Ecoenzymatic Stoichiometry Reveals Microbial Carbon and Phosphorus Limitations under Elevated CO2, Warming and Drought at Different Winter Wheat Growth Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Soil Materials
2.2. Experimental Design and Treatments
2.3. Collections of Soil and Plant Samples
2.4. Soil Property Measurements
2.5. Vector Length and Vector Angle Calculations
2.6. Data Analysis
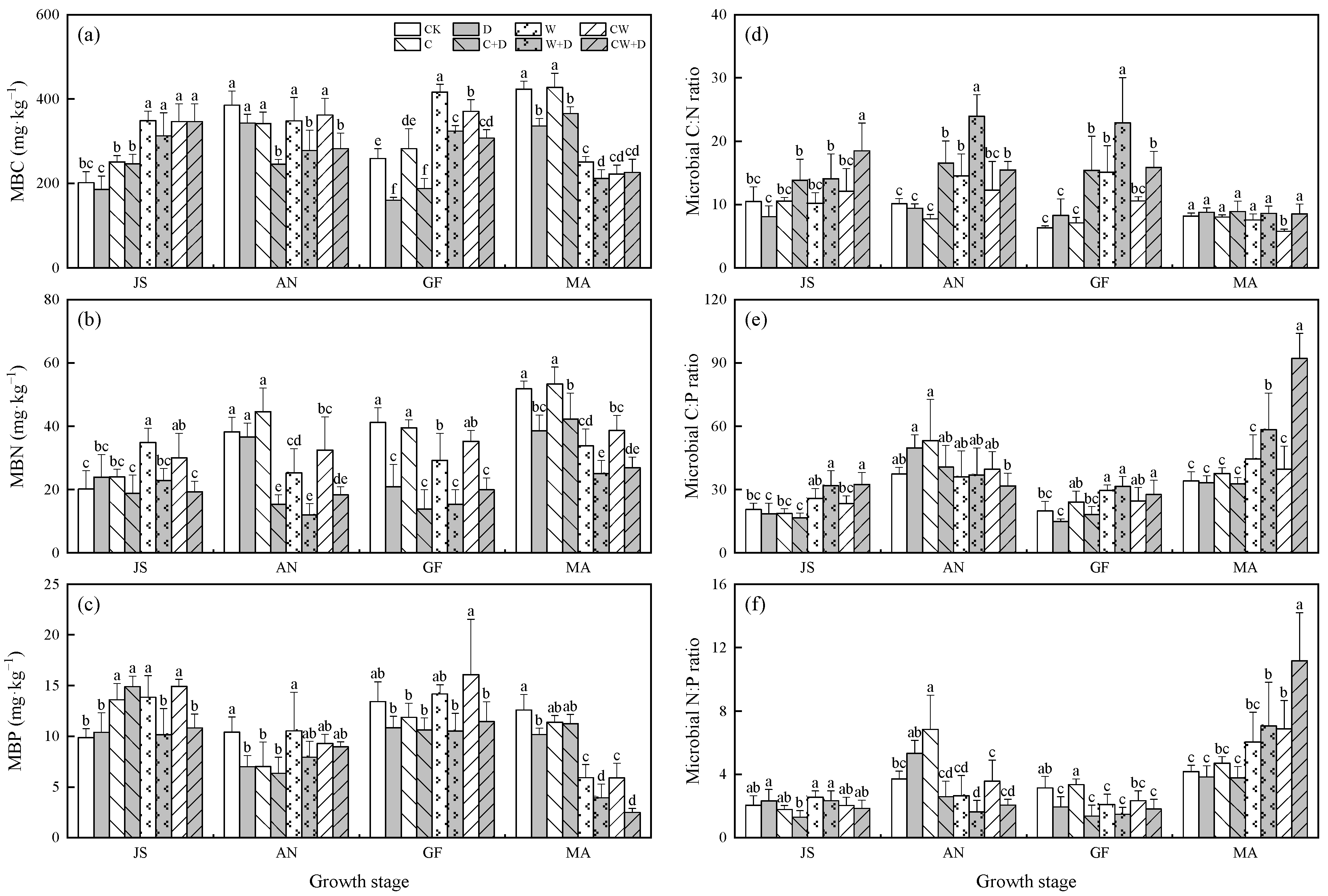
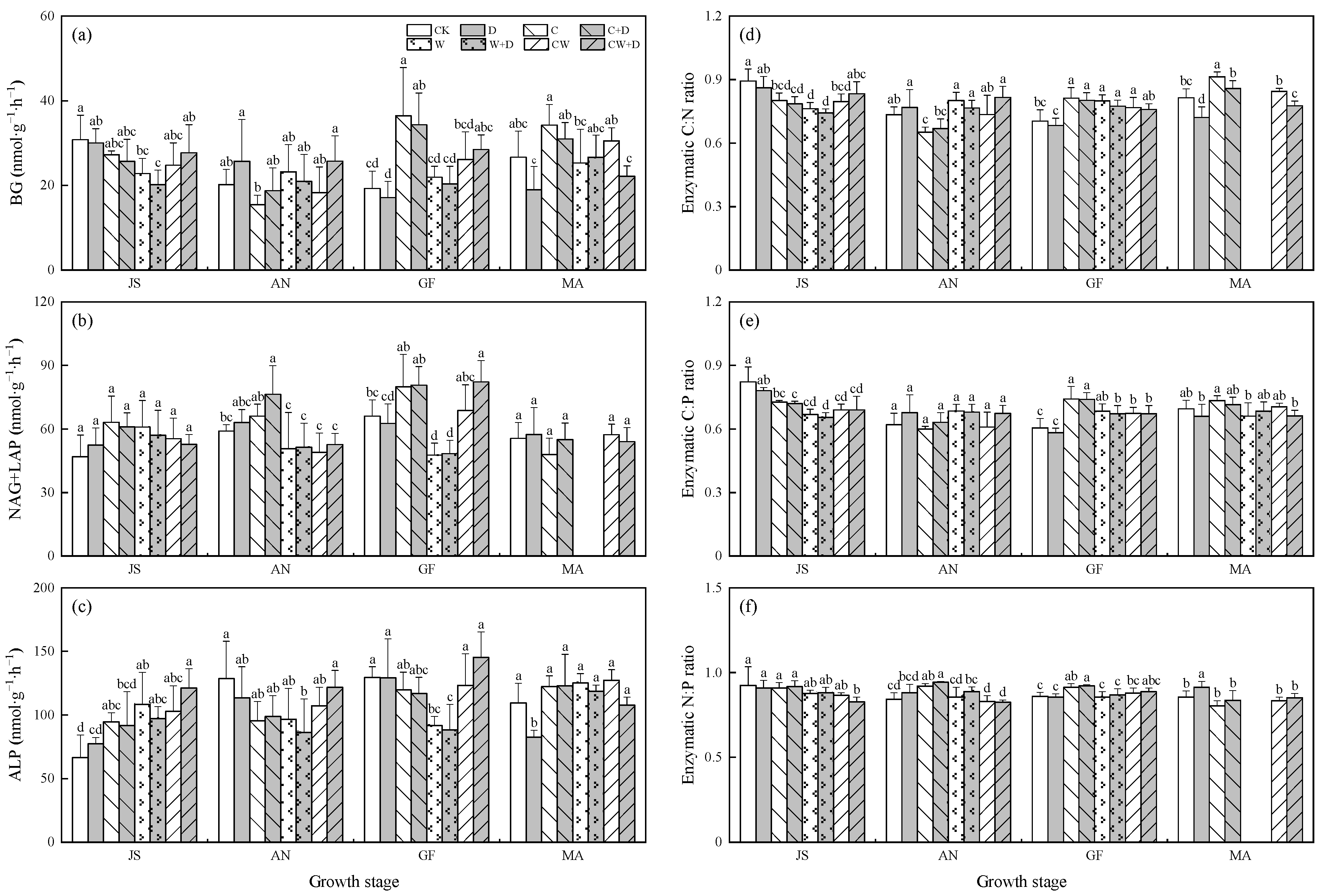
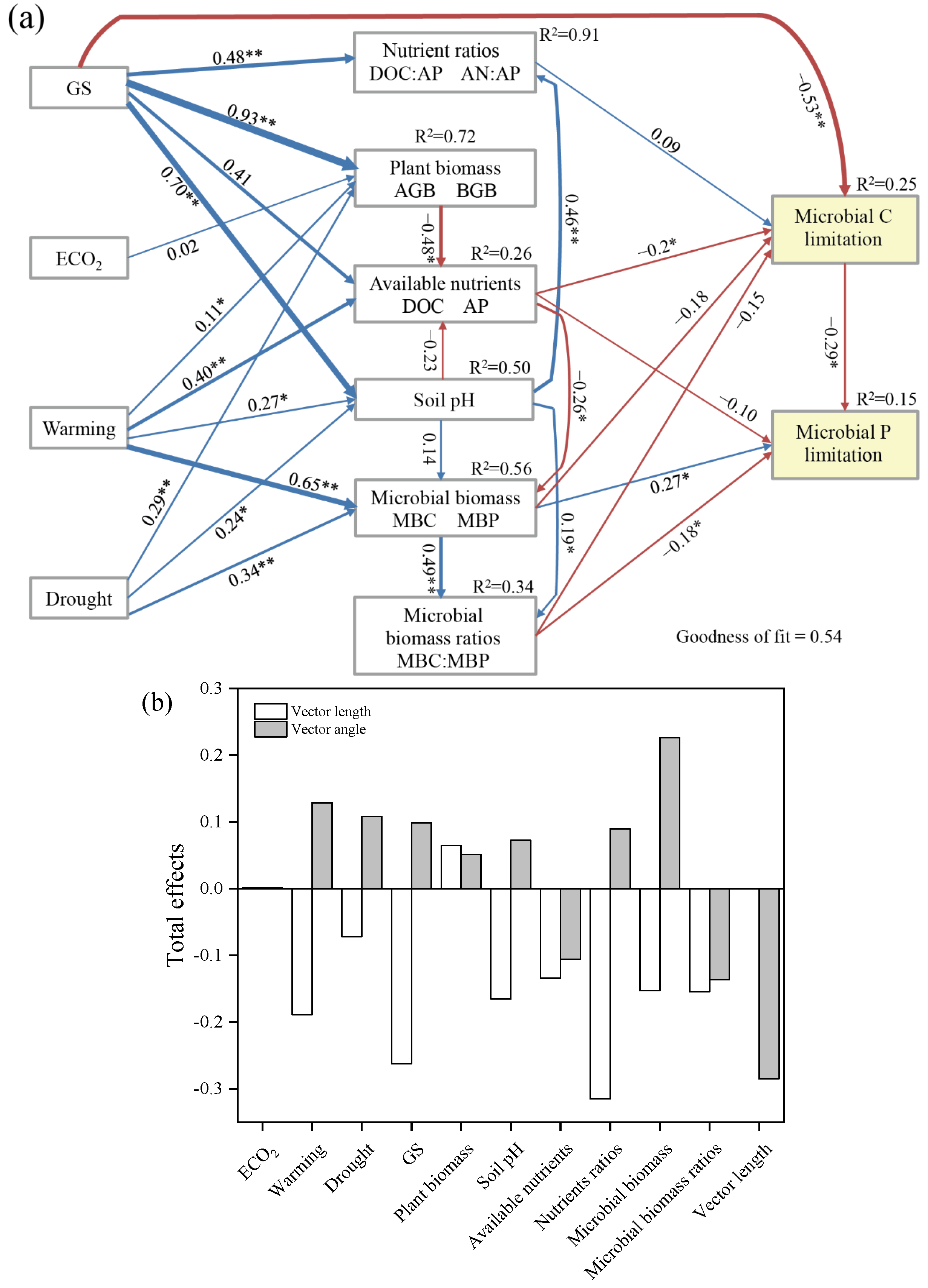
3. Results
3.1. Plant Biomass and Soil Physicochemical Properties
3.2. Soil Microbial Biomass and Stoichiometry
3.3. Soil Enzymatic Activity and Stoichiometry
3.4. Soil Microbial Metabolic Limitation
3.5. Factors Affecting Soil Microbial Metabolic Limitation
4. Discussion
4.1. Soil Microbial Metabolism Limitation under Climate Factors
4.1.1. Elevated CO2
4.1.2. Climate Warming
4.1.3. Drought
4.2. Soil Microbial Metabolism Limitation Responses to Interactions of Climate Factors
4.3. Potential Mechanism of the Effects of Climate Factors on Microbial Metabolic Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Soil C:N ratio | The ratio of DOC to AN |
| Soil C:P ratio | The ratio of DOC to AP |
| Soil N:P ratio | The ratio of AN to AP |
| Microbial C:N ratio | The ratio of MBC to MBN |
| Microbial C:P ratio | The ratio of MBC to MBP |
| Microbial N:P ratio | The ratio of MBN to MBP |
| Enzymatic C:N ratio | The ratio of ln(BG) to ln(NAG + LAP) |
| Enzymatic C:P ratio | The ratio of ln(BG) to ln(ALP) |
| Enzymatic N:P ratio | The ratio of ln(NAG + LAP) to ln(ALP) |
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Poulter, B.; Frank, D.; Ciais, P.; Myneni, R.B.; Andela, N.; Bi, J.; Broquet, G.; Canadell, J.G.; Chevallier, F.; Liu, Y.Y.; et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 2014, 509, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Haugwitz, M.S.; Bergmark, L.; Prieme, A.; Christensen, S.; Beier, C.; Michelsen, A. Soil microorganisms respond to five years of climate change manipulations and elevated atmospheric CO2 in a temperate heath ecosystem. Plant Soil 2014, 374, 211–222. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Q.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Cui, Y.X.; Fang, L.C.; Guo, X.B.; Han, F.; Ju, W.L.; Ye, L.P.; Wang, X.; Tan, W.F.; Zhang, X.C. Natural grassland as the optimal pattern of vegetation restoration in arid and semi-arid regions: Evidence from nutrient limitation of soil microbes. Sci. Total Environ. 2019, 648, 388–397. [Google Scholar] [CrossRef]
- Cui, Y.X.; Fang, L.C.; Deng, L.; Guo, X.B.; Han, F.; Ju, W.L.; Wang, X.; Chen, H.S.; Tan, W.F.; Zhang, X.C. Patterns of soil microbial nutrient limitations and their roles in the variation of soil organic carbon across a precipitation gradient in an arid and semi-arid region. Sci. Total Environ. 2019, 658, 1440–1451. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, X.; Green, S.M.; Dungait, J.A.J.; Wen, X.; Quine, T.A. Soil enzyme activity and stoichiometry along a gradient of vegetation restoration at the Karst Critical Zone Observatory in Southwest China. Land Degrad. Dev. 2019, 30, 1916–1927. [Google Scholar] [CrossRef]
- Montiel-Gonzalez, C.; Tapia-Torres, Y.; Souza, V.; Garcia-Oliva, F. The response of soil microbial communities to variation in annual precipitation depends on soil nutritional status in an oligotrophic desert. PeerJ 2017, 5, e4007. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.L.; Bing, H.J.; Chang, R.Y.; Cui, Y.X.; Shen, G.T.; Wang, X.X.; Zhang, S.P.; Fang, L.C. Microbial metabolic limitation response to experimental warming along an altitudinal gradient in alpine grasslands, eastern Tibetan Plateau. Catena 2022, 214, 106243. [Google Scholar] [CrossRef]
- Wang, X.L.; Gong, X.W.; Li, X.Y.; Sun, S.T.; Dang, K.; Feng, B.L. Microbial nutrient limitation in rhizosphere soils of different food crop families: Evidence from ecoenzymatic stoichiometry. Land Degrad. Dev. 2022, 34, 1019–1034. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Cheng, W. Interactions between soil and tree roots accelerate long-term soil carbon decomposition. Ecol. Lett. 2007, 10, 1046–1053. [Google Scholar] [CrossRef]
- Stone, M.M.; DeForest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Alarcon-Gutierrez, E.; Floch, C.; Ziarelli, F.; Augur, C.; Criquet, S. Drying-rewetting cycles and gamma-irradiation effects on enzyme activities of distinct layers from a Quercus ilex L. litter. Soil Biol. Biochem. 2010, 42, 283–290. [Google Scholar] [CrossRef]
- Thakur, M.P.; Del Real, I.M.; Cesarz, S.; Steinauer, K.; Reich, P.B.; Hobbie, S.; Ciobanu, M.; Rich, R.; Worm, K.; Eisenhauer, N. Soil microbial, nematode, and enzymatic responses to elevated CO2 N fertilization, warming, and reduced precipitation. Soil Biol. Biochem. 2019, 135, 184–193. [Google Scholar] [CrossRef]
- Guenet, B.; Lenhart, K.; Leloup, J.; Giusti-Miller, S.; Pouteau, V.; Mora, P.; Nunan, N.; Abbadie, L. The impact of long-term CO2 enrichment and moisture levels on soil microbial community structure and enzyme activities. Geoderma 2012, 170, 331–336. [Google Scholar] [CrossRef]
- Alvarez, R.; Lavado, R.S. Climate, organic matter and clay content relationships in the Pampa and Chaco soils, Argentina. Geoderma 1998, 83, 127–141. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.D.; Gao, D.X.; Wang, X.; Liu, W.C.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Ecoenzymatic stoichiometry and nutrient dynamics along a revegetation chronosequence in the soils of abandoned land and Robinia pseudoacacia plantation on the Loess Plateau, China. Soil Biol. Biochem. 2019, 134, 1–14. [Google Scholar] [CrossRef]
- Banerjee, S.; Bora, S.; Thrall, P.H.; Richardson, A.E. Soil C and N as causal factors of spatial variation in extracellular enzyme activity across grassland-woodland ecotones. Appl. Soil Ecol. 2016, 105, 1–8. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Henry, H.A.L. Soil extracellular enzyme dynamics in a changing climate. Soil Biol. Biochem. 2012, 47, 53–59. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef]
- Khan, F.N.; Lukac, M.; Turner, G.; Godbold, D.L. Elevated atmospheric CO2 changes phosphorus fractions in soils under a short rotation poplar plantation (EuroFACE). Soil Biol. Biochem. 2008, 40, 1716–1723. [Google Scholar] [CrossRef]
- Williams, M.A. Response of microbial communities to water stress in irrigated and drought-prone tallgrass prairie soils. Soil Biol. Biochem. 2007, 39, 2750–2757. [Google Scholar] [CrossRef]
- Adair, E.C.; Reich, P.B.; Trost, J.J.; Hobbie, S.E. Elevated CO2 stimulates grassland soil respiration by increasing carbon inputs rather than by enhancing soil moisture. Glob. Change Biol. 2011, 17, 3546–3563. [Google Scholar] [CrossRef]
- Ebersberger, D.; Niklaus, P.A.; Kandeler, E. Long term CO2 enrichment stimulates N-mineralisation and enzyme activities in calcareous grassland. Soil Biol. Biochem. 2003, 35, 965–972. [Google Scholar] [CrossRef]
- Wall, G.W.; Garcia, R.L.; Kimball, B.A.; Hunsaker, D.J.; Pinter, P.J.; Long, S.P.; Osborne, C.P.; Hendrix, D.L.; Wechsung, F.; Wechsung, G.; et al. Interactive effects of elevated carbon dioxide and drought on wheat. Agron. J. 2006, 98, 354–381. [Google Scholar] [CrossRef]
- Pinay, G.; Barbera, P.; Carreras-Palou, A.; Fromin, N.; Sonie, L.; Couteaux, M.M.; Roy, J.; Philippot, L.; Lensi, R. Impact of atmospheric CO2 and plant life forms on soil microbial activities. Soil Biol. Biochem. 2007, 39, 33–42. [Google Scholar] [CrossRef]
- Mueller, C.; Ruetting, T.; Abbasi, M.K.; Laughlin, R.J.; Kammann, C.; Clough, T.J.; Sherlock, R.R.; Kattge, J.; Jaeger, H.-J.; Watson, C.J.; et al. Effect of elevated CO2 on soil N dynamics in a temperate grassland soil. Soil Biol. Biochem. 2009, 41, 1996–2001. [Google Scholar] [CrossRef]
- Inselsbacher, E.; Umana, N.H.-N.; Stange, F.C.; Gorfer, M.; Schueller, E.; Ripka, K.; Zechmeister-Boltenstern, S.; Hood-Novotny, R.; Strauss, J.; Wanek, W. Short-term competition between crop plants and soil microbes for inorganic N fertilizer. Soil Biol. Biochem. 2010, 42, 360–372. [Google Scholar] [CrossRef]
- Hu, S.; Chapin, F.S.; Firestone, M.K.; Field, C.B.; Chiariello, N.R. Nitrogen limitation of microbial decomposition in a grassland under elevated CO2. Nature 2001, 409, 188–191. [Google Scholar] [CrossRef]
- Keane, J.B.; Hoosbeek, M.R.; Taylor, C.R.; Miglietta, F.; Phoenix, G.K.; Hartley, I.P. Soil C, N and P cycling enzyme responses to nutrient limitation under elevated CO2. Biogeochemistry 2020, 151, 221–235. [Google Scholar] [CrossRef]
- Bradford, M.A.; Davies, C.A.; Frey, S.D.; Maddox, T.R.; Melillo, J.M.; Mohan, J.E.; Reynolds, J.F.; Treseder, K.K.; Wallenstein, M.D. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol. Lett. 2008, 11, 1316–1327. [Google Scholar] [CrossRef]
- Steinweg, J.M.; Dukes, J.S.; Paul, E.A.; Wallenstein, M.D. Microbial responses to multi-factor climate change: Effects on soil enzymes. Front. Microbiol. 2013, 4, 146. [Google Scholar] [CrossRef]
- Romero-Olivares, A.L.; Allison, S.D.; Treseder, K.K. Soil microbes and their response to experimental warming over time: A meta-analysis of field studies. Soil Biol. Biochem. 2017, 107, 32–40. [Google Scholar] [CrossRef]
- Jing, X.; Wang, Y.; Chung, H.; Mi, Z.; Wang, S.; Zeng, H.; He, J.S. No temperature acclimation of soil extracellular enzymes to experimental warming in an alpine grassland ecosystem on the Tibetan Plateau. Biogeochemistry 2014, 117, 39–54. [Google Scholar] [CrossRef]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.H.C.; Gurevitch, J.; Gcte, N. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, S.E.; Nadelhoffer, K.J.; Hogberg, P. A synthesis: The role of nutrients as constraints on carbon balances in boreal and arctic regions. Plant Soil 2002, 242, 163–170. [Google Scholar] [CrossRef]
- Verburg, P.S.J.; Van Loon, W.K.P.; Lukewille, A. The CLIMEX soil-heating experiment: Soil response after 2 years of treatment. Biol. Fertil. Soils 1999, 28, 271–276. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Change Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Zheng, H.F.; Liu, Y.; Chen, Y.M.; Zhang, J.; Li, H.J.; Wang, L.F.; Chen, Q. Short-term warming shifts microbial nutrient limitation without changing the bacterial community structure in an alpine timberline of the eastern Tibetan Plateau. Geoderma 2020, 360, 113985. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Sardans, J.; Penuelas, J.; Estiarte, M. Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland. Appl. Soil Ecol. 2008, 39, 223–235. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Kandeler, E.; Boddy, L. Interactive effects of temperature and soil moisture on fungal-mediated wood decomposition and extracellular enzyme activity. Soil Biol. Biochem. 2014, 70, 151–158. [Google Scholar] [CrossRef]
- Tapia-Torres, Y.; Elser, J.J.; Souza, V.; Garcia-Oliva, F. Ecoenzymatic stoichiometry at the extremes: How microbes cope in an ultra-oligotrophic desert soil. Soil Biol. Biochem. 2015, 87, 34–42. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Li, Y.; Wu, H.D.; Zhang, K.R.; Hao, Y.B.; Wang, J.Z.; Zhang, X.D.; Yan, L.; Kang, X.M. Different responses of soil hydrolases and oxidases to extreme drought in an alpine peatland on the Qinghai-Tibet Plateau, China. Eur. J. Soil Biol. 2020, 99, 103195. [Google Scholar] [CrossRef]
- Sun, Y.; Liao, J.H.; Zou, X.M.; Xu, X.; Yang, J.Y.; Chen, H.Y.H.; Ruan, H.H. Coherent responses of terrestrial C:N stoichiometry to drought across plants, soil, and microorganisms in forests and grasslands. Agric. For. Meteorol. 2020, 292, 108104. [Google Scholar] [CrossRef]
- Steinweg, J.M.; Dukes, J.S.; Wallenstein, M.D. Modeling the effects of temperature and moisture on soil enzyme activity: Linking laboratory assays to continuous field data. Soil Biol. Biochem. 2012, 55, 85–92. [Google Scholar] [CrossRef]
- Henry, H.A.L.; Juarez, J.D.; Field, C.B.; Vitousek, P.M. Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a California annual grassland. Glob. Change Biol. 2005, 11, 1808–1815. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Zhang, X.C.; Zheng, B.Y.; Yue, S.C.; Zhang, X.C.; Zhai, B.N.; Wang, Z.H.; Zheng, W.; Li, Z.Y.; Zamanian, K.; et al. Effects of plastic and straw mulching on soil microbial P limitations in maize fields: Dependency on soil organic carbon demonstrated by ecoenzymatic stoichiometry. Geoderma 2021, 388, 114928. [Google Scholar] [CrossRef]
- Li, Y.F.; Xiao, M.L.; Yuan, H.C.; Zhu, Z.K.; Wang, J.R.; Li, K.L.; Ge, T.D.; Wu, J.S. Effects of doubled concentration of CO2 on soil hydrolase activities related to turnover of soil C and N in a rice-cropping system. China Environ. Sci. 2018, 38, 3474–3480. (In Chinese) [Google Scholar]
- Padhy, S.R.; Nayak, S.; Dash, P.K.; Das, M.; Roy, K.S.; Nayak, A.K.; Neogi, S.; Bhattacharyya, P. Elevated carbon dioxide and temperature imparted intrinsic drought tolerance in aerobic rice system through enhanced exopolysaccharide production and rhizospheric activation. Agric. Ecosyst. Environ. 2018, 268, 52–60. [Google Scholar] [CrossRef]
- Cui, Y.X.; Zhang, Y.L.; Duan, C.J.; Wang, X.; Zhang, X.C.; Ju, W.L.; Chen, H.S.; Yue, S.C.; Wang, Y.Q.; Li, S.Q.; et al. Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage Res. 2020, 197, 104463. [Google Scholar] [CrossRef]
- Zhao, G.C.; Chang, X.H.; Wang, D.M.; Tao, Z.Q.; Wang, Y.J.; Yang, Y.S.; Zhu, Y.J. General situation and development of wheat production. Crops 2018, 34, 1–7. (In Chinese) [Google Scholar]
- Lyu, J.Y.; Jiang, Y.N.; Xu, C.; Liu, Y.J.; Su, Z.H.; Liu, J.C.; He, J.Q. Multi-objective winter wheat irrigation strategies optimization based on coupling AquaCrop-OSPy and NSGA-III: A case study in Yangling, China. Sci. Total Environ. 2022, 843, 157104. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). World Reference Base for Soil Resources 2014 (Updated 2015); World soil resources reports; FAO: Rome, Italy, 2015; p. 106. Available online: https://www.isric.org/explore/wrb (accessed on 16 October 2019).
- Lane, J.M.; Delavaux, C.S.; Koppen, L.V.; Lu, P.N.; Cade-Menun, B.J.; Tremblay, J.; Bainard, D. Soil sample storage conditions impact extracellular enzyme activity and bacterial amplicon diversity metrics in a semi-arid ecosystem. Soil Biol. Biochem. 2022, 175, 108858. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000; pp. 29–34, 44–48, 74–76, 81–83. (In Chinese) [Google Scholar]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org (accessed on 20 May 2022).
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martinez, V.; Calderon, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Norby, R.J. Issues and perspectives for investigating root responses to elevated atmospheric carbon-dioxide. Plant Soil 1994, 165, 9–20. [Google Scholar] [CrossRef]
- Sulman, B.N.; Phillips, R.P.; Oishi, A.C.; Shevliakova, E.; Pacala, S.W. Microbe-driven turnover offsets mineral-mediated storage of soil carbon under elevated CO2. Nat. Clim. Change 2014, 4, 1099–1102. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Pendall, E.; Morgan, J.A.; Blumenthal, D.M.; Carrillo, Y.; LeCain, D.R.; Follett, R.F.; Williams, D.G. Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol. 2012, 196, 807–815. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic stoichiometry and ecological theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Reed, S.C.; Townsend, A.R. Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 2006, 87, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of soil microbial and enzyme activities are linked to soil C, N and P stoichiometry in afforested ecosystems. For. Ecol. Manag. 2018, 427, 289–295. [Google Scholar] [CrossRef]
- Walker, T.W.N.; Kaiser, C.; Strasser, F.; Herbold, C.W.; Leblans, N.I.W.; Woebken, D.; Janssens, I.A.; Sigurdsson, B.D.; Richter, A. Microbial temperature sensitivity and biomass change explain soil carbon loss with warming. Nat. Clim. Change 2018, 8, 885–889. [Google Scholar] [CrossRef]
- Giardina, C.P.; Litton, C.M.; Crow, S.E.; Asner, G.P. Warming-related increases in soil CO2 effux are explained by increased below-ground carbon flux. Nat. Clim. Change 2014, 4, 822–827. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Schnecker, J.; Takriti, M.; Borken, W.; Wanek, W. Microbial physiology and soil CO2 efflux after 9 years of soil warming in a temperate forest—No indications for thermal adaptations. Glob. Change Biol. 2015, 21, 4265–4277. [Google Scholar] [CrossRef]
- Young, L.W.; Wilen, R.W.; Bonham-Smith, P.C. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 2004, 55, 485–495. [Google Scholar] [CrossRef]
- Jing, Z.W.; Chen, R.R.; Wei, S.P.; Feng, Y.Z.; Zhang, J.B.; Lin, X.G. Response and feedback of C mineralization to P availability driven by soil microorganisms. Soil Biol. Biochem. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Emmett, B.A.; Beier, C.; Estiarte, M.; Tietema, A.; Kristensen, H.L.; Williams, D.; Penuelas, J.; Schmidt, I.; Sowerby, A. The response of soil processes to climate change: Results from manipulation studies of shrublands across an environmental gradient. Ecosystems 2004, 7, 625–637. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Poll, C.; Ingwersen, J.; Stemmer, M.; Gerzabek, M.H.; Kandeler, E. Mechanisms of solute transport affect small-scale abundance and function of soil microorganisms in the detritusphere. Eur. J. Soil Sci. 2006, 57, 583–595. [Google Scholar] [CrossRef]
- Liu, J.L.; Yang, Z.L.; Dang, P.; Zhu, H.L.; Gao, Y.; Vu Ngoc, H.; Zhao, Z. Response of soil microbial community dynamics to Robinia pseudoacacia L. afforestation in the loess plateau: A chronosequence approach. Plant Soil 2018, 423, 327–338. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Jing, X.; Chen, X.; Xiao, W.; Lin, L.; Wang, C.; He, J.S.; Zhu, B. Soil enzymatic responses to multiple environmental drivers in the Tibetan grasslands: Insights from two manipulative field experiments and a meta-analysis. Pedobiologia 2018, 71, 50–58. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Cesarz, S.; Koller, R.; Worm, K.; Reich, P.B. Global change belowground: Impacts of elevated CO2, nitrogen, and summer drought on soil food webs and biodiversity. Glob. Change Biol. 2012, 18, 435–447. [Google Scholar] [CrossRef]
- Kardol, P.; Cregger, M.A.; Campany, C.E.; Classen, A.T. Soil ecosystem functioning under climate change: Plant species and community effects. Ecology 2010, 91, 767–781. [Google Scholar] [CrossRef]
- Garten, C.T., Jr.; Iversen, C.M.; Norby, R.J. Litterfall N-15 abundance indicates declining soil nitrogen availability in a free-air CO2 enrichment experiment. Ecology 2011, 92, 133–139. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource-allocation to extracellular enzyme-production- A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Capek, P.T.; Manzoni, S.; Kastovska, E.; Wild, B.; Diakova, K.; Barta, J.; Schnecker, J.; Blasi, C.; Martikainen, P.J.; Alves, R.J.E.; et al. A plant-microbe interaction framework explaining nutrient effects on primary production. Nat. Ecol. Evol. 2018, 2, 1588–1596. [Google Scholar] [CrossRef]
- Spohn, M.; Kuzyakov, Y. Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol. Biochem. 2013, 61, 69–75. [Google Scholar] [CrossRef]
- Ru, J.Y.; Zhou, Y.Q.; Hui, D.F.; Zheng, M.M.; Wan, S.Q. Shifts of growing-season precipitation peaks decrease soil respiration in a semiarid grassland. Glob. Change Biol. 2018, 24, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.S.; Zhang, X.; Bilyera, N.; Guber, A.; Zarebanadkouki, M. Soil zymography: Simple and reliable? Review of current knowledge and optimization of the method. Rhizosphere 2019, 11, 100161. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.C.; Xu, M.P.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Cui, Y.X.; Wang, X.; Zhang, X.C.; Ju, W.L.; Duan, C.J.; Guo, X.B.; Wang, Y.Q.; Fang, L.C. Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]


| Treatment Codes | Experimental Treatments | Experimental Conditions | ||
|---|---|---|---|---|
| CO2 Concentration (μmol·mol−1) | Temperature (°C) | Soil Moisture Content (%) | ||
| CK | Control | 400 | T0 | 60% FC |
| D | Light drought | T0 | 80% FC | |
| W | Warming | 400 | T0 + 4 °C | 60% FC |
| W + D | Warming + light drought | T0 + 4 °C | 80% FC | |
| C | Elevated CO2 concentration | 800 | T0 | 60% FC |
| C + D | Elevated CO2 concentration + light drought | T0 | 80% FC | |
| CW | Elevated CO2 concentration + warming | 800 | T0 + 4 °C | 60% FC |
| CW + D | Elevated CO2 concentration + warming + light drought | T0 + 4 °C | 80% FC | |
| Treatment Codes | Growth Days at Sampling | |||
|---|---|---|---|---|
| Jointing Stage | Anthesis Stage | Grain Filling Stage | Maturity Stage | |
| CK | 148 | 174 | 192 | 218 |
| D | 148 | 169 | 187 | 216 |
| C | 146 | 174 | 191 | 218 |
| C + D | 146 | 168 | 186 | 216 |
| W | 144 | 155 | 172 | 198 |
| W + D | 144 | 153 | 170 | 197 |
| CW | 142 | 156 | 173 | 198 |
| CW + D | 142 | 154 | 171 | 197 |
| Treatments Codes | Growth Stage | Parameters | |||||
|---|---|---|---|---|---|---|---|
| AGB (g) | BGB (g) | pH | SOC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | ||
| CK | JS | 10.81 ± 0.88 ab | 1.02 ± 0.06 a | 7.86 ± 0.12 ab | 9.08 ± 0.35 b | 1.08 ± 0.02 a | 1.41 ± 0.05 a |
| AN | 38.8 ± 2.02 a | 1.87 ± 0.28 a | 8.35 ± 0.15 a | 8.94 ± 0.16 abc | 1.05 ± 0.03 abc | 1.45 ± 0.02 a | |
| GF | 63.6 ± 6.12 a | 2.24 ± 0.13 a | 8.19 ± 0.03 bc | 10.24 ± 0.15 a | 1.03 ± 0.01 cd | 1.5 ± 0.04 abc | |
| MA | 79.91 ± 4.39 a | 1.13 ± 0.2 a | 8.23 ± 0.04 ab | 10 ± 0.09 a | 1.08 ± 0.03 abc | 1.44 ± 0.02 ab | |
| D | JS | 7.63 ± 1.57 d | 0.59 ± 0.07 c | 7.89 ± 0.13 ab | 8.43 ± 0.32 c | 1.12 ± 0.02 a | 1.42 ± 0.06 a |
| AN | 22.12 ± 2.55 b | 1.11 ± 0.16 bc | 8.1 ± 0.09 b | 9.14 ± 0.24 ab | 1.08 ± 0.02 abc | 1.43 ± 0.03 ab | |
| GF | 35.2 ± 2.54 bc | 1.33 ± 0.23 b | 8.1 ± 0.09 de | 9.41 ± 0.23 bcd | 1.07 ± 0.01 abc | 1.5 ± 0.05 ab | |
| MA | 52.42 ± 1.43 cd | 0.88 ± 0.1 b | 8.11 ± 0.04 b | 9.91 ± 0.34 ab | 1.1 ± 0.01 abc | 1.5 ± 0.03 a | |
| C | JS | 11.41 ± 0.53 ab | 0.9 ± 0.18 a | 7.95 ± 0.03 ab | 9.78 ± 0.08 a | 1.09 ± 0.01 a | 1.41 ± 0.01 a |
| AN | 39.54 ± 2.96 a | 2.14 ± 0.25 a | 8.41 ± 0.04 a | 9.14 ± 0.35 a | 1.01 ± 0.07 c | 1.47 ± 0.05 a | |
| GF | 62.08 ± 3.68 a | 2.37 ± 0.13 a | 8.18 ± 0.01 bcd | 9.85 ± 0.15 ab | 1.02 ± 0.04 d | 1.52 ± 0.01 a | |
| MA | 78.05 ± 1 a | 1.18 ± 0.21 a | 8.16 ± 0.22 b | 9.84 ± 0.2 abc | 1.07 ± 0.03 c | 1.5 ± 0.05 a | |
| C + D | JS | 8.82 ± 1.26 cd | 0.36 ± 0.03 d | 7.81 ± 0.03 b | 9.45 ± 0.09 ab | 1.11 ± 0.01 a | 1.41 ± 0.04 a |
| AN | 23.09 ± 2.66 b | 1.31 ± 0.05 b | 8.17 ± 0.07 b | 9.2 ± 0.48 a | 1.11 ± 0.01 a | 1.43 ± 0.04 ab | |
| GF | 33.11 ± 4.07 cd | 1.23 ± 0.1 b | 8.11 ± 0.04 cde | 9.78 ± 0.57 bc | 1.09 ± 0.02 a | 1.5 ± 0.01 ab | |
| MA | 51.46 ± 3.97 d | 0.77 ± 0.14 b | 8.16 ± 0.07 b | 10.08 ± 0.14 a | 1.09 ± 0.04 abc | 1.43 ± 0.08 b | |
| W | JS | 12.27 ± 1.27 a | 0.34 ± 0.06 d | 7.97 ± 0.04 a | 9.55 ± 0.64 ab | 1.1 ± 0.04 a | 1.41 ± 0.03 a |
| AN | 23.56 ± 2.49 b | 1.11 ± 0.26 bc | 8.13 ± 0.09 b | 8.62 ± 0.4 bc | 1.03 ± 0.03 c | 1.38 ± 0.04 bc | |
| GF | 39.94 ± 4.05 b | 1.24 ± 0.05 b | 8.23 ± 0.04 b | 9.41 ± 0.09 bcd | 1.09 ± 0.02 a | 1.48 ± 0.03 abc | |
| MA | 56.58 ± 4.11 c | 0.72 ± 0.14 bc | 8.21 ± 0.06 ab | 9.6 ± 0.13 cd | 1.07 ± 0.01 bc | 1.48 ± 0.02 ab | |
| W + D | JS | 9.81 ± 1.15 bc | 0.18 ± 0.04 e | 7.9 ± 0.04 ab | 9.45 ± 0.17 ab | 1.11 ± 0.04 a | 1.44 ± 0.03 a |
| AN | 15.98 ± 1.2 c | 0.76 ± 0.15 d | 8.15 ± 0.07 b | 8.84 ± 0.39 abc | 1.1 ± 0.04 ab | 1.46 ± 0.03 a | |
| GF | 26.7 ± 4.14 d | 0.79 ± 0.14 c | 8.09 ± 0.05 e | 9.36 ± 0.41 cd | 1.11 ± 0.04 a | 1.46 ± 0.02 bc | |
| MA | 34.34 ± 1.86 e | 0.48 ± 0.02 d | 8.09 ± 0.04 b | 9.67 ± 0.17 bcd | 1.11 ± 0.01 ab | 1.45 ± 0.04 ab | |
| CW | JS | 10.76 ± 1.34 ab | 0.74 ± 0.14 b | 7.93 ± 0.14 ab | 9.78 ± 0.28 a | 1.12 ± 0.02 a | 1.39 ± 0.04 a |
| AN | 22.48 ± 2.62 b | 1.19 ± 0.08 bc | 8.12 ± 0.07 b | 8.53 ± 0.23 c | 1.04 ± 0.05 bc | 1.41 ± 0.03 ab | |
| GF | 40.08 ± 4.96 b | 1.43 ± 0.18 b | 8.37 ± 0.05 a | 9.57 ± 0.25 bcd | 1.04 ± 0.03 bcd | 1.45 ± 0.03 bc | |
| MA | 61.35 ± 5.52 b | 0.86 ± 0.15 b | 8.31 ± 0.02 a | 9.54 ± 0.27 cd | 1.07 ± 0.02 bc | 1.5 ± 0.03 a | |
| CW + D | JS | 8.55 ± 1.01 cd | 0.52 ± 0.07 c | 7.94 ± 0.03 ab | 9.09 ± 0.22 b | 1.09 ± 0.02 a | 1.46 ± 0.09 a |
| AN | 17.23 ± 2.19 c | 0.97 ± 0.17 cd | 8.16 ± 0.09 b | 8.6 ± 0.39 bc | 1.09 ± 0.04 abc | 1.35 ± 0.02 c | |
| GF | 29.9 ± 4.75 cd | 0.99 ± 0.14 c | 8.03 ± 0.09 e | 9.22 ± 0.23 d | 1.08 ± 0.03 ab | 1.45 ± 0.04 c | |
| MA | 34.82 ± 1.79 e | 0.54 ± 0.11 cd | 8.11 ± 0.02 b | 9.43 ± 0.13 d | 1.12 ± 0.04 a | 1.48 ± 0.03 ab | |
| Treatment Codes | Growth Stage | Parameters | |||||
|---|---|---|---|---|---|---|---|
| DOC (g·kg−1) | AN (g·kg−1) | AP (g·kg−1) | Soil C:N Ratio | Soil C:P Ratio | Soil N:P Ratio | ||
| CK | JS | 36.72 ± 2.27 cd | 119.23 ± 8.53 b | 13.47 ± 0.19 d | 0.31 ± 0.04 b | 2.72 ± 0.13 a | 8.86 ± 0.75 b |
| AN | 45.49 ± 1.95 a | 11.36 ± 2.39 e | 11.08 ± 1.32 d | 4.2 ± 1.24 a | 4.15 ± 0.52 a | 1.03 ± 0.25 e | |
| GF | 40.86 ± 3.9 a | 10.7 ± 1.94 bc | 11.97 ± 1.07 ab | 3.94 ± 0.91 b | 3.42 ± 0.2 a | 0.91 ± 0.26 bc | |
| MA | 58.83 ± 2.79 a | 5.93 ± 1.31 d | 11.65 ± 0.54 c | 10.38 ± 2.68 a | 5.06 ± 0.37 a | 0.51 ± 0.1 d | |
| D | JS | 38.3 ± 1.01 bc | 66.87 ± 4.38 d | 13.79 ± 0.83 cd | 0.58 ± 0.05 a | 2.78 ± 0.16 a | 4.87 ± 0.54 d |
| AN | 42.69 ± 6.7 a | 87.25 ± 14.17 b | 14.01 ± 0.5 bc | 0.5 ± 0.12 c | 3.05 ± 0.51 b | 6.24 ± 1.09 ab | |
| GF | 33.5 ± 4.43 bc | 54.19 ± 19.31 a | 12.63 ± 1.51 ab | 0.71 ± 0.35 c | 2.71 ± 0.66 b | 4.23 ± 1.2 a | |
| MA | 46.35 ± 2.36 b | 12.31 ± 1.73 c | 11.55 ± 0.66 c | 3.83 ± 0.6 c | 4.02 ± 0.3 b | 1.07 ± 0.2 c | |
| C | JS | 28.67 ± 2.46 f | 91.39 ± 11.05 c | 13.91 ± 0.61 cd | 0.32 ± 0.06 b | 2.06 ± 0.18 c | 6.59 ± 0.97 c |
| AN | 40.06 ± 3.54 ab | 12.88 ± 3.98 e | 12.46 ± 0.2 cd | 3.24 ± 0.63 b | 3.22 ± 0.31 b | 1.03 ± 0.32 e | |
| GF | 39.26 ± 5.76 ab | 4.66 ± 1.06 c | 13.75 ± 0.72 a | 8.93 ± 3.03 a | 2.85 ± 0.28 ab | 0.34 ± 0.1 c | |
| MA | 45.02 ± 4.35 b | 5.82 ± 0.99 d | 12.18 ± 0.33 c | 7.89 ± 1.27 b | 3.69 ± 0.29 b | 0.48 ± 0.08 d | |
| C + D | JS | 42.45 ± 3.61 a | 156.11 ± 12.89 a | 14.8 ± 0.13 ab | 0.27 ± 0.03 bc | 2.87 ± 0.25 a | 10.54 ± 0.78 a |
| AN | 33.43 ± 2.34 bc | 68.02 ± 10.16 c | 13.82 ± 0.59 bc | 0.5 ± 0.08 c | 2.42 ± 0.21 c | 4.94 ± 0.85 bc | |
| GF | 37.45 ± 1.15 abc | 25.39 ± 0.5 bc | 12.87 ± 1.11 ab | 1.48 ± 0.07 c | 2.92 ± 0.2 ab | 1.99 ± 0.23 b | |
| MA | 50.49 ± 10.54 b | 18.98 ± 4.34 b | 12.09 ± 1 c | 2.72 ± 0.53 cd | 4.24 ± 1.2 b | 1.58 ± 0.38 b | |
| W | JS | 26.36 ± 1.16 f | 121.91 ± 24.24 b | 14.42 ± 0.3 abc | 0.22 ± 0.04 cd | 1.83 ± 0.11 d | 8.44 ± 1.58 b |
| AN | 35.4 ± 5.97 bc | 58.54 ± 8.88 c | 15.44 ± 0.92 bc | 0.61 ± 0.13 c | 2.29 ± 0.37 cd | 3.78 ± 0.37 cd | |
| GF | 39.05 ± 4.45 ab | 26.49 ± 11.95 bc | 12.95 ± 0.55 ab | 1.8 ± 0.98 c | 3.02 ± 0.32 ab | 2.05 ± 0.92 b | |
| MA | 36.24 ± 1.48 c | 13.87 ± 4.24 c | 14.57 ± 0.32 ab | 2.83 ± 0.95 cd | 2.49 ± 0.1 c | 0.95 ± 0.29 c | |
| W + D | JS | 31.79 ± 1.27 e | 159.22 ± 19.3 a | 14.21 ± 0.58 bcd | 0.2 ± 0.02 d | 2.24 ± 0.1 bc | 11.22 ± 1.47 a |
| AN | 30.83 ± 3.94 c | 107.36 ± 15.96 a | 18.2 ± 3.39 a | 0.29 ± 0.04 c | 1.72 ± 0.28 d | 6.09 ± 1.6 b | |
| GF | 34.99 ± 4.22 abc | 75.89 ± 21.14 a | 13.33 ± 1.18 ab | 0.51 ± 0.26 c | 2.64 ± 0.4 b | 5.69 ± 1.54 a | |
| MA | 33.77 ± 2.09 c | 31.48 ± 3.34 a | 13.89 ± 0.79 b | 1.09 ± 0.19 d | 2.44 ± 0.2 c | 2.27 ± 0.26 a | |
| CW | JS | 34.79 ± 1.68 d | 109.6 ± 18.08 bc | 14.84 ± 0.26 ab | 0.32 ± 0.06 b | 2.34 ± 0.11 b | 7.39 ± 1.22 bc |
| AN | 34.77 ± 5.25 bc | 41.59 ± 5.85 d | 15.79 ± 1.86 b | 0.86 ± 0.27 c | 2.23 ± 0.47 cd | 2.65 ± 0.37 d | |
| GF | 36.66 ± 1.5 abc | 28.83 ± 5.34 b | 11.72 ± 1 b | 1.3 ± 0.23 c | 3.14 ± 0.17 ab | 2.46 ± 0.37 b | |
| MA | 37.62 ± 2.38 c | 11.98 ± 5.47 c | 16 ± 2.34 a | 3.53 ± 1.18 c | 2.38 ± 0.25 c | 0.74 ± 0.25 cd | |
| CW + D | JS | 41.04 ± 0.59 ab | 120.69 ± 2.82 b | 15.12 ± 0.8 a | 0.34 ± 0.01 b | 2.72 ± 0.15 a | 7.99 ± 0.35 bc |
| AN | 35.7 ± 3.06 bc | 105.57 ± 17.47 a | 14.07 ± 0.21 bc | 0.35 ± 0.08 c | 2.54 ± 0.2 bc | 7.52 ± 1.34 a | |
| GF | 32.63 ± 2.89 c | 63.93 ± 27.45 a | 11.99 ± 1.21 ab | 0.6 ± 0.28 c | 2.76 ± 0.5 b | 5.24 ± 1.9 a | |
| MA | 30.64 ± 2.99 c | 31.94 ± 2.68 a | 15.22 ± 0.94 ab | 0.97 ± 0.14 d | 2.03 ± 0.32 c | 2.11 ± 0.24 a | |
| Source of Variation | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| BG | NAG + LAP | ALP | Enzymatic C:N Ratio | Enzymatic C:P Ratio | Enzymatic N:P Ratio | Vector Length | Vector Angle | |
| Stage | 6.77 (0.002) ** | 10.46 (<0.001) *** | 11.42 (<0.001) *** | 21.98 (<0.001) *** | 16.20 (<0.001) *** | 1.48 (0.234) | 21.49 (<0.001) *** | 3.90 (0.025) * |
| CO2 | 7.85 (0.007) ** | 24.97 (<0.001) *** | 7.44 (0.008) ** | 0.30 (0.587) | 0.07 (0.787) | 2.13 (0.149) | 0.06 (0.806) | 0.01 (0.934) |
| Warming | 2.68 (0.106) | 16.59 (<0.001) *** | 0.30 (0.585) | 4.19 (0.044) * | 1.86 (0.177) | 23.66 (<0.001) *** | 0.36 (0.548) | 17.02 (<0.001) *** |
| Drought | 0.21 (0.647) | 1.38 (0.244) | 0.18 (0.676) | 0.07 (0.792) | 0.06 (0.809) | 0.72 (0.398) | 0.002 (0.961) | 0.02 (0.897) |
| CO2 × Stage | 14.27 (<0.001) *** | 8.03 (<0.001) *** | 1.80 (0.173) | 14.91 (<0.001) *** | 8.15 (<0.001) *** | 4.84 (0.011) * | 6.33 (0.003) ** | 2.48 (0.091) |
| Warming × Stage | 2.56 (0.085) | 5.43 (0.006) ** | 8.45 (<0.001) *** | 16.11 (<0.001) *** | 13.97 (<0.001) *** | 2.23 (0.115) | 17.84 (<0.001) *** | 3.10 (0.051) |
| Drought × Stage | 1.19 (0.311) | 0.62 (0.542) | 0.27 (0.763) | 3.08 (0.052) | 1.36 (0.265) | 1.52 (0.226) | 0.65 (0.527) | 0.92 (0.404) |
| CO2 × Warming | 0.13 (0.723) | 1.75 (0.19) | 14.38 (<0.001) *** | 1.28 (0.261) | 2.00 (0.161) | 15.23 (<0.001) *** | 1.53 (0.220) | 14.06 (<0.001) *** |
| CO2 × Drought | 1.04 (0.312) | 0.68 (0.412) | 2.73 (0.103) | 1.03 (0.313) | 2.43 (0.123) | 0.35 (0.558) | 1.20 (0.277) | 1.03 (0.313) |
| Warming × Drought | 0.21 (0.652) | 0.01 (0.914) | 0.73 (0.394) | 0.11 (0.744) | 0.49 (0.486) | 0.31 (0.581) | 0.03 (0.860) | 0.77 (0.383) |
| CO2 × Warming × Stage | 7.06 (0.002) ** | 4.58 (0.013) * | 7.08 (0.002) ** | 17.93 (<0.001) *** | 20.46 (<0.001) *** | 3.03 (0.055) | 25.03 (<0.001) *** | 3.07 (0.053) |
| CO2 × Drought × Stage | 0.05 (0.954) | 0.72 (0.489) | 0.23 (0.799) | 0.03 (0.972) | 0.29 (0.75) | 0.25 (0.783) | 0.67 (0.513) | 0.10 (0.910) |
| Warming × Drought × Stage | 0.28 (0.759) | 1.21 (0.304) | 0.21 (0.811) | 0.31 (0.737) | 0.34 (0.713) | 0.34 (0.715) | 1.92 (0.155) | 0.003 (0.997) |
| CO2 ×Warming Drought | 2.64 (0.108) | 0.36 (0.550) | 2.84 (0.096) | 0.36 (0.553) | 2.51 (0.117) | 1.60 (0.210) | 2.82 (0.098) | 0.004 (0.948) |
| CO2 × Warming × Drought × Stage | 0.26 (0.774) | 0.24 (0.790) | 0.44 (0.645) | 1.04 (0.359) | 0.96 (0.389) | 0.22 (0.800) | 0.81 (0.451) | 1.12 (0.331) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, X.; Zheng, F.; Wei, H.; Zhao, M.; Jiao, J. Ecoenzymatic Stoichiometry Reveals Microbial Carbon and Phosphorus Limitations under Elevated CO2, Warming and Drought at Different Winter Wheat Growth Stages. Sustainability 2023, 15, 9037. https://doi.org/10.3390/su15119037
Wang J, Wang X, Zheng F, Wei H, Zhao M, Jiao J. Ecoenzymatic Stoichiometry Reveals Microbial Carbon and Phosphorus Limitations under Elevated CO2, Warming and Drought at Different Winter Wheat Growth Stages. Sustainability. 2023; 15(11):9037. https://doi.org/10.3390/su15119037
Chicago/Turabian StyleWang, Jing, Xuesong Wang, Fenli Zheng, Hanmei Wei, Miaomiao Zhao, and Jianyu Jiao. 2023. "Ecoenzymatic Stoichiometry Reveals Microbial Carbon and Phosphorus Limitations under Elevated CO2, Warming and Drought at Different Winter Wheat Growth Stages" Sustainability 15, no. 11: 9037. https://doi.org/10.3390/su15119037
APA StyleWang, J., Wang, X., Zheng, F., Wei, H., Zhao, M., & Jiao, J. (2023). Ecoenzymatic Stoichiometry Reveals Microbial Carbon and Phosphorus Limitations under Elevated CO2, Warming and Drought at Different Winter Wheat Growth Stages. Sustainability, 15(11), 9037. https://doi.org/10.3390/su15119037




