Valorization of Face Masks Produced during COVID-19 Pandemic through Hydrothermal Carbonization (HTC): A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
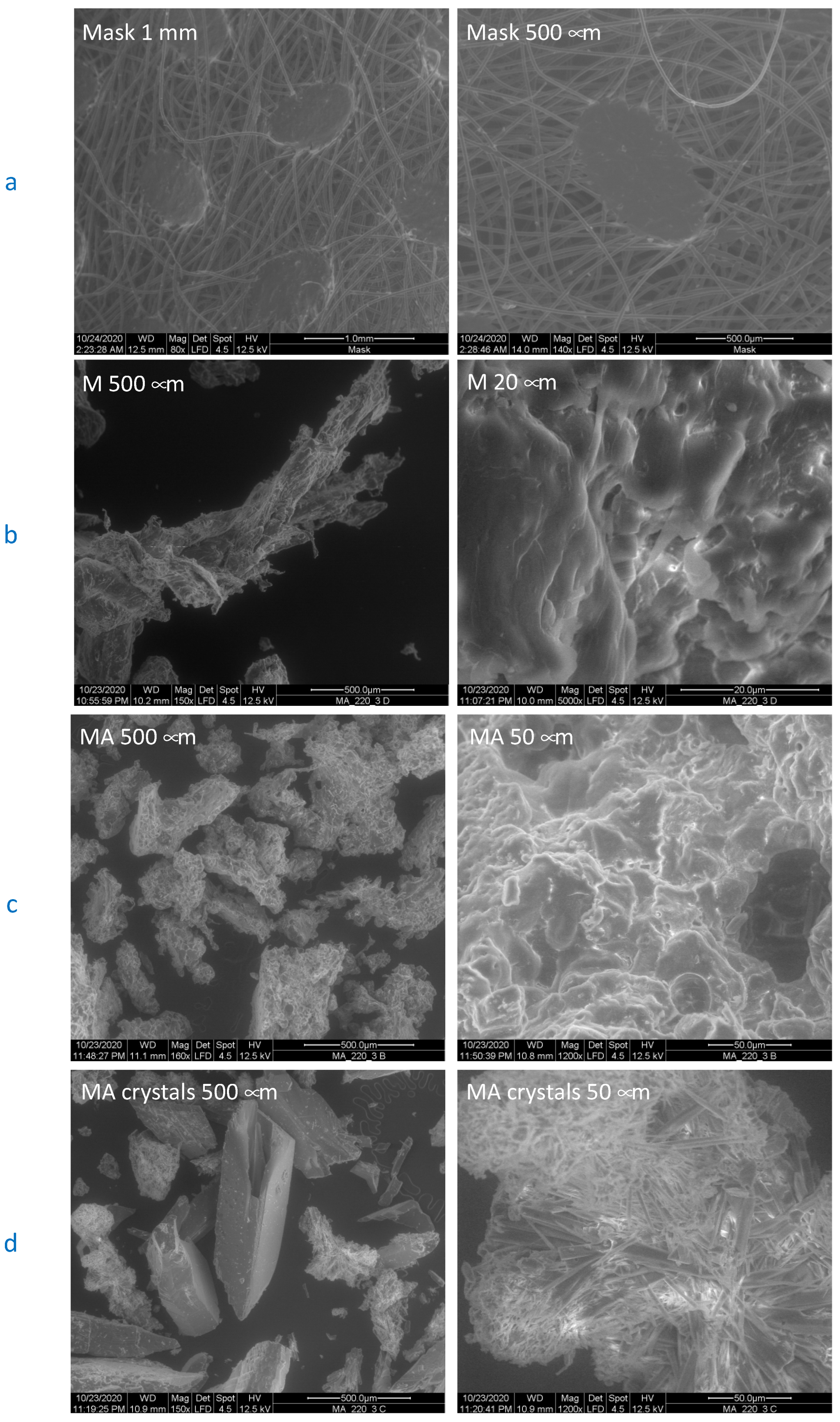
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| ARG | Antibiotic Resistance Gene |
| BET | Brunauer–Emmett–Teller |
| EC | Electrical Conductivity |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HC | Hydrochar |
| HHV | Higher Heating Value |
| HTC | Hydrothermal Carbonization |
| IC | Inorganic Carbon |
| M | Hydrochar from surgical masks |
| MA | Hydrochar from surgical masks in presence of acetic acid |
| PC | Polycarbonate |
| PE | Polyethylene |
| PET | Polyethylene Terephthalate |
| PP | Polypropylene |
| PPE | Personal Protective Equipment |
| PS | Polystyrene |
| PTFE | Polytetrafluoroethylene |
| PVC | Polyvinyl Chloride |
| PW | Process Water |
| PXRD | Powder X-ray Diffraction |
| SEM | Scanning Electron Microscopy |
| TC | Total Carbon |
| TGA | Thermogravimetric Analysis |
| TOC | Total Organic Carbon |
| TPA | Terephthalic Acid |
| XRD | X-ray Diffraction |
References
- World Health Organization; The United Nations Children’s Fund (UNICEF). Water, Sanitation, Hygiene, and Waste Management for the COVID-19 Virus: Interim Guidance; World Health Organization: Geneva, Switzerland, 2020.
- Dharmaraj, S.; Ashokkumar, V.; Pandiyan, R.; Halimatul Munawaroh, H.S.; Chew, K.W.; Chen, W.-H.; Ngamcharussrivichai, C. Pyrolysis: An Effective Technique for Degradation of COVID-19 Medical Wastes. Chemosphere 2021, 275, 130092. [Google Scholar] [CrossRef]
- Liang, Y.; Song, Q.; Wu, N.; Li, J.; Zhong, Y.; Zeng, W. Repercussions of COVID-19 Pandemic on Solid Waste Generation and Management Strategies. Front. Environ. Sci. Eng. 2021, 15, 115. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Pyrolysis Kinetic Behaviour and TG-FTIR-GC–MS Analysis of Coronavirus Face Masks. J. Anal. Appl. Pyrolysis 2021, 156, 105118. [Google Scholar] [CrossRef]
- Crespo, C.; Ibarz, G.; Sáenz, C.; Gonzalez, P.; Roche, S. Study of Recycling Potential of FFP2 Face Masks and Characterization of the Plastic Mix-Material Obtained. A Way of Reducing Waste in Times of COVID-19. Waste Biomass Valor. 2021, 12, 6423–6432. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef]
- Patrício Silva, A.L.; Prata, J.C.; Duarte, A.C.; Barcelò, D.; Rocha-Santos, T. An Urgent Call to Think Globally and Act Locally on Landfill Disposable Plastics under and after COVID-19 Pandemic: Pollution Prevention and Technological (Bio) Remediation Solutions. Chem. Eng. J. 2021, 426, 131201. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of Potential Applications of Hydrochar Derived from Hydrothermal Carbonization of Biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Brillard, A.; Kehrli, D.; Douguet, O.; Gautier, K.; Tschamber, V.; Bueno, M.-A.; Brilhac, J.-F. Pyrolysis and Combustion of Community Masks: Thermogravimetric Analyses, Characterizations, Gaseous Emissions, and Kinetic Modeling. Fuel 2021, 306, 121644. [Google Scholar] [CrossRef]
- Duangchan, A.; Samart, C. Tertiary Recycling of PVC-Containing Plastic Waste by Copyrolysis with Cattle Manure. Waste Manag. 2008, 28, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Eui Lee, M.; Kim, S.-S.; Joh, H.-I.; Lee, S. Efficient Upcycling of Polypropylene-Based Waste Disposable Masks into Hard Carbons for Anodes in Sodium Ion Batteries. J. Ind. Eng. Chem. 2022, 105, 268–277. [Google Scholar] [CrossRef]
- Ro, K.S.; Hunt, P.G.; Jackson, M.A.; Compton, D.L.; Yates, S.R.; Cantrell, K.; Chang, S. Co-Pyrolysis of Swine Manure with Agricultural Plastic Waste: Laboratory-Scale Study. Waste Manag. 2014, 34, 1520–1528. [Google Scholar] [CrossRef]
- Adolfsson, K.H.; Lin, C.; Hakkarainen, M. Microwave Assisted Hydrothermal Carbonization and Solid State Postmodification of Carbonized Polypropylene. ACS Sustain. Chem. Eng. 2018, 6, 11105–11114. [Google Scholar] [CrossRef]
- Ducey, T.F.; Collins, J.C.; Ro, K.S.; Woodbury, B.L.; Griffin, D.D. Hydrothermal Carbonization of Livestock Mortality for the Reduction of Pathogens and Microbially-Derived DNA. Front. Environ. Sci. Eng. 2017, 11, 9. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Bioref. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y. A Review on Hydrothermal Carbonization of Biomass and Plastic Wastes to Energy Products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.-D. Hydrothermal Carbonization of Poly(Vinyl Chloride). Chemosphere 2015, 119, 682–689. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Ge, S.; Chen, X.; Ge, X.; Chen, M. Hydrothermal Carbonization of Medical Wastes and Lignocellulosic Biomass for Solid Fuel Production from Lab-Scale to Pilot-Scale. Energy 2017, 118, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Ma, X. Characteristics of Co-Hydrothermal Carbonization on Polyvinyl Chloride Wastes with Bamboo. Bioresour. Technol. 2018, 247, 302–309. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, X. Microplastic Degradation in Sewage Sludge by Hydrothermal Carbonization: Efficiency and Mechanisms. Chemosphere 2022, 297, 134203. [Google Scholar] [CrossRef]
- Wei, Y.; Fakudze, S.; Zhang, Y.; Ma, R.; Shang, Q.; Chen, J.; Liu, C.; Chu, Q. Co-Hydrothermal Carbonization of Pomelo Peel and PVC for Production of Hydrochar Pellets with Enhanced Fuel Properties and Dechlorination. Energy 2022, 239, 122350. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Wedwitschka, H.; Baskyr, I.; Koehler, R.; Kopinke, F.-D. Characterization of Biocoals and Dissolved Organic Matter Phases Obtained upon Hydrothermal Carbonization of Brewer’s Spent Grain. Bioresour. Technol. 2014, 164, 162–169. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-Based Chemical Looping Technologies: The Good, the Bad and the Future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef] [Green Version]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.-X. Bio-Based Polymers with Performance-Advantaged Properties. Nat. Rev. Mater. 2021, 7, 83–103. [Google Scholar] [CrossRef]
- Huang, Y.W.; Chen, M.Q.; Li, Q.H.; Xing, W. A Critical Evaluation on Chemical Exergy and Its Correlation with High Heating Value for Single and Multi-Component Typical Plastic Wastes. Energy 2018, 156, 548–554. [Google Scholar] [CrossRef]
- Linstrom, P. NIST Chemistry WebBook, NIST Standard Reference Database 69. J. Phys. Chem. Ref. Data Monogr. 1998, 9, 1–1951. [Google Scholar]
- Ali, L.; Kuttiyathil, M.S.; Altarawneh, M. Catalytic Upgrading of the Polymeric Constituents in COVID-19 Masks. J. Environ. Chem. Eng. 2022, 10, 106978. [Google Scholar] [CrossRef]
- Krylova, V.; Dukštienė, N. Synthesis and Characterization of Ag2S Layers Formed on Polypropylene. J. Chem. 2013, 2013, 987879. [Google Scholar] [CrossRef] [Green Version]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; Analytical techniques in the sciences; J. Wiley: Chichester, UK; Hoboken, NJ, USA, 2004; ISBN 978-0-470-85427-3. [Google Scholar]
- Chiu, F.-C.; Chu, P.-H. Characterization of Solution-Mixed Polypropylene/Clay Nanocomposites without Compatibilizers. J Polym Res 2006, 13, 73–78. [Google Scholar] [CrossRef]
- Ravichandran, S.A.; Rajan, V.P.; Aravind, P.V.; Seenivasan, A.; Prakash, D.G.; Ramakrishnan, K. Characterization of Terephthalic Acid Monomer Recycled from Post-Consumer PET Polymer Bottles. Macromol. Symp. 2016, 361, 30–33. [Google Scholar] [CrossRef]
- van Leeuwen, P.W.N.M. Catalysis, Homogeneous. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 457–490. ISBN 978-0-12-227410-7. [Google Scholar]
- Zhou, J.-H.; Shen, G.-Z.; Zhu, J.; Yuan, W.-K. Terephthalic Acid Hydropurification over Pd/C Catalyst. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2006; Volume 159, pp. 293–296. ISBN 978-0-444-51733-3. [Google Scholar]
- Cosimbescu, L.; Merkel, D.R.; Darsell, J.; Petrossian, G. Simple But Tricky: Investigations of Terephthalic Acid Purity Obtained from Mixed PET Waste. Ind. Eng. Chem. Res. 2021, 60, 12792–12797. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into Terephthalic Acid in Neutral Media Catalyzed by the ZSM-5 Acidic Catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Lee, H.L.; Chiu, C.W.; Lee, T. Engineering Terephthalic Acid Product from Recycling of PET Bottles Waste for Downstream Operations. Chem. Eng. J. Adv. 2021, 5, 100079. [Google Scholar] [CrossRef]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product? Biomass Valor. 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Alhnidi, M.-J.; Wüst, D.; Funke, A.; Hang, L.; Kruse, A. Fate of Nitrogen, Phosphate, and Potassium during Hydrothermal Carbonization and the Potential for Nutrient Recovery. ACS Sustain. Chem. Eng. 2020, 8, 15507–15516. [Google Scholar] [CrossRef]
- Crossley, O.P.; Thorpe, R.B.; Peus, D.; Lee, J. Phosphorus Recovery from Process Waste Water Made by the Hydrothermal Carbonisation of Spent Coffee Grounds. Bioresour. Technol. 2020, 301, 122664. [Google Scholar] [CrossRef] [PubMed]
- Farru, G.; Cappai, G.; Carucci, A.; De Gioannis, G.; Asunis, F.; Milia, S.; Muntoni, A.; Perra, M.; Serpe, A. A Cascade Biorefinery for Grape Marc: Recovery of Materials and Energy through Thermochemical and Biochemical Processes. Sci. Total Environ. 2022, 846, 157464. [Google Scholar] [CrossRef] [PubMed]
- Farru, G.; Asquer, C.; Cappai, G.; De Gioannis, G.; Melis, E.; Milia, S.; Muntoni, A.; Piredda, M.; Scano, E.A. Hydrothermal Carbonization of Hemp Digestate: Influence of Operating Parameters. Biomass Conv. Bioref. 2022, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Wang, J.; Zheng, C. Effects of Aqueous Phase Recirculation in Hydrothermal Carbonization of Sweet Potato Waste. Bioresour. Technol. 2018, 267, 167–174. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Olszewski, M.P.; Wang, X.; Pfersich, J.; Sebastian, V.; Manyà, J.; Hedin, N.; Kruse, A. Assessment of the Effects of Process Water Recirculation on the Surface Chemistry and Morphology of Hydrochar. Renew. Energy 2020, 155, 1173–1180. [Google Scholar] [CrossRef]

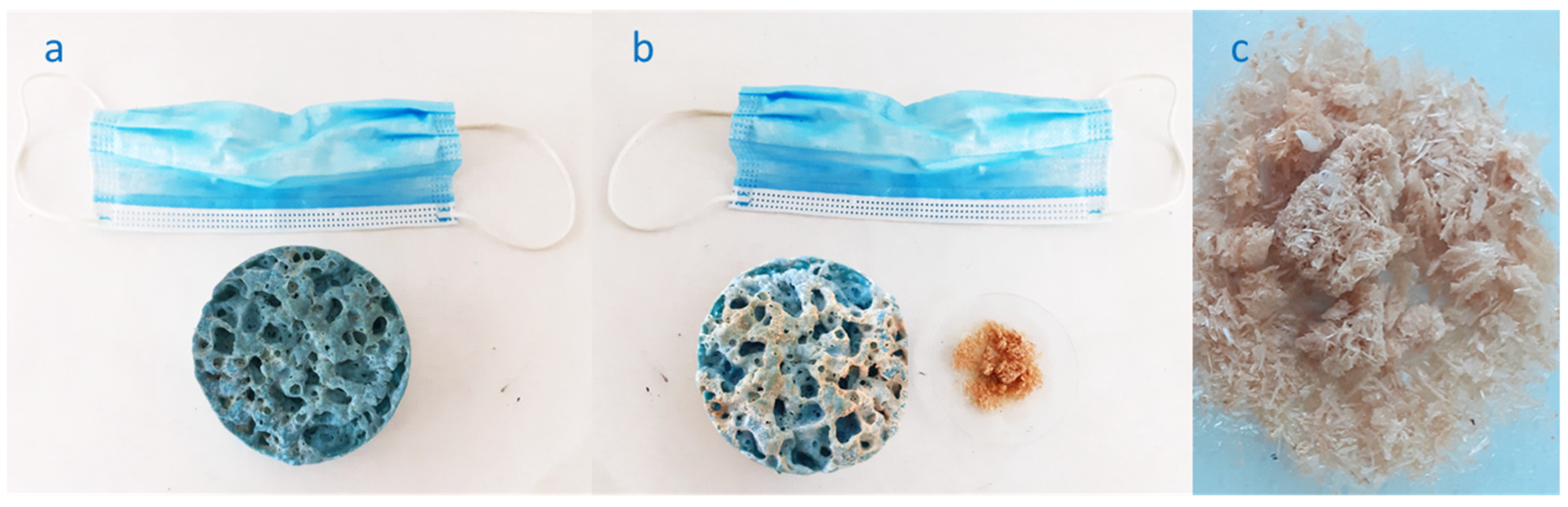

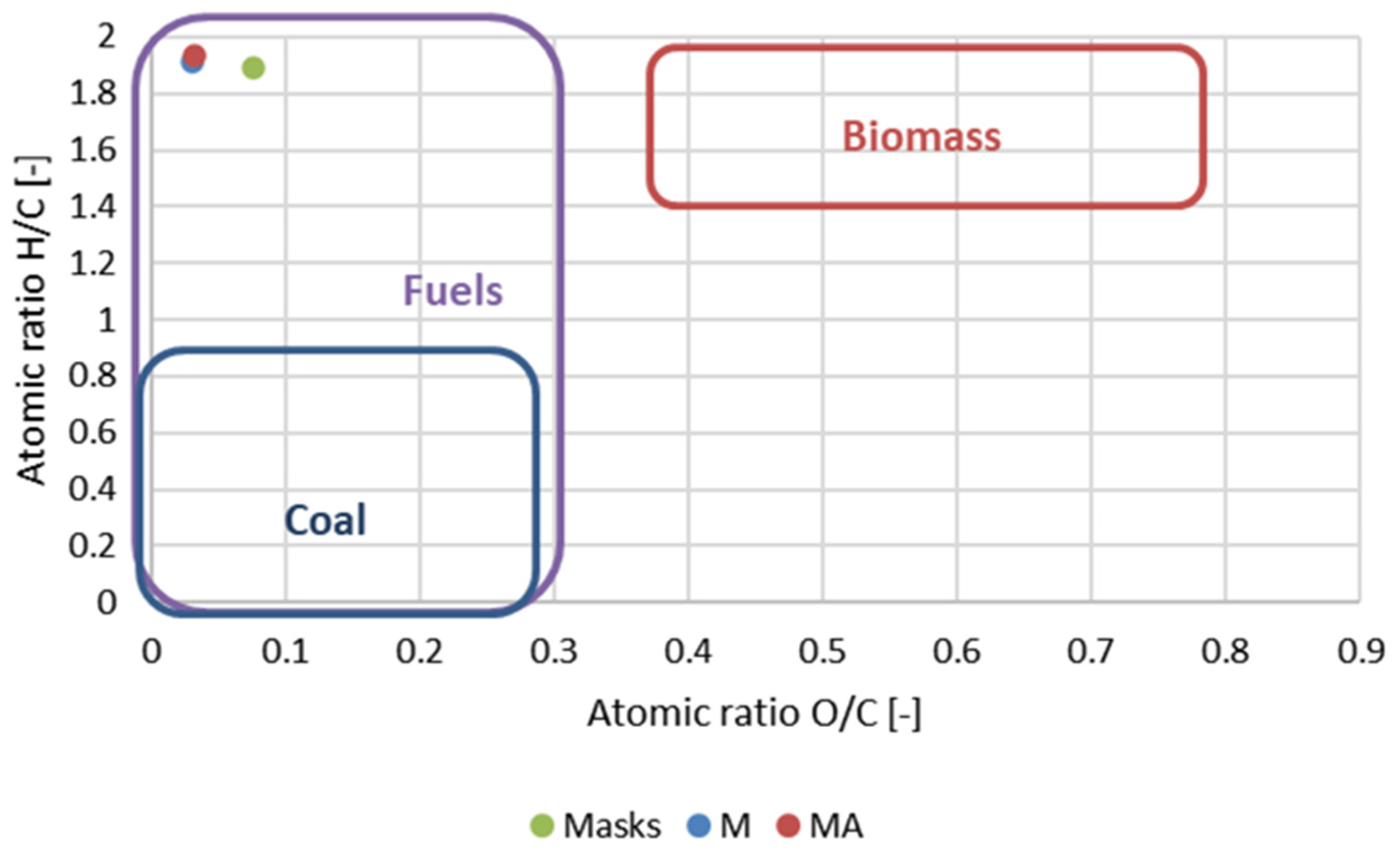


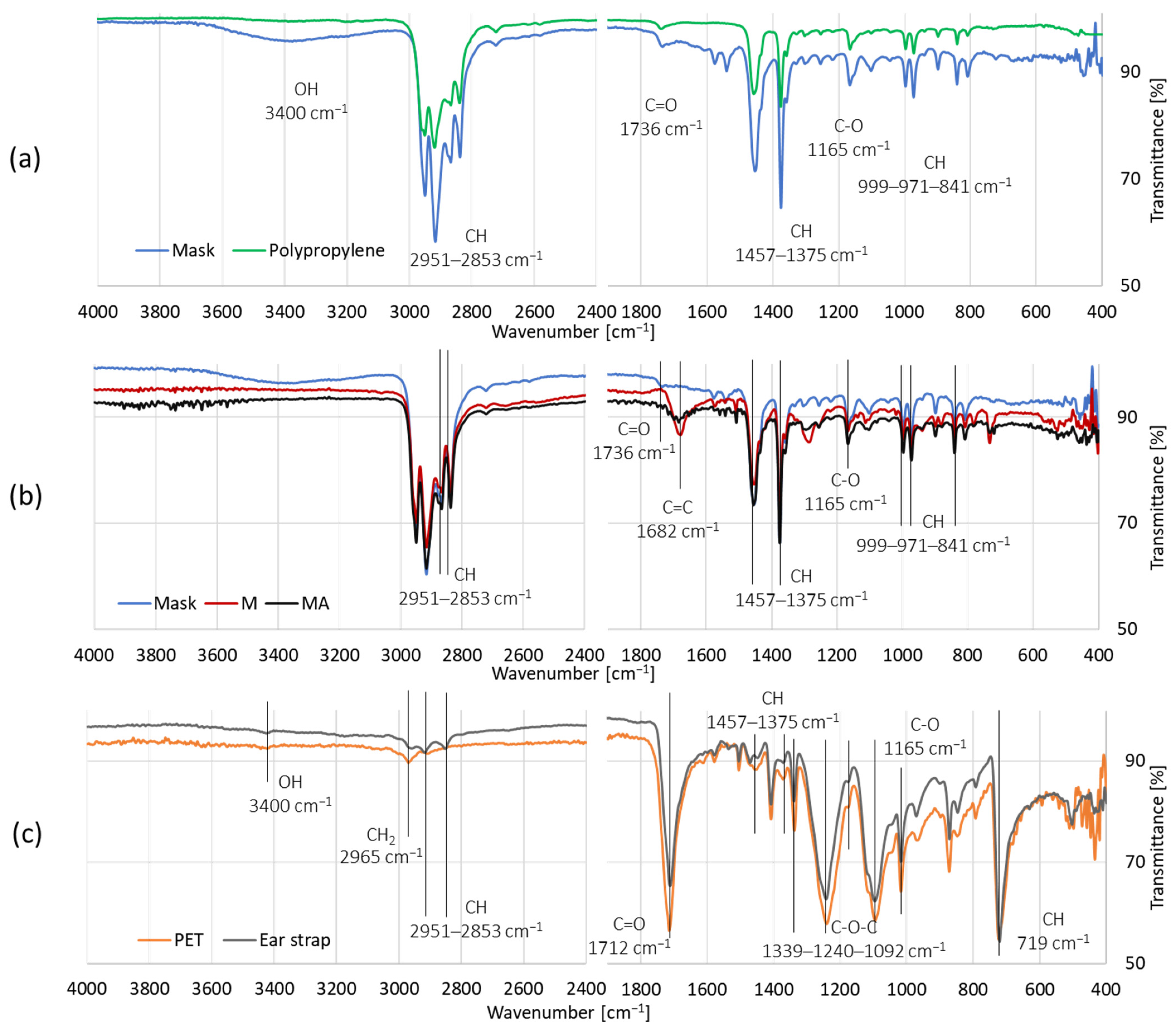

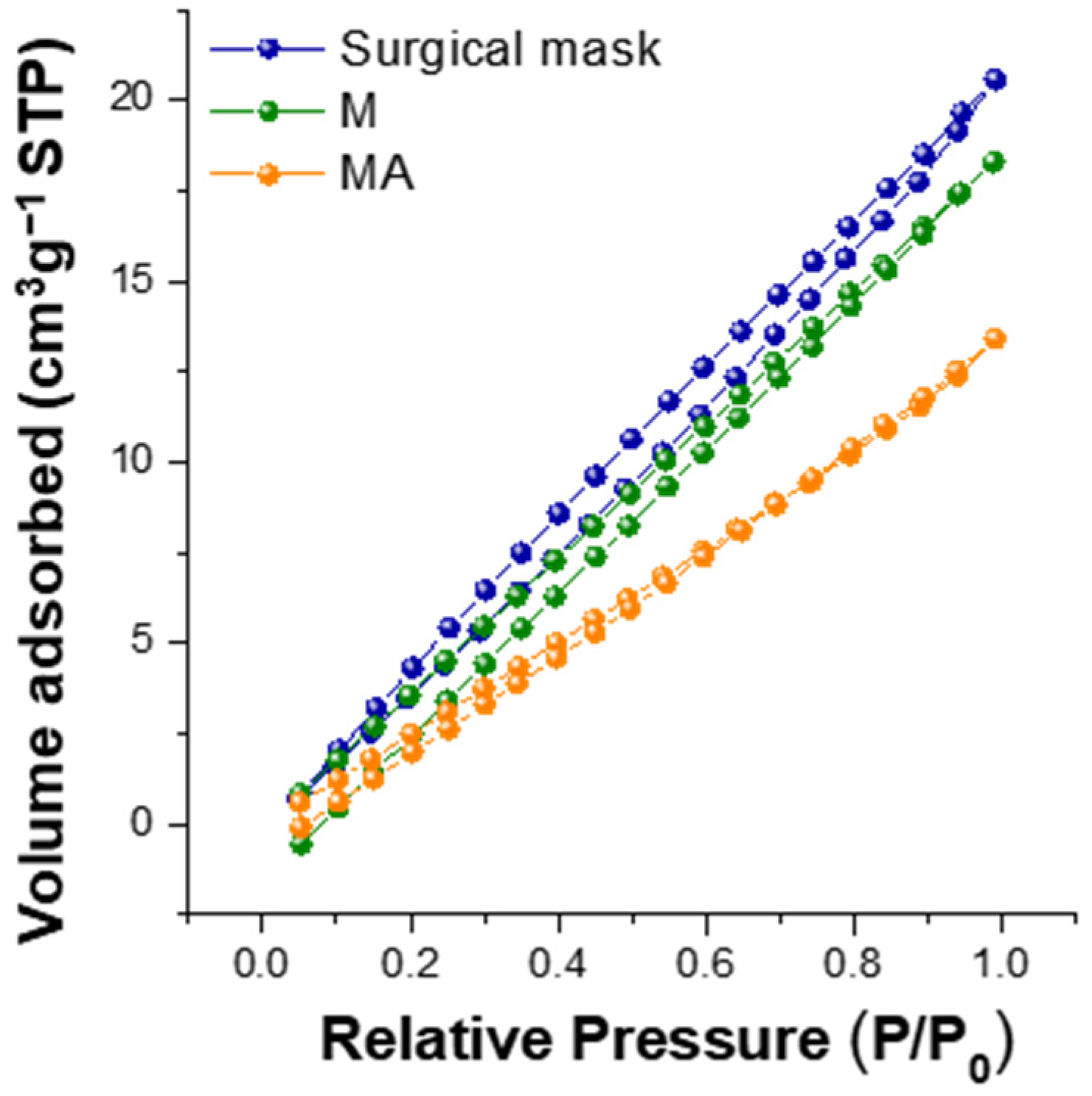
| Sample | Ash [%] | C [%] | H [%] | N [%] | O [%] | H/C [-] | O/C [-] | HHV [MJ/kg] | LHV [MJ/kg] | Energy Yield [-] |
|---|---|---|---|---|---|---|---|---|---|---|
| Masks | 1.92 (0.04) | 76.21 (0.89) | 12.02 (0.53) | 2.08 (0.20) | 7.77 (1.22) | 1.892 | 0.077 | 43.12 (0.51) | 40.48 | - |
| M | 0.21 (0.02) | 82.99 (1.14) | 13.22 (0.35) | 0.17 (0.03) | 3.41 (1.47) | 1.911 | 0.031 | 45.29 (0.21) | 42.41 | 0.94 |
| MA | 0.59 (0.04) | 82.41 (0.09) | 13.31 (0.19) | 0.17 (0.04) | 3.68 (0.26) | 1.938 | 0.032 | 45.33 (0.17) | 42.41 | 0.88 |
| Sample | Surface Area [m2/g] | Micro-Mesoporous Volume [cm3/g] |
|---|---|---|
| Surgical mask | 44.0 | 0.03 |
| M | 37.7 | 0.03 |
| MA | 24.5 | 0.02 |
| Sample | pH | Electrical Conductivity [mS/cm] | TOC [g/L] | TC [g/L] | IC [g/L] |
|---|---|---|---|---|---|
| Before HTC treatment | |||||
| M | 6.11 | 0.02 | 0.00 | 0.01 | 0.01 |
| MA | 2.58 | 1.51 | 20.49 | 20.50 | 0.01 |
| After HTC treatment | |||||
| M | 3.82 | 0.51 | 2.65 | 2.67 | 0.02 |
| MA | 2.70 | 2.57 | 26.58 | 26.75 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farru, G.; Libra, J.A.; Ro, K.S.; Cannas, C.; Cara, C.; Muntoni, A.; Piredda, M.; Cappai, G. Valorization of Face Masks Produced during COVID-19 Pandemic through Hydrothermal Carbonization (HTC): A Preliminary Study. Sustainability 2023, 15, 9382. https://doi.org/10.3390/su15129382
Farru G, Libra JA, Ro KS, Cannas C, Cara C, Muntoni A, Piredda M, Cappai G. Valorization of Face Masks Produced during COVID-19 Pandemic through Hydrothermal Carbonization (HTC): A Preliminary Study. Sustainability. 2023; 15(12):9382. https://doi.org/10.3390/su15129382
Chicago/Turabian StyleFarru, Gianluigi, Judy A. Libra, Kyoung S. Ro, Carla Cannas, Claudio Cara, Aldo Muntoni, Martina Piredda, and Giovanna Cappai. 2023. "Valorization of Face Masks Produced during COVID-19 Pandemic through Hydrothermal Carbonization (HTC): A Preliminary Study" Sustainability 15, no. 12: 9382. https://doi.org/10.3390/su15129382







