Novel Application of MnO2–H2O2 System for Highly Efficient Arsenic Adsorption and Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
3. Results
3.1. Characterization of MnO2
3.2. Performance of MnO2–H2O2 System with Respect to As Removal
3.2.1. Key Operating Parameters for As Removal
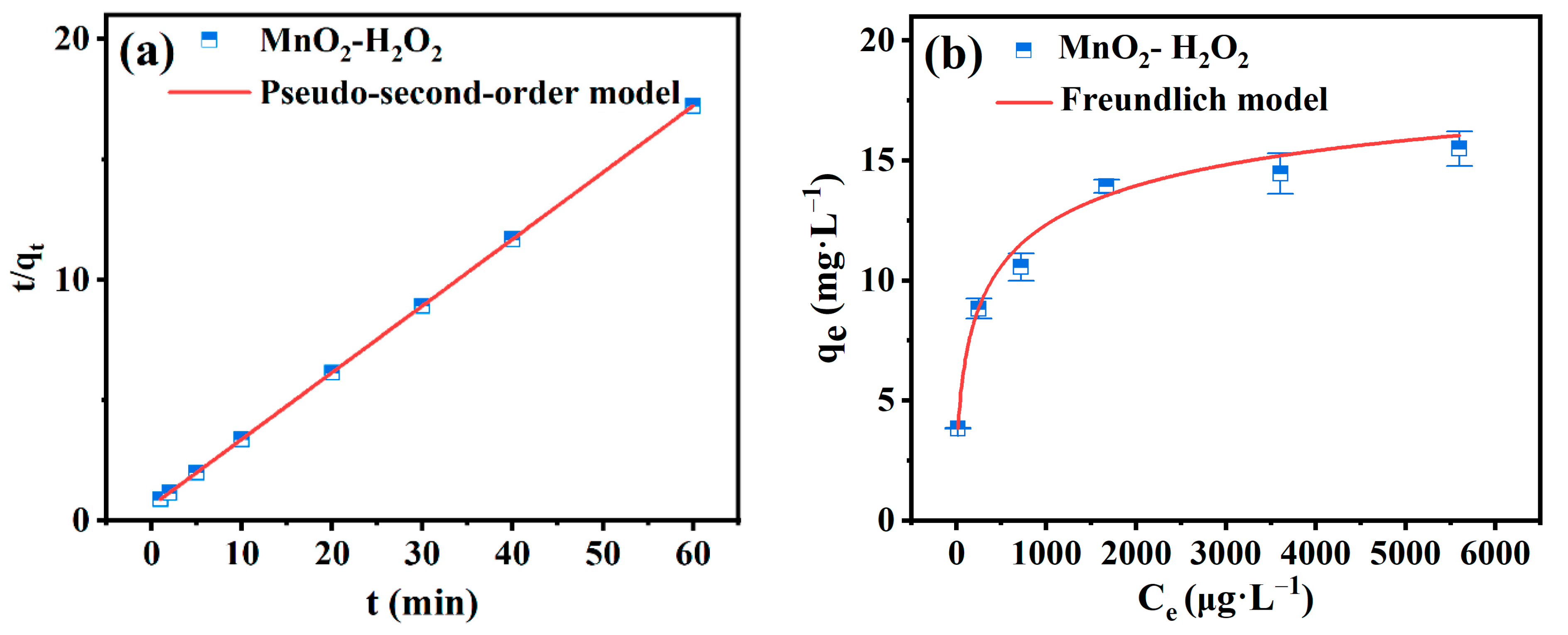
3.2.2. Simulations of Adsorption Kinetics, Isotherms, and Thermodynamics
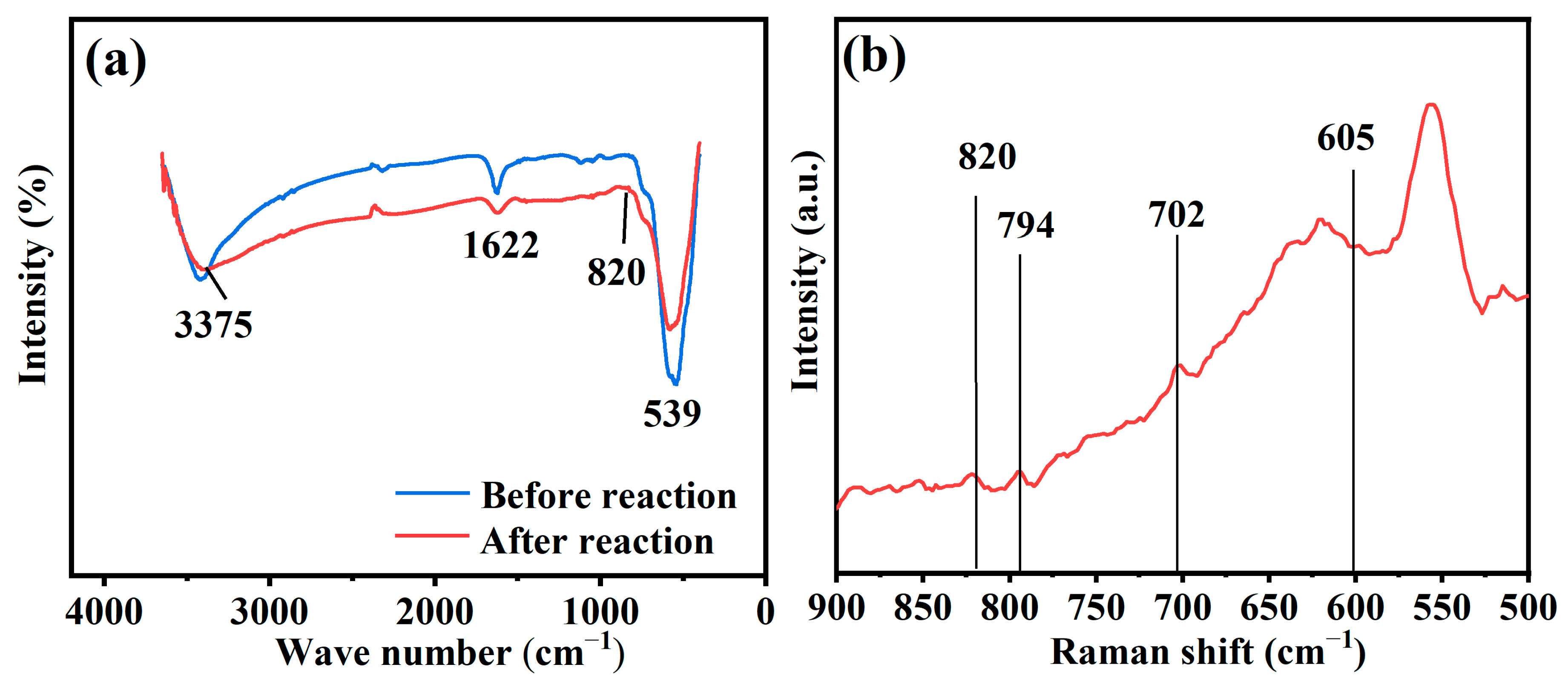
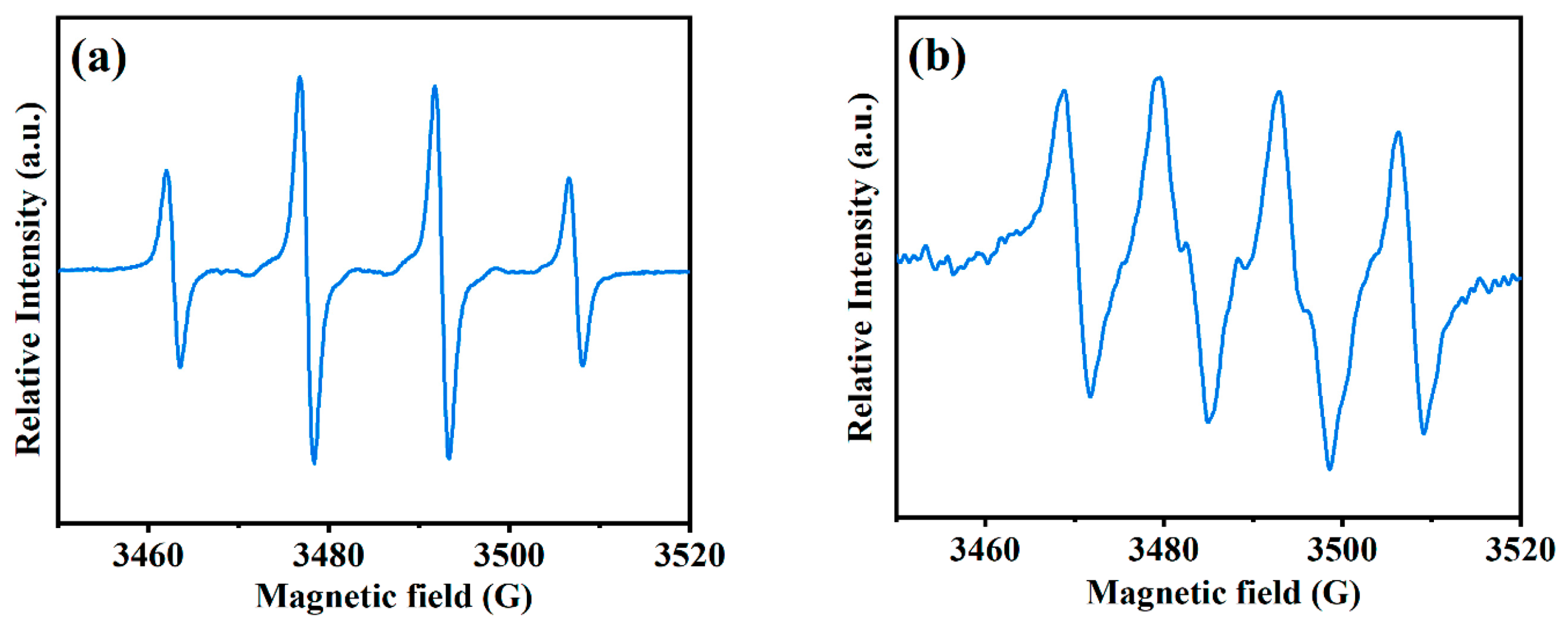
3.3. Mechanisms for As Removal
3.4. Influences of Environmental Factors and Practical Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Williams, M. Arsenic in mine waters: An international study. Environ. Geol. 2001, 40, 267–278. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-water Quality, 4th ed.; WHO Press: Geneva, Switzerland, 2011; Volume 38, pp. 104–108. [Google Scholar]
- Jain, C.K.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- Gibbon-Walsh, K.; Salaun, P.; Berg, C.M.V.D. Arsenic speciation in natural waters by cathodic stripping voltammetry. Anal. Chim. Acta 2010, 662, 1–8. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Singh, N.; Nagpal, G.; Agrawal, S. Rachna Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Mondal, P.; Bhowmick, S.; Chatterjee, D.; Figoli, A.; Van der Bruggen, B. Remediation of inorganic arsenic in groundwater for safe water supply: A critical assessment of technological solutions. Chemosphere 2013, 92, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Bissen, M.; Frimmel, F.H. Arsenic— a Review. Part II: Oxidation of Arsenic and its Removal in Water Treatment. Acta Hydrochim. et Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Nujić, M. Arsenic removal by nanoparticles: A review. Environ. Sci. Pollut. Res. 2015, 22, 8094–8123. [Google Scholar] [CrossRef]
- Hou, S.; Ding, W.; Liu, S.; Zheng, H.; Zhai, J.; Yang, L.; Zhong, Z. Fast oxidation and deep removal of As(III) by integrating metal–organic framework ZIF-67 and sulfite: Performance and mechanism. Chem. Eng. J. 2023, 460. [Google Scholar] [CrossRef]
- Lin, Z.; Deng, F.; Ren, W.; Wang, Z.; Xiao, X.; Shao, P.; Zou, J.; Luo, X. Integration of adsorption and simultaneous heterogeneous catalytic oxidation by defective CoFe2O4 activated peroxymonosulfate for efficient As (III) removal: Performance and new insight into the mechanism. Chem. Eng. J. 2023, 454, 139960. [Google Scholar] [CrossRef]
- Park, H.; Choi, H. As(III) removal by hybrid reactive membrane process combined with ozonation. Water Res. 2011, 45, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Sorlini, S.; Gialdini, F. Conventional oxidation treatments for the removal of arsenic with chlorine dioxide, hypochlorite, potassium permanganate and monochloramine. Water Res. 2010, 44, 5653–5659. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, G.; Liu, C.; Li, J.; Zheng, T.; Ma, J.; Wang, L.; Jiang, J.; Zhai, X. Enhanced removal of arsenite and arsenate by a multifunctional Fe-Ti-Mn composite oxide: Photooxidation, oxidation and adsorption. Water Res. 2018, 147, 264–275. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, G.; Wu, Q.; Wang, D. Facile fabrication of nanostructured cerium-manganese binary oxide for enhanced arsenite removal from water. Chem. Eng. J. 2018, 334, 1518–1526. [Google Scholar] [CrossRef]
- Gupta, K.; Ghosh, U.C. Arsenic removal using hydrous nanostructure iron(III)–titanium(IV) binary mixed oxide from aqueous solution. J. Hazard. Mater. 2009, 161, 884–892. [Google Scholar] [CrossRef]
- Ding, W.; Wan, X.; Zheng, H.; Wu, Y.; Muhammad, S. Sulfite-assisted oxidation/adsorption coupled with a TiO2 supported CuO composite for rapid arsenic removal: Performance and mechanistic studies. J. Hazard. Mater. 2021, 413, 125449. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.; Hoffmann, M.R.; Hering, J.G. TiO2-Photocatalyzed As(III) Oxidation in Aqueous Suspensions: Reaction Kinetics and Effects of Adsorption. Environ. Sci. Technol. 2005, 39, 1880–1886. [Google Scholar] [CrossRef]
- Cai, X.; Li, Y.; Guo, J.; Liu, S.; Na, P. Mn(IV) promotion mechanism for the photocatalytic oxidation of arsenite by anatase-TiO2. Chem. Eng. J. 2014, 248, 9–17. [Google Scholar] [CrossRef]
- Zhao, Z.; Jia, Y.; Xu, L.; Zhao, S. Adsorption and heterogeneous oxidation of As(III) on ferrihydrite. Water Res. 2011, 45, 6496–6504. [Google Scholar] [CrossRef]
- Manning, B.A.; Fendorf, S.E.; Bostick, B.; Suarez, D.L. Arsenic(III) Oxidation and Arsenic(V) Adsorption Reactions on Synthetic Birnessite. Environ. Sci. Technol. 2002, 36, 976–981. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Z.; Yan, J.; Liu, Y.; Wu, Y.; Fang, Y.; Yu, L.; Cheng, G.; Pan, Z.; Hu, G. Adsorption and oxidation of arsenic by two kinds of β-MnO2. J. Hazard. Mater. 2019, 373, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.J.; Morgan, J.J. Reactions at Oxide Surfaces. 1. Oxidation of As(III) by Synthetic Birnessite. Environ. Sci. Technol. 1995, 29, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Do, S.-H.; Batchelor, B.; Lee, H.-K.; Kong, S.-H. Hydrogen peroxide decomposition on manganese oxide (pyrolusite): Kinetics, intermediates, and mechanism. Chemosphere 2009, 75, 8–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Xu, B.; Qi, F.; Chu, W. Degradation of benzotriazole by a novel Fenton-like reaction with mesoporous Cu/MnO2: Combination of adsorption and catalysis oxidation. Appl. Catal. B Environ. 2016, 199, 447–457. [Google Scholar] [CrossRef]
- He, X.; Sun, B.; He, M.; Chi, H.; Wang, Z.; Zhang, W.; Ma, J. Highly efficient simultaneous catalytic degradation and defluorination of perfluorooctanoic acid by the H2O2-carbon/MnO2 system generating O2- and OH synchronously. Appl. Catal. B Environ. 2020, 277, 119219. [Google Scholar] [CrossRef]
- He, X.; Li, B.; Wang, P.; Ma, J. Novel H2O2–MnO2 system for efficient physico-chemical cleaning of fouled ultrafiltration membranes by simultaneous generation of reactive free radicals and oxygen. Water Res. 2019, 167, 115111. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bahk, Y.K.; Wang, J. Organic dye removal by MnO2 and Ag micromotors under various ambient conditions: The comparison between two abatement mechanisms. Chemosphere 2017, 184, 601–608. [Google Scholar] [CrossRef]
- Chang, Y.; Cho, Y.-C.; Lin, Y.-P. Degradation of PFOS by a MnO2/H2O2 process. Environ. Sci. Water Res. Technol. 2020, 6, 3476–3487. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Fu, W.; Tien, S.; Yang, Y.; Yang, K.; Xu, X.; Zhang, X. Oriented generation of singlet oxygen in H2O2 activation for water decontamination: Regulation of oxygen vacancy over α-MnO2 nanocatalyst. Environ. Sci. Nano 2023. [Google Scholar] [CrossRef]
- Wang, J.; Jing, Y. Inhibition of iron chelate degradation in liquid redox desulfurization by nanoscale MnO2 through H2O2 decomposition. J. Chem. Technol. Biotechnol. 2023, 98, 1395–1401. [Google Scholar] [CrossRef]
- Lu, Y.; Deng, H.; Pan, T.; Liao, X.; Zhang, C.; He, H. Effective Toluene Ozonation over δ-MnO2: Oxygen Vacancy-Induced Reactive Oxygen Species. Environ. Sci. Technol. 2023, 57, 2918–2927. [Google Scholar] [CrossRef]
- Le, S.; Yan, B.; Mao, Y.; Chi, D.; Zhu, M.; Jia, H.; Zhao, G.; Zhu, X.; Zhang, N. N-doped δ-MnO2 coated N-doped carbon cloth as stable cathode for aqueous zinc-ion batteries. Int. J. Electrochem. Sci. 2023, 18, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Liang, M.; Gao, J.; Ma, C.; He, Z.; Zhao, Y.; Miao, Z. Robust structural stability of flower-like δ-MnO2 as cathode for aqueous zinc ion battery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128804. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die Adsorption in Losungen. Z. fÜR Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Unuabonah, E.; Adebowale, K.; Olu-Owolabi, B. Kinetic and thermodynamic studies of the adsorption of lead (II) ions onto phosphate-modified kaolinite clay. J. Hazard. Mater. 2007, 144, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, M.; Dou, X.-M.; He, H.; Wang, D.-S. Arsenate Adsorption on an Fe−Ce Bimetal Oxide Adsorbent: Role of Surface Properties. Environ. Sci. Technol. 2005, 39, 7246–7253. [Google Scholar] [CrossRef]
- Goldberg, S.; Johnston, C. Mechanisms of Arsenic Adsorption on Amorphous Oxides Evaluated Using Macroscopic Measurements, Vibrational Spectroscopy, and Surface Complexation Modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W. Arsenate and Arsenite Removal by Zerovalent Iron: Kinetics, Redox Transformation, and Implications for in Situ Groundwater Remediation. Environ. Sci. Technol. 2001, 35, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, H.; Canning, G.; Bancroft, G. XPS study of reductive dissolution of 7Å-birnessite by H3AsO3, with constraints on reaction mechanism. Geochim. et Cosmochim. Acta 1998, 62, 2097–2110. [Google Scholar] [CrossRef]
- Khalaf, M.M.; El-Lateef, H.M.A.; A Mohamed, I.M. Novel electrocatalysts for ethylene glycol oxidation based on functionalized phosphates of bimetals Mn/Ni: Morphology, crystallinity, and electrocatalytic performance. Surfaces Interfaces 2023, 38. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Banerjee, D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2precipitation. Am. Miner. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Guo, Z.; Li, C.; Gao, M.; Han, X.; Zhang, Y.; Zhang, W.; Li, W. Mn−O Covalency Governs the Intrinsic Activity of Co-Mn Spinel Oxides for Boosted Peroxymonosulfate Activation. Angew. Chem. Int. Ed. 2020, 60, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Timmins, G.; Monske, M.; Burdick, A.; Kalyanaraman, B.; Liu, Y.; Clément, J.-L.; Burchiel, S.; Liu, K.J. Evaluation of spin trapping agents and trapping conditions for detection of cell-generated reactive oxygen species. Arch. Biochem. Biophys. 2005, 437, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Sorge, A.R.; Turco, M.; Pilone, G.; Bagnasco, G. Decomposition of Hydrogen Peroxide on MnO2/TiO2 Catalysts. J. Propuls. Power 2004, 20, 1069–1075. [Google Scholar] [CrossRef]
- Stumm, W. Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems. John Wiley & Son Inc.: New York, NY, USA, 1992; p. 520. [Google Scholar]
- Ren, Z.; Zhang, G.; Chen, J.P. Adsorptive removal of arsenic from water by an iron–zirconium binary oxide adsorbent. J. Colloid Interface Sci. 2011, 358, 230–237. [Google Scholar] [CrossRef] [PubMed]






| Temperature (K) | ΔG0 (kJ·mol−1) | ΔH0 (kJ·mol−1) | ΔS0 (kJ·mol−1·K) |
|---|---|---|---|
| 288.15 | −4.421 | −0.939 | 12.112 |
| 298.15 | −4.557 | ||
| 308.15 | −4.686 | ||
| 318.15 | −4.780 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wang, Y.; Zhao, Y.; Sun, Z.; Ma, J.; He, X. Novel Application of MnO2–H2O2 System for Highly Efficient Arsenic Adsorption and Oxidation. Sustainability 2023, 15, 9080. https://doi.org/10.3390/su15119080
Liu Q, Wang Y, Zhao Y, Sun Z, Ma J, He X. Novel Application of MnO2–H2O2 System for Highly Efficient Arsenic Adsorption and Oxidation. Sustainability. 2023; 15(11):9080. https://doi.org/10.3390/su15119080
Chicago/Turabian StyleLiu, Qingliang, Yu Wang, Ying Zhao, Zhiqiang Sun, Jun Ma, and Xu He. 2023. "Novel Application of MnO2–H2O2 System for Highly Efficient Arsenic Adsorption and Oxidation" Sustainability 15, no. 11: 9080. https://doi.org/10.3390/su15119080
APA StyleLiu, Q., Wang, Y., Zhao, Y., Sun, Z., Ma, J., & He, X. (2023). Novel Application of MnO2–H2O2 System for Highly Efficient Arsenic Adsorption and Oxidation. Sustainability, 15(11), 9080. https://doi.org/10.3390/su15119080








