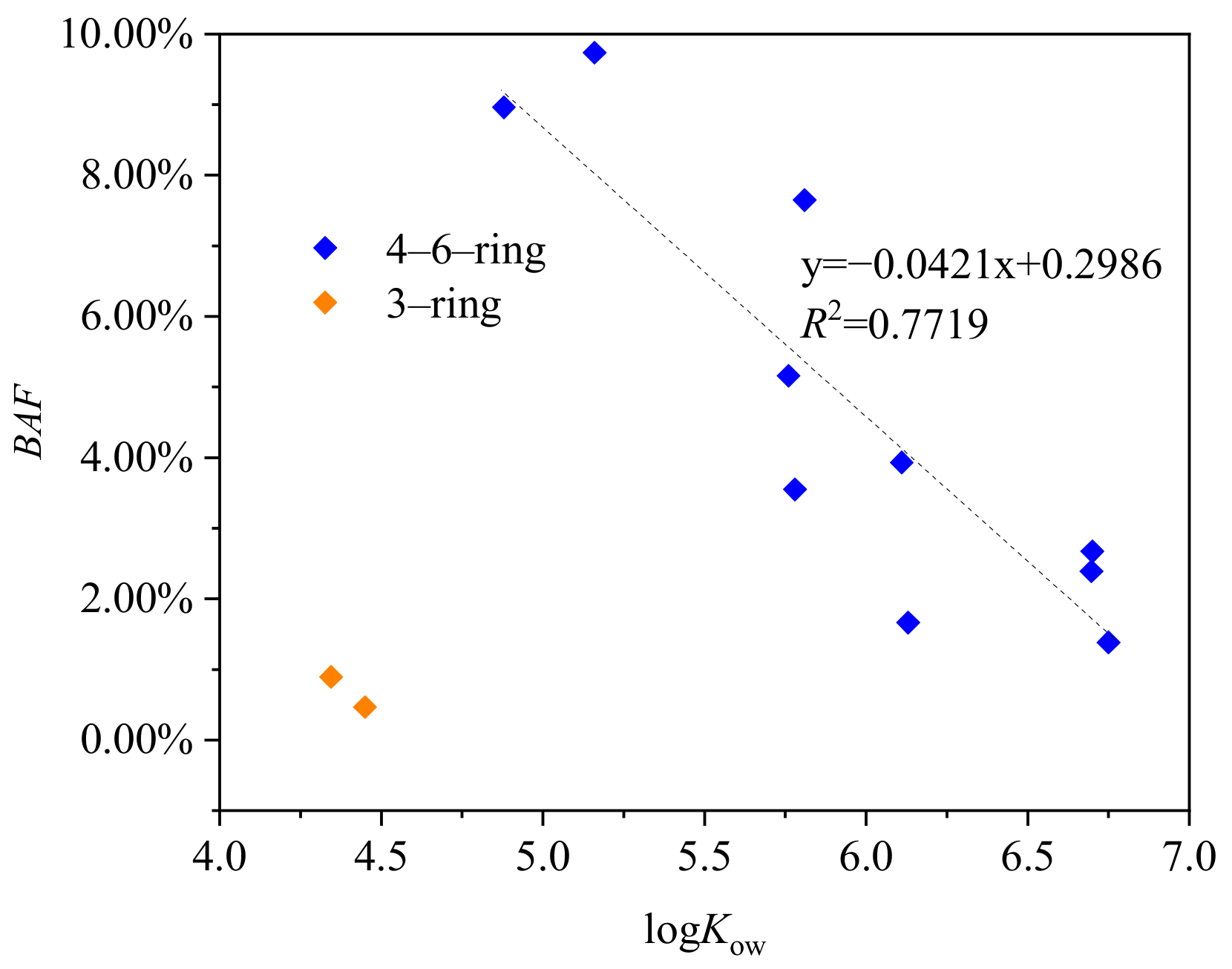
3.2.3. Bioavailability Factor
3.3. Human Health Risk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Contaminants | KPDMS-Water [19] mL-Water/mL-PDMS | Koc [54,64] L/kg | logKow [54,64] L/kg | H [54,64] Dimensionless |
|---|---|---|---|---|
| Naphthalene | 7.24 × 102 | 1.54 × 103 | 3.30 | 1.80 × 10−2 |
| Acenaphthylene | 5.62 × 102 | 6.92 × 103 | 3.94 | 4.74 × 10−3 |
| Acenaphthene | 2.51 × 103 | 5.03 × 103 | 3.92 | 7.52 × 10−3 |
| Fluorene | 3.63 × 103 | 9.16 × 103 | 4.18 | 3.93 × 10−3 |
| Phenanthrene | 6.31 × 103 | 1.41 × 104 | 4.35 | 5.40 × 10−3 |
| Anthracene | 6.76 × 103 | 1.64 × 104 | 4.45 | 2.27 × 10−3 |
| Fluoranthene | 1.58 × 104 | 5.55 × 104 | 5.16 | 3.62 × 10−4 |
| Pyrene | 1.86 × 104 | 5.43 × 104 | 4.88 | 4.91 × 10−4 |
| Benzo(a)anthracene | 5.89 × 104 | 1.77 × 105 | 5.76 | 4.91 × 10−4 |
| Chrysene | 4.90 × 104 | 1.81 × 105 | 5.81 | 2.14 × 10−4 |
| Benzo(b)fluoranthene | 1.62 × 105 | 5.99 × 105 | 5.78 | 2.69 × 10−5 |
| Benzo(k)fluoranthene | 1.78 × 105 | 5.87 × 105 | 6.11 | 2.39 × 10−5 |
| Benzo(a)pyrene | 1.74 × 105 | 5.87 × 105 | 6.13 | 1.87 × 10−5 |
| Dibenzo(a,h)anthracene | 3.31 × 105 | 1.95 × 106 | 6.75 | 1.42 × 10−5 |
| Benzo(g,h,i)perylene | 4.07 × 105 | 1.91 × 106 | 6.70 | 5.76 × 10−6 |
| Indeno(1,2,3-cd)pyrene | 4.37 × 105 | 1.58 × 106 | 6.70 | 5.82 × 10−6 |
| Symbol | Unit | Value | Symbol | Unit | Value |
|---|---|---|---|---|---|
| OSIRc | mg·d−1 | 200 | OSIRa | mg·d−1 | 100 |
| EDc | a | 6 | EDa | a | 24 |
| EFc | d·a−1 | 350 | EFa | d·a−1 | 350 |
| BWc | kg | 19.2 | BWa | kg | 61.8 |
| ATca | d | 27740 | ATnc | d | 2190 |
| SAF | dimensionless | 0.5 | BAF | dimensionless | measured |
| SFo | (mg·kg−1·d−1)−1 | Benzo(a)anthracene: 1.0 × 10−1 Chrysene:1.0 × 10−3 Benzo(b)fluoranthene: 1.0 × 10−1 Benzo(k)fluoranthene: 1.0 × 10−2 Benzo(a)pyrene: 1 Dibenzo(a,h)anthracene: 1 Indeno(1,2,3-cd)pyrene: 1.0 × 10−1 | RfDo | mg·kg−1·d−1 | Fluoranthene: 4.0 × 10−2 Pyrene: 3.0 × 10−2 Benzo(a)pyrene: 3.0 × 10−4 Benzo(g,h,i)Perylene: 3.0 × 10−1 |
| Number of Rings | Contaminants | Cfree μg/mL | Cw μg/mL | BAF |
|---|---|---|---|---|
| 2-ring | Naphthalene | - | 1.18 | - |
| 3-ring | Acenaphthylene | - | 6.90 × 10−2 | - |
| Acenaphthene | - | 9.36 × 10−1 | - | |
| Fluorene | - | 6.72 × 10−2 | - | |
| Phenanthrene | 6.11 × 10−4 | 6.84 × 10−2 | 0.89% | |
| Anthracene | 1.10 × 10−3 | 2.36 × 10−1 | 0.46% | |
| 4-ring | Fluoranthene | 6.34 × 10−3 | 6.51 × 10−2 | 9.74% |
| Pyrene | 3.42 × 10−3 | 3.81 × 10−2 | 8.96% | |
| Benzo(a)anthracene | 5.57 × 10−4 | 1.08 × 10−2 | 5.16% | |
| Chrysene | 7.48 × 10−4 | 9.77 × 10−3 | 7.65% | |
| 5-ring | Benzo(b)fluoranthene | 1.88 × 10−4 | 5.29 × 10−3 | 3.55% |
| Benzo(k)fluoranthene | 4.89 × 10−5 | 1.24 × 10−3 | 3.93% | |
| Benzo(a)pyrene | 1.29 × 10−4 | 7.74 × 10−3 | 1.66% | |
| 6-ring | Dibenzo(a,h)anthracene | 2.51 × 10−6 | 1.82 × 10−4 | 1.38% |
| Benzo(g,h,i)perylene | 1.85 × 10−5 | 7.75 × 10−4 | 2.39% | |
| Indeno(1,2,3-cd)pyrene | 1.93 × 10−5 | 7.22 × 10−4 | 2.67% |
References
- Blumer, M. Polycyclic aromatic compounds in nature. Sci. Am. 1976, 234, 35–45. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Polynuclear Aromatic Compounds, Part I, Chemical, Environmental and Experimental Data; International Agency for Research on Cancer: Lyon, France, 1983; Volume 32, pp. 35–91. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs); U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 1995.
- Jia, X.Y.; Jiang, L.; Xia, T.X.; Yao, J.J.; Liang, J.; Zhong, M.S. Analysis on accumulation, distribution and origin of polycyclic aromatic hydrocarbons in soils under a coking plant. J. CIESC 2011, 62, 3525–3531. [Google Scholar]
- Feng, Y.; Lv, Y.L.; Jiao, W.T.; Wang, T.Y.; Wang, G.; Shi, Y.J.; Luo, W. Distribution and risk of polycyclic aromatic hydro-carbons in soils from different workshops of an abandoned coking factory in Beijing. Asian J. Ecotoxicol. 2009, 4, 399–407. [Google Scholar]
- Tian, J.; Zhu, J.; Yang, H.B.; Wu, G.P.; Wei, F.S. Investigation, assessment and source analysis of polycyclic aromatic hydrocarbons (PAHs) pollution in soil from a large iron and steel plant and its surrounding areas, in China. Environ. Chem. 2013, 32, 1002–1008. [Google Scholar]
- Meng, X.S. Study on Pollution Characteristic of Polycyclic Aromatic Hydrocarbons at a Typical Iron and Steel Site in the North. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2020. [Google Scholar]
- Yao, W.C.; Su, Y.Y.; Zhang, E.Y.; Li, Q.; Liu, G. Pollution characteristic, source apportionment and risk assessment of polycyclic aromatic hydrocarbons in the soil of an iron and steel industrial area and its surroundings. J. Environ. Pollut. Control 2022, 44, 625–630+638. [Google Scholar]
- Orecchio, S. Contamination from polycyclic aromatic hydrocarbons (PAHs) in the soil of a botanic garden localized next to a former manufacturing gas plant in Palermo (Italy). J. Hazard. Mater. 2010, 180, 590–601. [Google Scholar] [CrossRef]
- Jennings, A. Worldwide regulatory guidance values for surface soil exposure to noncarcinogenic polycyclic aromatic hydrocarbons. J. Environ. Manag. 2012, 101, 173–190. [Google Scholar] [CrossRef]
- Yu, J.; Luo, H.; Yang, B.; Wang, M.; Gong, Y.; Wang, P.; Jiao, Y.; Liang, T.; Cheng, H.; Ma, F.; et al. Risk Control Values and Remediation Goals for Benzo[a]pyrene in Contaminated Sites: Sectoral Characteristics, Temporal Trends, and Empirical Implications. Environ. Sci. Technol. 2023, 57, 2064–2074. [Google Scholar] [CrossRef]
- Cui, X.Y.; Xiang, P.; He, R.W.; Juhasz, A.; Ma, L.Q. Advances in in vitro methods to evaluate oral bioaccessibility of PAHs and PBDEs in environmental matrices. Chemosphere 2016, 150, 378–389. [Google Scholar] [CrossRef]
- Zhang, S.J.; Li, C.; Li, Y.Z.; Zhang, R.R.; Gao, P.; Cui, X.Y.; Ma, L.Q. Bioaccessibility of PAHs in contaminated soils: Comparison of five in vitro methods with Tenax as a sorption sink. Sci. Total Environ. 2017, 601–602, 968–974. [Google Scholar] [CrossRef]
- Zhang, R.H.; Han, D.; Jiang, L.; Zhong, M.S.; Liang, J.; Xia, T.X.; Zhao, Y. Derivation of site-specific remediation goals by incorporating the bioaccessibility of polycyclic aromatic hydrocarbons with the probabilistic analysis method. J. Hazard. Mater. 2020, 384, 121239. [Google Scholar] [CrossRef]
- Rong, Y.Q.; Wang, L.F.; Xiao, Y.Y.; Dang, J.H. Evaluation on Models Applied to Health Risk Assessment of PAHs in Coking Plant. Environ. Sci. Technol. 2021, 44, 217–225. [Google Scholar]
- Shen, C.J. Practical Research on Investigation and Risk Assessment of a Coking Site. Master’s Thesis, Guangdong University of Technology, Guangzhou, China, 2019. [Google Scholar]
- Zhang, S.Y. Pollution characteristics and health risk assessment of heavy metals and polycyclic aromatic hydrocarbons in soils of remaining site left by a steel plant. Environ. Pollut. Control 2022, 44, 1336–1342. [Google Scholar]
- Northcott, G.L.; Jones, K.C. Partitioning, extractability, and formation of nonextractable PAH residues in soil. 1. Compound differences in aging and sequestration. Environ. Sci. Technol. 2001, 35, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Ruby, M.V.; Lowney, Y.W.; Bunge, A.L.; Roberts, S.M.; Gomez-Eyles, J.L.; Ghosh, U.; Kissel, J.C.; Tomlinson, P.; Menzie, C. Oral Bioavailability, Bioaccessibility, and Dermal Absorption of PAHs from Soil—State of the Science. Environ. Sci. Technol. 2016, 50, 2151–2164. [Google Scholar] [CrossRef]
- Van der Heijden, S.A.; Jonker, M.T.O. PAH Bioavailability in Field Sediments: Comparing Different Methods for Predicting in Situ Bioaccumulation. Environ. Sci. Technol. 2009, 43, 3757–3763. [Google Scholar] [CrossRef]
- Jonker, M.T.O.; Van der Heijden, S.A.; Kreitinger, J.P.; Hawthorne, S.B. Predicting PAH Bioaccumulation and Toxicity in Earthworms Exposed to Manufactured Gas Plant Soils with Solid-Phase Microextraction. Environ. Sci. Technol. 2007, 41, 7472–7478. [Google Scholar] [CrossRef]
- Ruby, M.V.; Schoof, R.; Brattin, W.; Goldade, M.; Post, G.; Harnois, M.D.; Mosby, E.S.; Casteel, W.; Berti, W.; Carpenter, M.; et al. Advances in Evaluating the Oral Bioavailability of Inorganics in Soil for Use in Human Health Risk Assessment. Environ. Sci. Technol. 1999, 33, 3697–3705. [Google Scholar] [CrossRef]
- Duan, L.; Naidu, R.; Liu, Y.; Dong, Z.; Mallavarapu, M.; Herde, P.; Kuchel, T.; Semple, K.T. Comparison of oral bioavailability of benzo[a]pyrene in soils using rat and swine and the implications for human health risk assessment. Environ. Int. 2016, 94, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Grøn, C.; Oomen, A.; Weyand, E.; Wittsiepe, J. Bioaccessibility of PAH from Danish soils. J. Environ. Sci. Health Part A 2007, 42, 1233–1239. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Weber, J.; Stevenson, G.; Slee, D.; Gancarz, D.; Rofe, A.; Smith, E. In vivo measurement, in vitro estimation and fugacity prediction of PAH bioavailability in post-remediated creosote-contaminated soil. Sci. Total Environ. 2014, 473–474, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Koganti, A.; Spina, D.A.; Rozett, K.; Ma, B.L.; Weyand, E.; Taylor, B.B.; Mauro, D.M. Studies on the Applicability of Biomarkers in Estimating the Systemic Bioavailability of Polynuclear Aromatic Hydrocarbons from Manufactured Gas Plant Tar-Contaminated Soils. Environ. Sci. Technol. 1998, 32, 3104–3112. [Google Scholar] [CrossRef]
- Chen, X.X.; Li, C.; Cao, X.Y.; Selvaraj, K.K.; Li, H.M.; Zhu, X.Y.; Yang, S.G.; Li, S.Y.; Zhang, L.M.; He, H. Bioaccessibility and bioavailability of NPAHs in soils using in vitro-in vivo assays: Comparison of laboratory and outdoor environmental aging effect. Sci. Total Environ. 2023, 868, 161619. [Google Scholar] [CrossRef] [PubMed]
- Oomen, A.G.; Hack, A.; Minekus, M.; Zeijdner, E.; Cornelis, C.; Schoeters, G.; Verstraete, W.; Van de Wiele, T.; Wragg, J.; Rompelberg, C.J.; et al. Comparison of Five In Vitro Digestion Models to Study the Bioaccessibility of Soil Contaminants. Environ. Sci. Technol. 2002, 36, 3326–3334. [Google Scholar] [CrossRef]
- BARGE (The Bioaccessibility Research Group of Europe). The BARGE Unified Bioaccessibility Method; BARGE: London, UK, 2016. [Google Scholar]
- Hack, A.; Selenka, F. Mobilization of PAH and PCB from contaminated soil using a digestive tract model. Toxicol. Lett. 1996, 88, 199–210. [Google Scholar] [CrossRef]
- Van de Wiele, T.R.; Verstraete, W.; Siciliano, S.D. Polycyclic Aromatic Hydrocarbon Release from a Soil Matrix in the In Vitro Gastrointestinal Tract. J. Environ. Qual. 2004, 33, 1343–1353. [Google Scholar] [CrossRef]
- Lu, M.; Li, G.; Yang, Y.; Yu, Y. A review on in-vitro oral bioaccessibility of organic pollutants and its application in human exposure assessment. Sci. Total Environ. 2021, 752, 142001. [Google Scholar] [CrossRef]
- Yu, Y.X.; Li, J.L.; Zhang, X.Y.; Yu, Z.Q.; Van de Wiele, T.; Han, S.Y.; Wu, M.H.; Sheng, G.Y.; Fu, J.M. Assessment of the Bioaccessibility of Polybrominated Diphenyl Ethers in Foods and the Correlations of the Bioaccessibility with Nutrient Contents. J. Agric. Food Chem. 2010, 58, 301–308. [Google Scholar] [CrossRef]
- Tang, X.Y.; Tang, L.; Zhu, Y.G.; Xing, B.S.; Duan, J.; Zheng, M.H. Assessment of the bioaccessibility of polycyclic aromatic hydrocarbons in soils from Beijing using an in vitro test. Environ. Pollut. 2006, 140, 279–285. [Google Scholar] [CrossRef]
- Burgess, R.M. Guidelines for Using Passive Samplers to Monitor Organic Contaminants at Superfund Sediment Sites; U.S. Environmental Protection Agency: Washington, DC, USA, 2012.
- Booij, K.; Robinson, C.D.; Burgess, R.M.; Mayer, P.; Roberts, C.A.; Ahrens, L.; Allan, I.J.; Brant, J.; Jones, L.; Kraus, U.R.; et al. Passive Sampling in Regulatory Chemical Monitoring of Nonpolar Organic Compounds in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 3–17. [Google Scholar] [CrossRef]
- Jahnke, A.; Mayer, P. Do complex matrices modify the sorptive properties of polydimethylsiloxane (PDMS) for non-polar organic chemicals? J. Chromatogr. A 2010, 1217, 4765–4770. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.Y. Estimation of Bioaccumulation Potential for Hydrophobic Organic Contaminants by Polydimethylsiloxane-Based Passive Sampling. Ph.D. Thesis, University of China Academy of Science (Guangzhou Institute of Geochemistry), Guangzhou, China, 2017. [Google Scholar]
- Ter Laak, T.L.; Barendregt, A.; Hermens, J.L.M. Freely dissolved pore water concentrations and sorption coefficients of PAHs in spiked aged, and field-contaminated soils. Environ. Sci. Technol. 2006, 40, 2184–2190. [Google Scholar] [CrossRef]
- Bergknut, M.; Sehlin, E.; Lundstedt, S.; Andersson, P.L.; Haglund, P.; Tysklind, M. Comparison of techniques for estimating PAH bioavailability: Uptake in Eisenia fetida, passive samplers and leaching using various solvents and additives. Environ. Pollut. 2007, 145, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Van der Wal, L.; Jager, T.; Fleuren, R.; Barendregt, A.; Sinnige, T.L.; Van Gestel, C.A.M.; Hermens, J.L.M. Solid-phase microextraction to predict bioavailability and accumulation of organic micropollutants in terrestrial organisms after exposure to a field-contaminated soil. Environ. Sci. Technol. 2004, 38, 4842–4848. [Google Scholar] [CrossRef]
- Wang, J.; Taylor, A.; Xu, C.; Schlenk, D.; Gan, J. Evaluation of different methods for assessing bioavailability of DDT residues during soil remediation. Environ. Pollut. 2018, 238, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Maruya, K.A.; Lao, W.; Tsukada, D.; Diehl, D.W. A passive sampler based on solid phase microextraction (SPME) for sediment-associated organic pollutants: Comparing freely-dissolved concentration with bioaccumulation. Chemosphere 2015, 137, 192–197. [Google Scholar] [CrossRef]
- Beijing Municipal Ecology and Environment Bureau. Site Investigation and Risk Assessment Guideline of Development Land; Beijing Municipal Ecology and Environment Bureau: Beijing, China, 2019.
- Jia, X.; Xia, T.; Liang, J.; Zhu, X.; Zhang, D.; Wang, J. Source Apportionment of Heavy Metals Based on Multiple Approaches for a Proposed Subway Line in the Southeast Industrial District of Beijing, China. Int. J. Environ. Res. Public Health 2022, 20, 683. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Method 8270E (SW-846): Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS); U.S. EPA: Washington, DC, USA, 2014.
- Ministry of Ecology and Environment of the People’s Republic of China. Technical Provisions on Quality Assurance and Quality Control for Enterprise Land Surveys in Key Industries (for Trial Implementation); Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2017.
- Research Institute of Forestry Chinese Academy of Forestry. Determination of Particle Density in Forest Soil; Research Institute of Forestry Chinese Academy of Forestry: Beijing, China, 1999. [Google Scholar]
- RPCMOA. Soil Testing Part 4: Method for Determination of Soil Bulk Density; RPCMOA: Beijing, China, 2006. [Google Scholar]
- RPCCAF. Determination of Forest Soil Water Content; RPCCAF: Beijing, China, 1999. [Google Scholar]
- RPCMOA. Soil Testing Part 6: Method for Determination of Soil Organic Matter; RPCMOA: Beijing, China, 2006. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China. Technical Guidelines for Risk Assessment of Soil Contamination of Land for Construction; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2019.
- Fan, J.J.; Zhou, Y.Y.; Wang, S.P.; Zhang, C.Y.; Guo, X.X. Bioaccessibility and Health Risk of PAHs in Site Soil Based on DIN Test. Res. Environ. Sci. 2020, 33, 2629–2638. [Google Scholar]
- Texas Commission on Environmental Quality. TRRP Protective Concentration Levels; Texas Commission on Environmental Quality: Austin, TX, USA, 2022.
- Meng, X.S.; Wu, M.M.; Chen, H.H.; Yue, X.; Tao, S.Y. Vertical pollution characteristics and sources of polycyclic aromatic hydrocarbons in a heterogeneous unsaturated zone under a coking plant. J. Environ. Sci. 2020, 41, 377–384. [Google Scholar]
- Guo, X.X.; Fan, J.J.; Zhou, Y.Y.; Zhang, C.Y.; Wang, S.P.; Yan, K. Xiong, J. Refined risk assessment of typical polycyclic aromatic hydrocarbons in a coking site. Asian J. Ecotoxicol. 2021, 16, 155–164. [Google Scholar]
- Li, Q.; Li, M.; Li, Y.; Zhou, Y.; Deng, S.P.; Zhang, S.T.; Fan, T.T.; Wu, Y.J. Analysis on distribution characteristics of soil polycyclic aromatic hydrocarbons (PHAs) in a large coking plant site. J. Ecol. Rural Environ. 2021, 37, 1623–1632. [Google Scholar]
- Hebei Provincial Academy of Ecological Environmental Science. Screening Value of Soil Pollution Risk of Construction Land; Hebei Provincial Academy of Ecological Environmental Science: Qinhuangdao, China, 2020. [Google Scholar]
- Shenzhen Academy of Ecological Environmental Science. Risk Screening Values and Intervention Values for Soil Contamination of Development Land in Shenzhen; Shenzhen Academy of Ecological Environmental Science: Shenzhen, China, 2020. [Google Scholar]
- Li, N.; Guo, M.X.; Gong, Z.Q.; Zhao, R.R. Prediction of the bioavailability of PAHs in earthworms by Tenax-TA and solid phase microextraction. Chin. J. Ecol. 2016, 35, 1963–1969. [Google Scholar]
- Li, J.Y.; Li, Z.H.; Cui, Y.; Hu, Q.; Chen, M.N.; Zheng, Y.X. Biomimetic Research on Bioavailability and Bioaccumulation of Sediment-associated Pyrethroids using Solid-Phase Microextraction. Asian J. Ecotoxicol. 2015, 10, 144–152. [Google Scholar]
- ChemSafety PRO. n-Octanol/Water Partition Coefficient (Kow/logKow); ChemSafety PRO: Piemonte, Italy, 2016. [Google Scholar]
- Xu, Y.Z. Study on Oral Bioaccessibility and Risk of Hydrophobic Organic Contaminants in Soils. Ph.D. Thesis, Guangdong University of Technology, Guangzhou, China, 2022. [Google Scholar]
- US EPA. Regional Screening Levels; OSWER: Washington, DC, USA, 2023.
- Tao, S.; Zhang, D.; Lu, Y.; Li, L.; Ding, J.; Yang, Y.; Yang, Y.; Wang, X.; Liu, W.; Xing, B. Mobility of Polycyclic Aromatic Hydrocarbons in the Gastrointestinal Tract Assessed Using an in Vitro Digestion Model with Sorption Rectification. Environ. Sci. Technol. 2010, 44, 5608–5612. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, Q.; Lin, A.J.; Zhu, Y.G. Concentrations and bioaccessibility of polycyclic aromatic hydrocarbons in wastewater-irrigated soil using in vitro gastrointestinal test. Environ. Sci. Pollut. Res. Int. 2008, 15, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Lou, S.; Wang, X.; Lu, S.; Ma, S.; Li, G.; Feng, Y.; Zhang, X.; An, T. Relationships between the bioavailability of polybrominated diphenyl ethers in soils measured with female C57BL/6 mice and the bioaccessibility determined using five in vitro methods. Environ. Int. 2019, 123, 337–344. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Tang, W.; Smith, E. Using in vitro bioaccessibility to refine estimates of human exposure to PAHs via incidental soil ingestion. Environ. Res. 2016, 145, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Sinha, A. Public health risk assessment with bioaccessibility considerations for soil PAHs at oil refinery vicinity areas in India. Sci. Total Environ. 2018, 616–617, 1477–1484. [Google Scholar] [CrossRef] [PubMed]




| Parameter | ρs | ρb | Pws | fom | foc | θT | θw | θa |
|---|---|---|---|---|---|---|---|---|
| g/cm3 | g/cm3 | kg-Water/kg-Soil | g/kg | Dimensionless | Dimensionless | Dimensionless | Dimensionless | |
| Value | 2.98 | 1.7 | 0.15 | 39.1 | 0.023 | 0.43 | 0.26 | 0.17 |
| Number of Rings | Contaminants | Content | Proportion | Screening Value | Exceeding Multiple | |
|---|---|---|---|---|---|---|
| 2-ring | Naphthalene | 41.10 | 5.50% | 5.50% | 25① | 0.64 |
| 3-ring | Acenaphthene | 10.75 | 1.44% | 31.97% | 2189 ② | - |
| Acenaphthylene | 106.00 | 14.18% | 2120 ③ | - | ||
| Fluorene | 13.85 | 1.85% | 1459 ② | - | ||
| Phenanthrene | 21.75 | 2.91% | 1060 ② | - | ||
| Anthracene | 86.75 | 11.60% | 10,000 ② | - | ||
| 4-ring | Fluoranthene | 81.25 | 10.87% | 28.15% | 1459 ② | - |
| Pyrene | 46.60 | 6.23% | 1094 ② | - | ||
| Benzo(a)anthracene | 42.95 | 5.74% | 5.5 ① | 6.81 | ||
| Chrysene | 39.70 | 5.31% | 490 ① | - | ||
| 5-ring | Benzo(b)fluoranthene | 71.30 | 9.54% | 25.41% | 5.5 ① | 11.96 |
| Benzo(k)fluoranthene | 16.45 | 2.20% | 55 ① | - | ||
| Benzo(a)pyrene | 102.25 | 13.67% | 0.55 ① | 184.91 | ||
| 6-ring | Indeno(1,2,3-cd)pyrene | 7.97 | 1.07% | 8.97% | 5.5 ① | 0.45 |
| Dibenzo(a,h)anthracene | 33.35 | 4.46% | 0.55 ① | 59.64 | ||
| Benzo(g,h,i)perylene | 25.75 | 3.44% | 1060 ② | - | ||
| - | ∑PAHs | 747.76 | - | - | - | - |
| Number of Rings | Contaminants | CL1 μg/mL | CL2 μg/mL | CL3 μg/mL | CL-average μg/mL | RSDs |
|---|---|---|---|---|---|---|
| 2-ring | Naphthalene | ND | ND | ND | ND | - |
| 3-ring | Acenaphthene | ND | ND | ND | ND | - |
| Acenaphthylene | ND | ND | ND | ND | - | |
| Fluorene | ND | ND | ND | ND | - | |
| Phenanthrene | 2.64 × 10−2 | 2.18 × 10−2 | 3.02 × 10−2 | 2.61 × 10−2 | 16.10% | |
| Anthracene | 5.58 × 10−2 | 4.05 × 10−2 | 5.42 × 10−2 | 5.02 × 10−2 | 16.76% | |
| 4-ring | Fluoranthene | 7.71 × 10−1 | 4.73 × 10−1 | 7.99 × 10−1 | 6.81 × 10−1 | 26.57% |
| Pyrene | 5.07 × 10−1 | 3.20 × 10−1 | 4.67 × 10−1 | 4.31 × 10−1 | 22.87% | |
| Benzo(a)anthracene | 1.87 × 10−1 | 2.42 × 10−1 | 2.37 × 10−1 | 2.22 × 10−1 | 13.59% | |
| Chrysene | 2.16 × 10−1 | 2.68 × 10−1 | 2.60 × 10−1 | 2.48 × 10−1 | 11.18% | |
| 5-ring | Benzo (b)fluoranthene | 1.84 × 10−1 | 2.16 × 10−1 | 2.19 × 10−1 | 2.06 × 10−1 | 9.42% |
| Benzo (k)fluoranthene | 5.08 × 10−2 | 6.31 × 10−2 | 6.30 × 10−2 | 5.90 × 10−2 | 11.99% | |
| Benzo (a)pyrene | 1.28 × 10−1 | 1.67 × 10−1 | 1.59 × 10−1 | 1.51 × 10−1 | 13.51% | |
| 6-ring | Indeno(1,2,3-cd)pyrene | 4.60 × 10−3 | 5.50 × 10−3 | 6.80 × 10−3 | 5.63 × 10−3 | 19.63% |
| Dibenzo(a,h)anthracene | 4.04 × 10−2 | 5.65 × 10−2 | 5.65 × 10−2 | 5.11 × 10−2 | 18.18% | |
| Benzo (g,h,i) perylene | 4.86 × 10−2 | 6.25 × 10−2 | 6.00 × 10−2 | 5.70 × 10−2 | 12.99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Yang, D.; Li, Y.; Zhu, X.; Zhang, L.; Wang, J. Application of the PDMS Passive Sampling Method to Assess Bioavailability and Health Risks Associated with PAH-Contaminated Soil. Sustainability 2023, 15, 9027. https://doi.org/10.3390/su15119027
Jia X, Yang D, Li Y, Zhu X, Zhang L, Wang J. Application of the PDMS Passive Sampling Method to Assess Bioavailability and Health Risks Associated with PAH-Contaminated Soil. Sustainability. 2023; 15(11):9027. https://doi.org/10.3390/su15119027
Chicago/Turabian StyleJia, Xiaoyang, Danhua Yang, Yandan Li, Xiaoying Zhu, Lina Zhang, and Jinsheng Wang. 2023. "Application of the PDMS Passive Sampling Method to Assess Bioavailability and Health Risks Associated with PAH-Contaminated Soil" Sustainability 15, no. 11: 9027. https://doi.org/10.3390/su15119027
APA StyleJia, X., Yang, D., Li, Y., Zhu, X., Zhang, L., & Wang, J. (2023). Application of the PDMS Passive Sampling Method to Assess Bioavailability and Health Risks Associated with PAH-Contaminated Soil. Sustainability, 15(11), 9027. https://doi.org/10.3390/su15119027





