Assessment of Cultivated Soil Contamination by Potentially Toxic Metals as a Result of a Galvanizing Plant Failure
Abstract
:1. Introduction
- -
- Assessing the condition of soils in terms of physical and chemical properties, with particular emphasis on the contents of Zn, Pb, Cd;
- -
- Determining the level of changes in relation to the geochemical background of the tested soils;
- -
- Determining the extent (range) of changes in the soils;
- -
- Determining environmental risks;
- -
- Organizing follow-up monitoring activities.
2. Materials and Methods
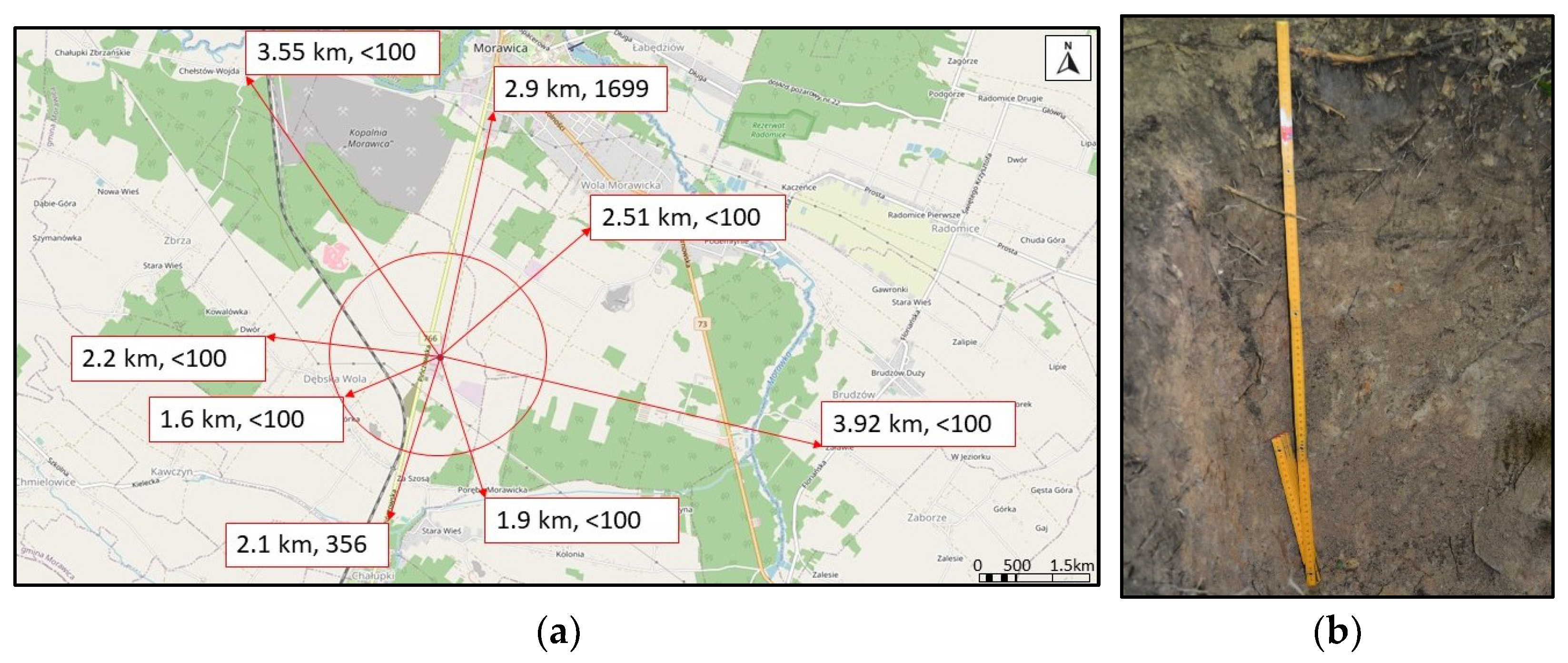
2.1. Study Area
2.2. Data Collection and Analysis
3. Results

| Sample No. | pHH2O | pHKCl | % Fraction Content (in mm) Bottom | Zn (mg·kg−1) | Pb (mg·kg−1) | Cd (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Top | Bottom | Top | Bottom | 2–0.05 | 0.05–0.02 | <0.002 | Top | Bottom | Top | Bottom | Top | Bottom | |
| 1 | 4.21 | 4.00 | 4.02 | 3.89 | 75 | 15 | 10 | 1761.08 | 909.45 | 509.70 | 290.67 | 17.11 | 10.90 |
| 2 | 4.19 | 4.04 | 4.00 | 3.88 | 68 | 16 | 16 | 2007.34 | 701.09 | 420.89 | 210.56 | 13.55 | 11.60 |
| 3 | 4.53 | 4.12 | 4.20 | 3.98 | 72 | 42 | 14 | 1093.66 | 400.64 | 307.56 | 230.87 | 14.88 | 7.90 |
| 4 | 4.06 | 4.00 | 3.89 | 3.87 | 82 | 32 | 14 | 1117.23 | 260.66 | 196.78 | 130.67 | 15.77 | 6.90 |
| 5 | 4.24 | 4.06 | 4.01 | 4.00 | 78 | 34 | 12 | 766.90 | 198.76 | 147.80 | 100.33 | 12.66 | 7.09 |
| 6 | 4.32 | 4.10 | 4.12 | 4.01 | 71 | 46 | 17 | 630.11 | 120.78 | 140.89 | 98.78 | 12.09 | 8.11 |
| 7 | 4.57 | 4.23 | 4.43 | 4.01 | 60 | 58 | 18 | 456.89 | 82.89 | 110.87 | 70.09 | 9.87 | 5.11 |
| 8 | 4.21 | 4.09 | 4.01 | 3.78 | 58 | 59 | 17 | 400.09 | 70.79 | 120.77 | 67.98 | 7.99 | 4.68 |
| 9 | 4.90 | 4.30 | 4.26 | 4.12 | 61 | 20 | 19 | 222.19 | 67.55 | 110.98 | 79.89 | 7.09 | 6.78 |
| 10 | 5.20 | 5.00 | 5.00 | 4.98 | 77 | 34 | 11 | 190.80 | 56.90 | 130.89 | 120.33 | 8.01 | 7.12 |
| 11 | 5.10 | 5.00 | 4.98 | 4.89 | 80 | 29 | 9 | 201.56 | 69.78 | 113.90 | 90.67 | 7.33 | 3.45 |
| 12 | 4.90 | 4.76 | 4.72 | 4.53 | 82 | 26 | 8 | 305.05 | 45.89 | 109.07 | 50.80 | 4.56 | 3.78 |
| 13 | 5.20 | 4.87 | 5.03 | 4.74 | 72 | 62 | 17 | 170.89 | 56.04 | 111.77 | 55.89 | 5.71 | 3.04 |
| 14 | 5.33 | 5.12 | 5.06 | 4.99 | 76 | 41 | 17 | 129.89 | 47.90 | 122.55 | 60.45 | 4.71 | 2.89 |
| 15 | 5.19 | 5.04 | 5.00 | 5.00 | 59 | 59 | 18 | 120.99 | 42.33 | 110.73 | 40.97 | 4.90 | 1.02 |
| Mean | 4.68 | 4.45 | 4.45 | 4.31 | 71.40 | 38.20 | 14.47 | 638.31 | 208.76 | 184.34 | 113.26 | 9.75 | 6.02 |
| Min | 4.06 | 4.00 | 3.89 | 3.78 | 58 | 15 | 8 | 120.99 | 42.33 | 109.07 | 40.97 | 4.56 | 1.02 |
| Max | 5.33 | 5.12 | 5.06 | 5.00 | 82 | 62 | 19 | 2007.34 | 909.45 | 509.70 | 290.67 | 17.11 | 11.60 |
| SD | 0.46 | 0.45 | 0.46 | 0.48 | 8.42 | 15.98 | 3.62 | 603.42 | 264.75 | 126.26 | 73.80 | 4.28 | 2.98 |
4. Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICP-OES | Inductively Coupled Plasma—optical emission spectrometry |
| Zn | Zinc |
| Pb | Lead |
| Cd | Cadmium |
| DM | Dry Mass |
| WRV | World Reference Values |
| Ap | Humus horizons (cultivated) |
| Bbr | Cambic horizons |
| Cca | Parent material of calcium carbonate |
| O | Organic horizons |
| A | Humus horizons |
| IMGW | Institute of Meteorology and Water Management |
| T | Top |
| B | Bottom |
References
- EEA. The European Environment—State and Outlook 2020. Knowledge for the Transition to a Sustainable Europe; Bruyninckx, H., Ed.; European Environment Agency: Prague, Czech Republic, 2020; Available online: https://www.cenia.cz/wp-content/uploads/2021/02/SOER2020_CZ-Launch_HB.pdf (accessed on 28 April 2023).
- UNECE. Annual Report 2022. United Nations Economic Commission for Europe, 2023. Available online: https://unece.org/sites/default/files/2023-04/UNECE_AR%202022_Web.pdf (accessed on 28 April 2023).
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 22. [Google Scholar]
- Wolterbeek, H. Biomonitoring of trace element air pollution: Principles, possibilities and perspectivites. Environ. Pollut. 2002, 120, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Shentu, J.; Zhang, T.; Yang, X.; Baligar, V.C.; He, Z. Sources, Indicators, and Assessment of Soil Contamination by Potentially Toxic Metals. Sustainability 2022, 14, 15878. [Google Scholar] [CrossRef]
- Szwed, M.; Żukowski, W.; Misztal, K.; Kozłowski, R. High-Energy Transformations of Fossil Fuels in the Cement Industry. Energies 2023, 16, 3634. [Google Scholar] [CrossRef]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; Araujo, A.; Singh, R.P. Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Zajęcka, E.; Świercz, A. Biomonitoring of the Urban Environment of Kielce and Olsztyn (Poland) Based on Studies of Total and Bioavailable Lead Content in Soils and Common Dandelion (Taraxacum officinale agg.). Minerals 2021, 11, 52. [Google Scholar] [CrossRef]
- Lasheen, E.S.R.; Zakaly, H.M.H.; Alotaibi, B.M.; Saadawi, D.A.; Ene, A.; Fathy, D.; Awad, H.A.; El Attar, R.M. Radiological Risk Parameters of the Phosphorite Deposits, Gebel Qulu El Sabaya: Natural Radioactivity and Geochemical Characteristics. Minerals 2022, 12, 1385. [Google Scholar] [CrossRef]
- Chang, S.E.; Stone, J.; Demes, K.; Piscitelli, M. Consequences of oil spills: A review and framework for informing planning. Ecol. Soc. 2014, 19, 26. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Tereza, V.K.; Salas-de-Leon, D.A.; Monreal-Jimenez, R.; Monreal-Gomez, M.A. The 2010 Gulf of Mexico oil spill: A modeling study. Arab. J. Geosci. 2021, 14, 636. [Google Scholar] [CrossRef]
- Orui, M.; Nakayama, C.; Moriyama, N.; Tsubokura, M.; Watanabe, K.; Nakayama, T.; Sugita, M.; Yasumura, S. Those Who Have Continuing Radiation Anxiety Show High Psychological Distress in Cases of High Post-Traumatic Stress: The Fukushima Nuclear Disaster. Int. J. Environ. Res. Public Health 2021, 18, 12048. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Lee, S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [Green Version]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; IZA: Brussels, Belgium; IFA: Paris, France, 2008. [Google Scholar]
- Dessureault-Rompré, J.; Luster, J.; Schulin, R.; Tercier-Waeber, M.-L.; Nowack, B. Decrease of labile Zn and Cd in the rhizosphere of hyperaccumulating Thlaspi caerulescens with time. Environ. Pollut. 2010, 158, 1955–1962. [Google Scholar] [CrossRef]
- Menzies, N.W.; Donn, M.J.; Kopittke, P.M. Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environ. Pollut. 2007, 145, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [Green Version]
- Al Jabri, H.; Saleem, M.H.; Rizwan, M.; Hussain, I.; Usman, K.; Alsafran, M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life 2022, 12, 594. [Google Scholar] [CrossRef]
- Kondracki, J. Geografia Regionalna Polski; Polish Scientific Publishers: Warsaw, Poland, 2000. [Google Scholar]
- Solon, J.; Borzyszkowski, J.; Bidłasik, M.; Richling, A.; Badora, K.; Balon, J.; Brzezinska-Wojcik, T.; Chabudziński, L.; Dobrowolski, L.; Grzegorczyk, I.; et al. Physico-geographical mesoregions of Poland: Verification and adjustment of boundaries on the basis of contemporary spatial data. Geogr. Pol. 2018, 91, 143–170. [Google Scholar] [CrossRef]
- Filonowicz, P. Explanations to Geological Map of Poland, Morawica Sheet; Polish Geological Publishers: Warsaw, Poland, 1968. [Google Scholar]
- Juszczyk, A.; Pasieczna, A.; Tomassi-Morawiec, H.; Nowacki, K.; Osendowska, E. Explanations to Geo-Environmental Map of Poland 1:50.000; Polish Geological Publishers: Warsaw, Poland, 2006. [Google Scholar]
- IUSS Working Group WRB 2015. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Systematyka Gleb Polski. Polskie Towarzystwo Gleboznawcze, Komisja Genezy Klasyfikacji i Kartografii Gleb; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu, Polskie Towarzystwo Gleboznawcze: Warsaw, Poland, 2019. [Google Scholar]
- PN-R-04032:1998—Polish Version; Soils and Mineral Formations. Division into Fractions and Granulometric Groups. Polish Committee for Standardization: Warsaw, Poland, 1998.
- PN-EN 15933:2013-02E—Polish Version; Soils and mineral formations. Sewage Sludge, Treated Bio-Waste, Soil and Waste—Determination of Dry Matter by Determination of Dry Matter Content or Water Content. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Salminen, R.; Batista, M.J.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W.; Duris, M.; Gilucis, A.; Gregorauskiene, V.; Halamic, J.; et al. FOREGS Geochemical Atlas of Europe, Part 1: Background Information, Methodology and Maps; Geological Survey of Finland: Espoo, Finland, 2005; pp. 140–347. [Google Scholar]
- Zhiyuan, W.; Dengfeng, W.; Huiping, Z.; Zhiping, Q. Assessment of soil heavy metal pollution with principal component analysis and geoaccumulation index. Procedia Environ. Sci. 2011, 10, 1946–1952. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, M. The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. J. Geol. Geophys. 2016, 5, 237. [Google Scholar] [CrossRef]
- Fodoué, Y.; Ismaila, A.; Yannah, M.; Wirmvem, M.J.; Mana, C.B. Heavy Metal Contamination and Ecological Risk Assessment in Soils of the Pawara Gold Mining Area, Eastern Cameroon. Earth 2022, 3, 907–924. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Biasioli, M.; Barberis, R.; Ajmone-Marsan, F. The influence of a large city on some soil properties and metals content. Sci. Total Environ. 2006, 356, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Biasioli, M.; Ajmone-Marsan, F. Organic and inorganic diffuse contamination in urban soils: The case of Torino (Italy). J. Environ. Monit. 2007, 9, 862–868. [Google Scholar] [CrossRef]
- Ajmone-Marsan, F.; Biasioli, M. Trace elements in soils of urban areas. Water Air Soil Pollut. 2010, 213, 121–143. [Google Scholar] [CrossRef]
- Rahman, M.S.; Saha, N.; Molla, A.H. Potential ecological risk assessment of heavy metal contamination in sedimen and water body around Dhaka export processing zone, Bangladesh. Environ. Earth Sci. 2014, 71, 2293–2308. [Google Scholar] [CrossRef]
- Cabała, J.; Warchulski, R.; Rozmus, D.; Środek, D.; Szełęg, E. Pb-Rich Slags, Minerals, and Pollution Resulted from a Medieval Ag-Pb Smelting and Mining Operation in the Silesian-Cracovian Region (Southern Poland). Minerals 2020, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Binner, H.; Sullivan, T.; Jansen, M.A.K.; McNamara, M.E. Metals in urban soils of Europe: A systematic review. Sci. Total Environ. 2023, 854, 158734. [Google Scholar] [CrossRef]
- Bosiacki, M.; Bednorz, L.; Spiżewski, T. Concentration of heavy metals in urban allotment soils and their uptake by selected vegetable crop species—A case study from Gorzów Wielkopolski, Poland. J. Elem. 2022, 27, 405–421. [Google Scholar] [CrossRef]
- Diatta, J.B.; Chudzińska, E.; Wirth, S. Assessment of heavy metal contamination of soils impacted by a zinc smelter activity. J. Elem. 2008, 13, 5–16. [Google Scholar]
- Gutiérrez Rodelo, C.; Salinas, R.A.; Armenta Jaime, E.; Armenta, S.; Galdámez-Martínez, A.; Castillo-Blum, S.E.; Astudillo-de la Vega, H.; Nirmala Grace, A.; Aguilar-Salinas, C.A.; Gutiérrez Rodelo, J.; et al. Zinc associated nanomaterials and their intervention in emerging respiratory viruses: Journey to the field of biomedicine and biomaterials. Coord. Chem. Rev. 2022, 457, 214402. [Google Scholar] [CrossRef] [PubMed]
- Hübner, C.; Haase, H. Interactions of zinc- and redox-signaling pathways. Redox. Biol. 2021, 41, 101916. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Khan, M.; Sheikh, T.M.M.; Mumtaz, M.Z.; Chohan, T.A.; Shamim, S.; Liu, Y. Zinc Essentiality, Toxicity, and Its Bacterial Bioremediation: A Comprehensive Insight. Front. Microbiol. 2023, 17, 1133733. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Lis, J.; Pasieczna, A.; Mojski, J.E.; Przenioslo, S.; Sylwestrzak, H.; Strzelecki, R.; Wołkiewicz, S. Atlas Geochemiczny Polski 1:250000, Warsaw, Poland. 2012. Available online: http://www.mapgeochem.pgi.gov.pl/poland/atlas.html (accessed on 28 April 2023).
- Pasieczna, A.; Markowski, W.; GEMAS. Badania Geochemiczne Gleb pól Uprawnych i Trwałych Użytków Zielonych w Polsce—Raport Krajowy. Warsaw, Poland, 2015. Available online: http://www.mapgeochem.pgi.gov.pl/gemas/atlas.html (accessed on 28 April 2023).
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Regulation of the Minister of the Environment. 1 September 2016 on the Method of Assessing Soil Contamination. J. Laws 2016, 1395. Available online: https://isap.sejm.gov.pl/ (accessed on 28 April 2023).
- Kabata-Pendias, A.; Motowicka-Terelak, T.; Piotrowska, M.; Terelak, H.; Witek, T. Evaluation of the Degree of Contamination of Soils and Plants by Heavy Metals and Sulfur—A Framework of Guidance for Agriculture; IUNG: Puławy, Poland, 1993. [Google Scholar]
- Kabata-Pendias, A.; Piotrowska, M. Basics of Assessment of Chemical Contamination of Soils. Heavy Metals, Sulfur and PAHs; Environment Monitoring Library, PIOŚ, IUNG: Warsaw, Poland, 1995. [Google Scholar]
- Świdawska-Urbańska, J.; Zalewski, M. Assessment of Selected Heavy Metals Content in Soil of Agricultural Activity. Geomat. Environ. Eng. 2019, 13, 103–113. [Google Scholar] [CrossRef]
- Baran, A.; Wieczorek, J. Content of zinc in the different elements of the environment in zone of potential impact galvanizing. Proc. ECOpole 2012, 6, 193–198. [Google Scholar] [CrossRef]
- Alsafran, M.; Usman, K.; Al Jabri, H.; Rizwan, M. Ecological and Health Risks Assessment of Potentially Toxic Metals and Metalloids Contaminants: A Case Study of Agricultural Soils in Qatar. Toxics 2021, 9, 35. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, Y.; Liu, Y.-J.; Xiao, L.; Li, K.; Li, M.-Y.; Tian, Y.; Wu, F. Source Apportionment and Ecological Risk Assessment of Potentially Toxic Elements in Cultivated Soils of Xiangzhou, China: A Combined Approach of Geographic Information System and Random Forest. Sustainability 2021, 13, 1214. [Google Scholar] [CrossRef]
- Al-Taani, A.A.; Nazzal, Y.; Howari, F.M.; Iqbal, J.; Bou Orm, N.; Xavier, C.M.; Bărbulescu, A.; Sharma, M.; Dumitriu, C.-S. Contamination Assessment of Heavy Metals in Agricultural Soil, in the Liwa Area (UAE). Toxics 2021, 9, 53. [Google Scholar] [CrossRef]
- Santos, H.F.; Soares, M.B.; Alleoni, L.D.F. Pristine and biochar-supported nano zero-valent iron to immobilize As, Zn and Pb in soil contaminated by smelting activities. J. Environ. Manag. 2022, 321, 116017. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Z.; Li, Q.; Hu, Q.; Yang, R.; Tang, H.; Li, M.; Huang, M.; Zhang, J.; Li, G. Canonical correspondence analysis of soil heavy metal pollution, microflora and enzyme activities in the Pb–Zn mine tailing dam collapse area of Sidi village, SW China. Environ. Earth Sci. 2015, 73, 267–274. [Google Scholar] [CrossRef]
- Hooda, P. Trace Elements in Soils; Wiley-Blackwell: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Amirmoradi, S.; Moghaddam, P.R.; Koocheki, A.; Danesh, S.; Fotovat, A. Effect of Cadmium and Lead on Quantitative and Essential Oil Traits of Peppermint (Mentha piperita L.). Not. Sci. Biol. 2012, 4, 101–109. [Google Scholar] [CrossRef] [Green Version]




| Metals | Measured Value (mg∙kg−1) ± SD | Certified Value (mg∙kg−1) ± SD | Relative Difference (%) |
|---|---|---|---|
| Cd | 0.90 ± 0.20 | 1.0 ± 0.15 | 99.0 |
| Pb | 10.60 ± 0.90 | 9.90 ± 0.40 | 115.0 |
| Zn | 42.23 ± 0.70 | 40.41 ± 0.30 | 98.0 |
| World Reference Values [51] | Polish Soil Value References 0–25 cm [52] | Sample 0–5 cm Mean (Min–Max) | Sample 5–20 cm Mean (Min–Max) | Igeo Mean (Top/Bottom) | Igeo Max (Top/Bottom) | Eri (Top/Bottom) | |
|---|---|---|---|---|---|---|---|
| pH | - | - | 4.68 (4.06–5.33) | 4.45 (4–5.12) | |||
| Cd (mg∙kg−1) | 1.1 | 2 | 9.75 (4.56–17.11) | 6.02 (1.02–11.6) | 5.24/4.54 | 6.05/5.49 | 97.5/60.2 |
| Pb (mg∙kg−1) | 25 | 100 | 184.34 (109.07–509.7) | 113.26 (40.97–290.67) | 2.71/2.01 | 4.18/3.37 | 921.7/566.3 |
| Zn (mg∙kg−1) | 62 | 300 | 638.31 (120.99–2007.34) | 208.76 (42.33–909.45) | 5.24/4.54 | 5.84/4.70 | 638.3/208.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świercz, A.; Szwed, M.; Bąk, Ł.; Gawlik, A.; Zamachowski, J. Assessment of Cultivated Soil Contamination by Potentially Toxic Metals as a Result of a Galvanizing Plant Failure. Sustainability 2023, 15, 9288. https://doi.org/10.3390/su15129288
Świercz A, Szwed M, Bąk Ł, Gawlik A, Zamachowski J. Assessment of Cultivated Soil Contamination by Potentially Toxic Metals as a Result of a Galvanizing Plant Failure. Sustainability. 2023; 15(12):9288. https://doi.org/10.3390/su15129288
Chicago/Turabian StyleŚwiercz, Anna, Mirosław Szwed, Łukasz Bąk, Adam Gawlik, and Jakub Zamachowski. 2023. "Assessment of Cultivated Soil Contamination by Potentially Toxic Metals as a Result of a Galvanizing Plant Failure" Sustainability 15, no. 12: 9288. https://doi.org/10.3390/su15129288
APA StyleŚwiercz, A., Szwed, M., Bąk, Ł., Gawlik, A., & Zamachowski, J. (2023). Assessment of Cultivated Soil Contamination by Potentially Toxic Metals as a Result of a Galvanizing Plant Failure. Sustainability, 15(12), 9288. https://doi.org/10.3390/su15129288








