Role of Biotransformation of Acacia nilotica Metabolites by Aspergillus subolivaceus in Boosting Lupinus termis Yield: A Promising Approach to Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Preparation of Metabolites
2.3. Experimental Design
2.4. Statistical Analysis
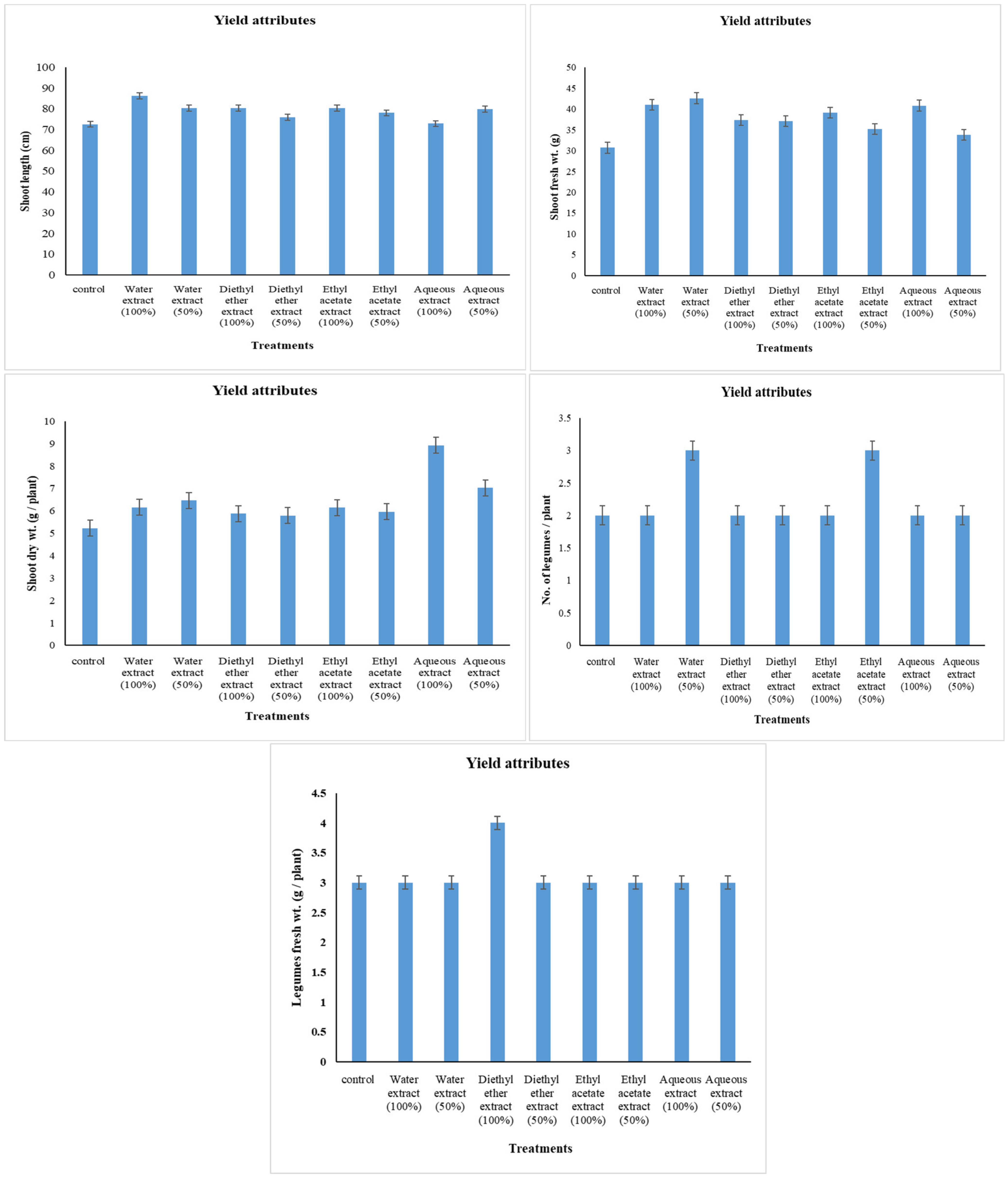
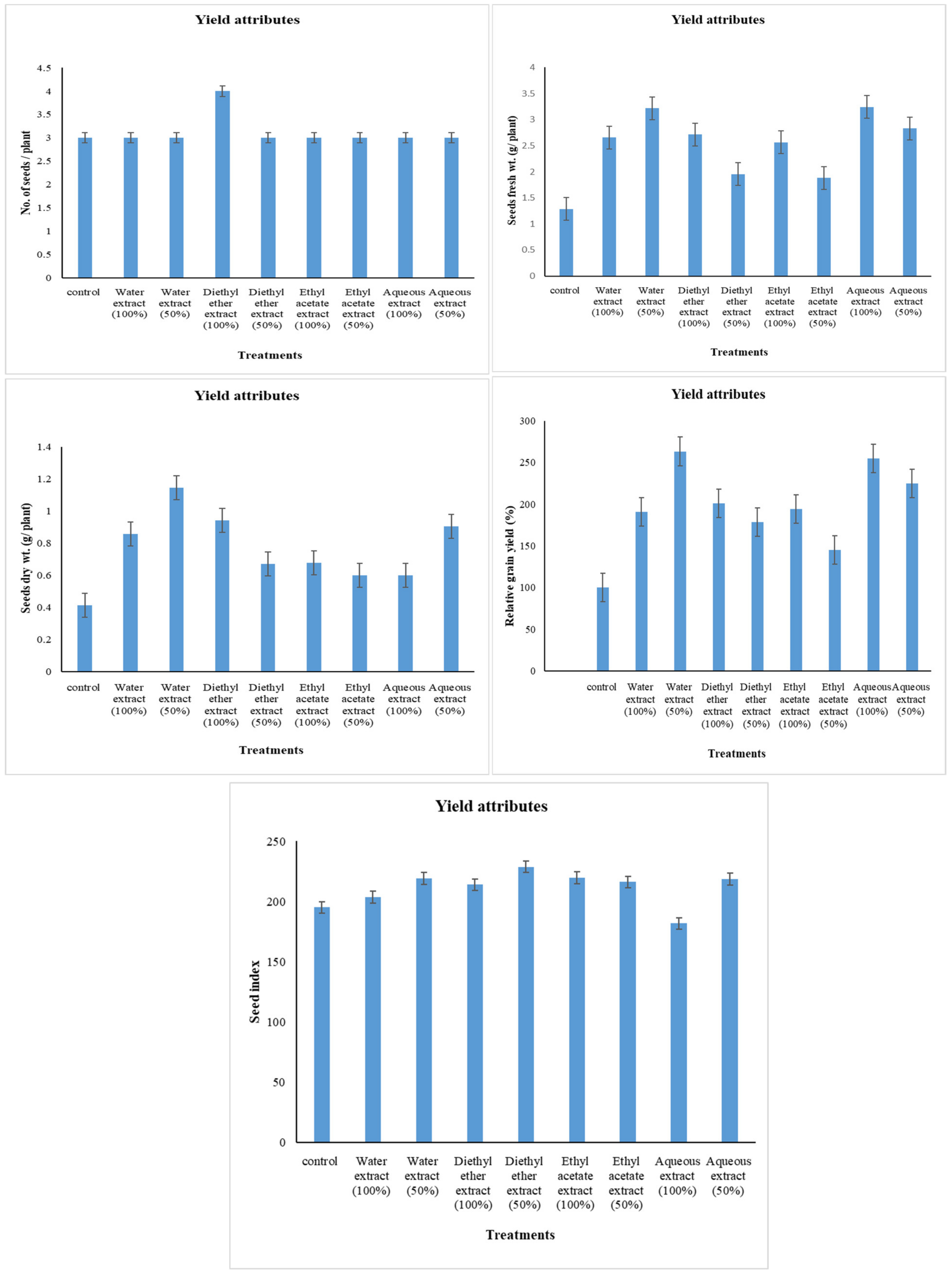
2.5. Yield Attributes
2.6. Physio-Biochemical Aspects
2.6.1. Estimation of Carbohydrates
2.6.2. Estimation of Nitrogenous Constituents
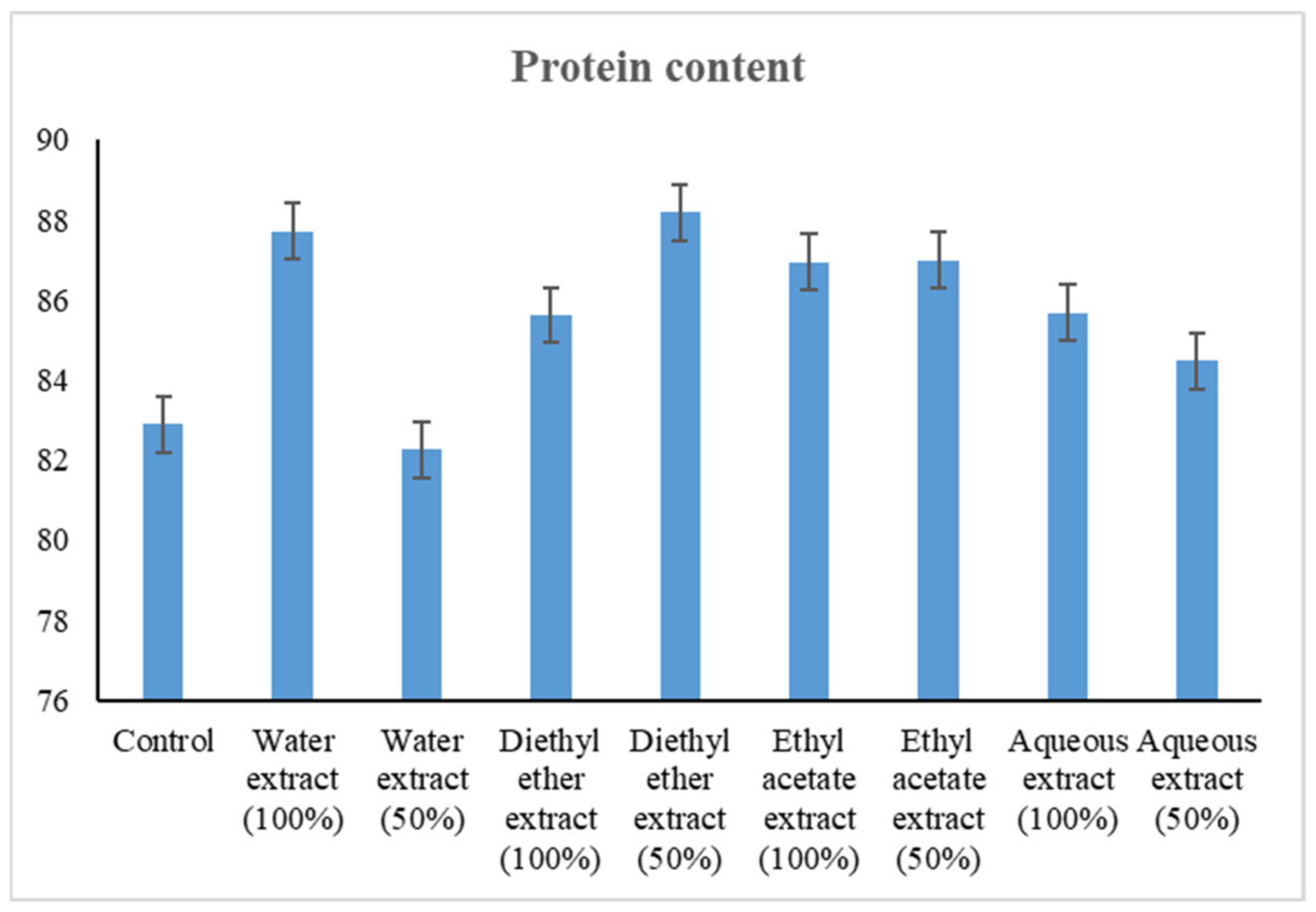
2.6.3. Estimation of Total Protein
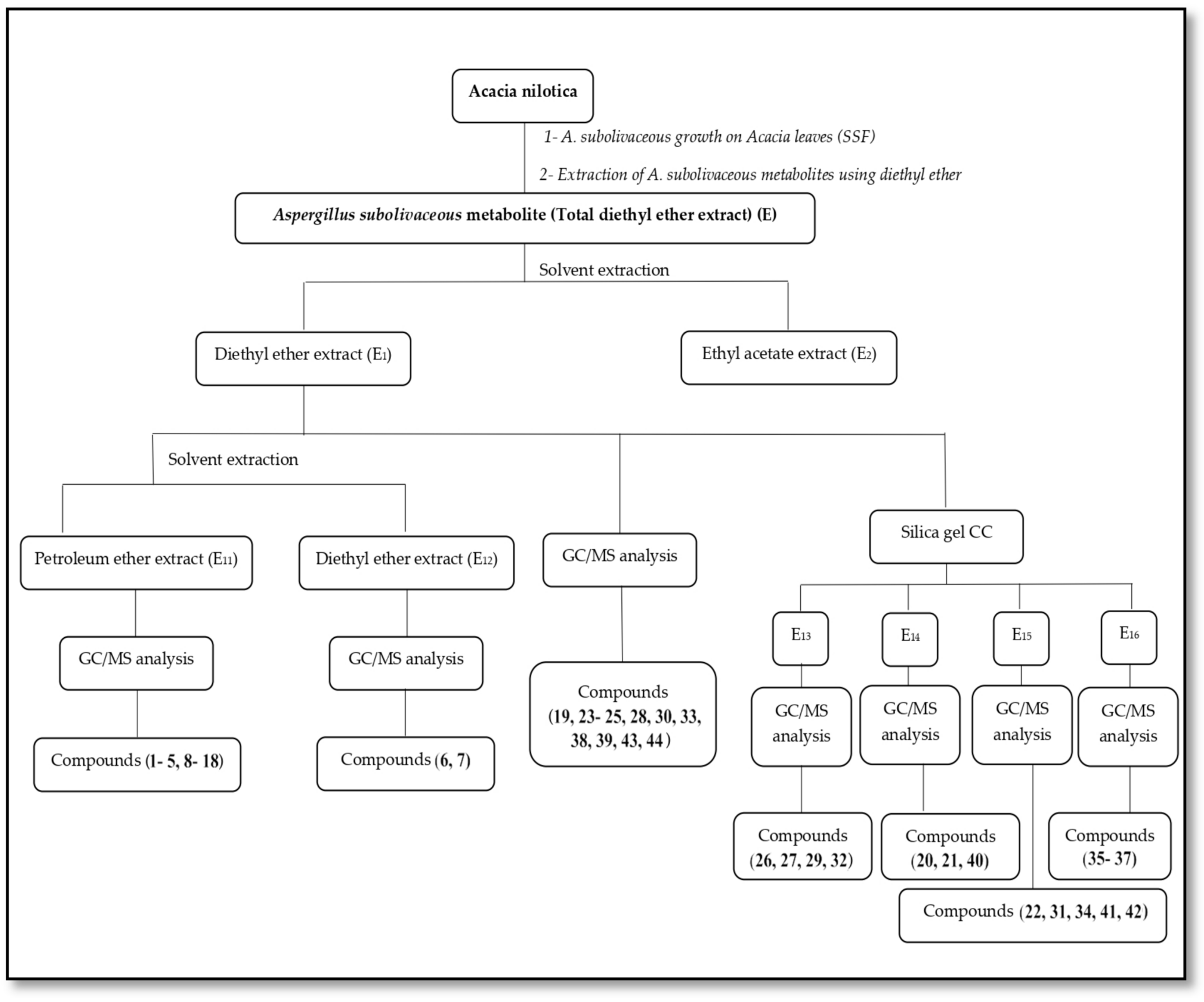
2.7. Chemical Constituents of Diethyl Ether Extract
Instrumentation
3. Results
3.1. Changes in Yield Attributes
3.2. Changes in Biochemical Aspects in L. termis L. yielded Seeds
3.2.1. Changes in Carbohydrate Content
3.2.2. Changes in Nitrogen Content
3.2.3. Changes in Protein Content
3.3. Processing of Total Diethyl Ether Extract (E) of A. subolivaceous/A. nilotica Metabolite
4. Discussion
4.1. Changes in Yield Attributes
4.2. Changes in Biochemical Aspects in L. termis L. yielded Seeds
4.2.1. Changes in Carbohydrate Content
4.2.2. Changes in Nitrogen Content
4.2.3. Changes in Protein Content
4.3. Processing of Total Diethyl Ether Extract (E) of A. subolivaceous/A. nilotica Metabolite
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grandini, A.; Summa, D.; Costa, S.; Buzzi, R.; Tamburini, E.; Sacchetti, G.; Guerrini, A. Biotransformation of Waste Bile Acids: A New Possible Sustainable Approach to Anti-Fungal Molecules for Crop Plant Bioprotection? Int. J. Mol. Sci. 2022, 23, 4152. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, L.F.; Arruda, M.F.C.; Vieira, S.R.; Campelo, P.M.S.; Gregio, A.M.T.; Rosa, E.A.R. Microbial biotransformation to obtain new antifungals. Front. Microbiol. 2015, 6, 1433. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Rosazza, J.P.N. Microbial and enzymatic transformations of flavonoids. J. Nat. Prod. 2006, 69, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Smitha, M.S.; Singh, V.; Singh, R. Microbial biotransformation: A process for chemical alterations. J. Bacteriol. Mycol. 2017, 4, 85. [Google Scholar]
- Rueda, M.G.M.; Guerrinia, A.; Giovanninib, P.P.; Medicib, A.; Grandinia, A.; Sacchettia, G.; Pedrini, P. Biotransformations of terpenes by fungi from Amazonian citrus plants. Chem. Biodivers. 2013, 10, 1909–1919. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G. Analysis of secondary metabolites from plant endophytic fungi. Methods Mol. Biol. 2018, 1848, 25–38. [Google Scholar]
- Hemamalini, G.; Jithesh, P.; Nirmala, P. In silico screening of phytochemicals identified from Acacia nilotica by GC/MS method for its anticancer activity. Int. J. Pharm. Sci. Res. 2014, 5, 2374–2381. [Google Scholar]
- Sadiq, M.B.; Tarning, J.; Aye, T.Z.; Anal, A.K. Antibacterial activities and possible modes of action of Acacia nilotica (L.) Del. against multidrug-resistant Escherichia coli and Salmonella. Molecules 2017, 22, 47. [Google Scholar] [CrossRef]
- Lim, T.K. Edible medicinal and non- medicinal plants. Springer Neth. 2012, 2, 763–769. [Google Scholar]
- Dubois, O.; Allanic, C.; Charvet, C.L.; Guégnard, F.; Février, H.; Théry-Koné, I.; Cortet, J.; Koch, C.; Bouvier, F.; Fassier, T.; et al. Lupin (Lupinus spp.) seeds exert anthelmintic activity associated with their alkaloid content. Sci. Rep. 2019, 9, 9070. [Google Scholar] [CrossRef] [PubMed]
- Sedláková, K.; Straková, E.; Suchý, P.; Krejcarová, J.; Herzig, I. Lupin as a perspective protein plant for animal and human nutrition. Areview. Acta Vet. Brno. 2016, 85, 165–175. [Google Scholar] [CrossRef]
- Zarei, M.; Asgarpanah, J.; Ziarati, P. Chemical composition profile of wild Acacia oerfota (Forssk) Schweinf seed growing in the south of Iran. Orient. J. Chem. 2015, 31, 2311–2318. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Ćavar, S.; Qayum, M.; Khan, I.; Ahmad, S. Chemical composition and antioxidant potential of Acacia leucophloea Roxb. Acta Bot. Croat. 2013, 72, 133–144. [Google Scholar] [CrossRef]
- Abduljawad, E.A. Review of some evidenced medicinal activities of Acacia nilotica. Arch. Pharm. Pract. 2020, 11, 20–25. [Google Scholar]
- Rajbir, S.; Bikram, S.; Sukhpreeet, S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicol. Vitro 2008, 22, 1965–1970. [Google Scholar]
- Banso, A. Phytochemical and antibacterial investigation of bark extracts of Acacia nilotica. J. Med. Plant Res. 2009, 3, 82–85. [Google Scholar]
- Wassel, G.M.; Abd-El-Wahab, S.M.; Aboutabl, E.A.; Ammar, N.M.; Afifi, M.S. Study of phenolic constituents and tannins isolated from Acacia nilotica L. Willd and Acacia farnesiana L. Willd growing in Egypt. Herba Hungarica 1990, 29, 43–50. [Google Scholar]
- Chaubal, R.; Tambe, A. Isolation of new straight chain compound from Acacia nilotica. Indian J. Chem. 2006, 45, 1231–1233. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, M.; Kumar, S.; Sharma, D.; Yadav, J.P. In vitro antioxidant activities and GC-MS analysis of different solvent extracts of Acacia nilotica leaves. Indian J. Pharm. Sci. 2018, 80, 892–902. [Google Scholar] [CrossRef]
- Bai, S.; Seasotiya, L.; Malik, A.; Bharti, P.; Dalal, S. GC-MS analysis of chloroform extract of Acacia nilotica L. leaves. J. Pharmacogn. Phytochem. 2014, 2, 79–82. [Google Scholar]
- Malviya, S.; Rawat, S.; Kharia, A.; Verma, M. Medicinal attributes of Acacia nilotica Linn. A comprehensive review on ethnopharmacological claims. Int. J. Pharm. Life Sci. 2011, 2, 830–837. [Google Scholar]
- Hemamalini, G.; Jithesh, P.; Nirmala, P. Phytochemical analysis of leafextract of plant Acacia nilotica by GCMS method. Adv. Biol. Res. 2013, 7, 141–144. [Google Scholar]
- Trevino-Cueto, B.; Luis, M.; Contreras-Esquivel, J.C.; Rodriguez, R.; Aguilera, A.; Aguilar, C.N. Gallic acid and tannase accumulation during fungal solid-state culture of a tannin-rich desert plant (Larrea tridentate Cov.). Bioresour. Technol. 2007, 98, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, G.; Banerjee, R. Biosynthesis of tannase and gallic acid from tannin rich substrates by Rhizopus oryzae and Aspergillus foetidus. J. Basic Microbiol. 2004, 44, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.; Prince, L.; Prabakaran, P. Antibacterial Activity of Marine derived Fungi Collected from South East Coast of Tamilnadu, India. J. Microbiol. Biotechnol. Res. 2011, 1, 86–94. [Google Scholar]
- Mohrig, J.R.; Hammond, C.N.; Schatz, P.F. Techniques in Organic Chemistry, 3rd ed.; Library of Congress: Washington, DC, USA, 2010; p. 200993463. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University: Ames, IA, USA, 1980. [Google Scholar]
- Gregory, P.J. Crop growth and development. In Ressell”s Soil Conditions and Plant Growth; Wild, A., Ed.; Longman Scientific Technical: London, UK, 1988; pp. 31–68. [Google Scholar]
- Beadle, C.L. Growth analysis. In Photosynthesis and Production in a Changing Environment. A Field and Laboratory Manual; Hall, D.C., Scurlock, J.M.O., Bolhar-Nordenkampf, H.R., Leegood, R.C., Long, S.P., Eds.; Chapman and Hall: London, UK, 1993; pp. 36–46. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Handel, E.V. Direct micro determination of sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- Fetris, A.W. A serum glucose method without protein precipitation. Am. J. Med. Technol. 1965, 31, 17–21. [Google Scholar]
- Riazi, A.; Matsuda, K.; Arslan, A. Water stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. J. Exp. Bot. 1985, 36, 1716–1725. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 2nd ed.; New Age Int. (P) Ltd.: Delhi, India, 1996. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The respiration of barely plants. IX. The metabolism of roots during the assimilation of nitrogen. New Phytol. 1956, 55, 229–252. [Google Scholar] [CrossRef]
- Delory, M. Colourimetric Estimation of Ammonia. In Inorganic Chemistry; Vogel, H.J., Ed.; Longman: London, UK, 1949; pp. 126–132. [Google Scholar]
- Naguib, M.I. Effect of sevin on carbohydrates and nitrogen metabolism during the germination of cotton seeds. Indian J. Exp. Biol. 1964, 2, 149–155. [Google Scholar]
- Muting, D.; Kaiser, E. Spectrophotometric method of determination of α-amino-N in biological material by means of the ninhydrin reaction. Hopper Seyler’s J. Physiol. Chem. 1963, 332, 276–289. [Google Scholar]
- El-Shahaby, O.A. Studies on Growth and Metabolism of Certain Plants. Ph.D. Thesis, Mansoura University, Mansoura, Egypt, 1981. [Google Scholar]
- Pirie, N.W. Proteins. In Modern Methods of Plant Analysis IV; Paech, K., Tracey, M.B., Eds.; Springer: Berlin, Germany, 1955; pp. 23–68. [Google Scholar]
- Rees, M.W.; Williams, E.F. The total nitrogen content of egg albumin and other proteins. J. Biochem. 1943, 37, 354–359. [Google Scholar]
- Haroun, S.A. Studies on Adaptation of Plants to Water Stress. Ph.D. Thesis, Mansoura University, Mansoura, Egypt, 1985. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitative of microorgan quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–251. [Google Scholar] [CrossRef]
- Adam, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 1995; ISBN 0-931710-42-1. [Google Scholar]
- Khalil, S.E.; Ismael, E.G. Growth, yield and seed quality of Lupinus termis as affected by different soil moisture levels and different ways of yeast application. Am. J. Sci. 2010, 6, 141–153. [Google Scholar]
- Anwer, M.D.A.; Khan, M.R. Aspergillus niger as tomato fruit (Lycopersicum esculentum Mill.) quality enhancer and plant health promoter. J. Postharvest Technol. 2013, 1, 36–51. [Google Scholar]
- Sen, B. Biocontrol: A success story. Indian Phytopathol. 2000, 53, 243–249. [Google Scholar]
- El-Shahawy, T.A.; El-Rokiek, K.G.; Balbaa, L.K.; Abbas, S.M. Micronutrients, B-vitamins and yeast in relation to increasing flax (Linum usitatissimum L.) growth, yield productivity and controlling associated weeds. Asian Res. J. Agric. 2008, 2, 1–14. [Google Scholar] [CrossRef]
- Mahfouz, S.A.; Sharaf-Eldin, M.A. Effect of mineral vs. biofertilizer on growth, yield, and essential oil content of fennel (Foeniculum vulgare Mill.). Int. Agrophys. 2007, 21, 361–366. [Google Scholar]
- Hayat, A.E.H. Physiological Studies on Hibiscus sabdariffa L. Production in New Reclamated Soils. Master’s Thesis, Zagazig University, Zagazig, Egypt, 2007. [Google Scholar]
- Mondal, M.M.A.; Puteh, A.B.; Ismail, M.R.; Rafii, M.Y. Optimizing plant spacing for modern rice varieties. Int. J. Agric. Biol. 2013, 15, 175–178. [Google Scholar]
- Gomaa, A.O.; Abou-Aly, H.E. Efficiency of bioferitlization in the presence of both inorganic and organic fertilizers on growth, yield and chemical constituents of anise plant Pimpinellaanisum L. In Proceedings of the 5th Arabian Horticulture Conference, Ismailia, Egypt, 24–28 March 2001. [Google Scholar]
- El-Sayed, A.A.; El-Leithy, A.S.; Bazraa, W.M.; Abdel-Latef, M.S. Effect of growing media, bio and organic fertilization on the flowering and chemical constituents of Calendula officinalis L. plants. Biosci. Res. 2018, 15, 2029–2040. [Google Scholar]
- Poschenrieder, F.J.C. Plant water relations as affected by heavy metal stress: A review. J. Plant Nutr. 1990, 13, 1–37. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: San Diego, CA, USA, 1995; p. 889. [Google Scholar]
- Nafie, E.M. The possible induction of resistance in Lupinus termis L. against Fusarium oxysporum by Streptomyces chibaensis and its mode of action: I. changes in certain morphological criteria and biochemical composition related to induced resistance. Int. J. Agric. Biol. 2003, 5, 463–472. [Google Scholar]
- Naghdi, A.A.; Piri, S.; Khaligi, A.; Moradi, P. Enhancing the qualitative and quantitative traits of potato by biological, organic and chemical fertilizers. J. Saudi Soc. Agric. Sci. 2022, 21, 87–92. [Google Scholar] [CrossRef]
- Wichrowska, D.; Szczepanek, M. Possibility of limiting mineral fertilization in potato cultivation by using bio-fertilizer and its influence on protein content in potato tubers. Agriculture 2020, 10, 442. [Google Scholar] [CrossRef]
- Abdeldaym, E.A.; El-Sawy, M.; El-Helaly, M. Combined application of different sources of nitrogen fertilizers for improvement of potato yield and quality. Plant Arch. 2019, 19, 2513–2521. [Google Scholar]
- Kim, A.J.; Choi, J.N.; Kim, J.; Park, S.B.; Yeo, S.H.; Choi, J.H.; Lee, H. GC-MS Based Metabolite Profiling of Rice Koji Fermentation by Various Fungi. Biosci. Biotechnol. Biochem. 2010, 74, 2267–2272. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Murugesan, S.; Babu, D.S.; Rajeshkannan, C. GC-MS analysis of the extract of endophytic fungus, Phomopsis sp. isolated from tropical tree species of India, Tectonagrandis L. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 10176–10179. [Google Scholar]
- Devi, N.N.; Prabakaran, J.J. Bioactive metabolites from an endophytic fungus Penicillium sp. isolated from Centellaasiatica. Environ. Appl. Mycol. 2014, 4, 34–43. [Google Scholar]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.; Kim, Y.; Lee, I. Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, S.; Cheong, B.E.; Taslima, K.; Kausar, H.; Hasan, M.M. Separation and Identification of Volatile Compounds from Liquid Cultures of Trichodermaharzianum by GC-MS using Three Different Capillary Columns. J. Chromatogr. Sci. 2011, 2, 358–367. [Google Scholar]
- Hosny, M.; Dhar, K.; Rosazza, J.P.N. Hydroxylations and methylations of quercetin, fisetin, and628 catechin by Streptomyces griseus. J. Nat. Prod. 2011, 64, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.D.; Pimenta, F.C.; Luz, W.C.; Oliveira, V.D. Selection of filamentous fungi of the Beauveria genus able to metabolize quercetin like mammalian cells. Braz. J. Microbiol. 2008, 39, 405–408. [Google Scholar] [CrossRef]
- Hegazy, M.F.; Mohamed, T.A.; ElShamy, A.I.; Mohamed, A.H.; Mahalel, U.A.; Reda, E.H.; Shaheen, A.M.; Tawfik, W.A.; Shahat, A.A.K.; Shams, A.; et al. Microbial biotransformation as a tool for drug development based on natural products from mevalonic acid pathway. A review. J. Adv. Res. 2015, 6, 17–33. [Google Scholar] [CrossRef]
- Velankar, H.R.; Heble, M.R. Biotransformation of (L)-citronellal to (L)-citronellol by free and immobilized Rhodotorula minuta. Electron. J. Biotechnol. 2003, 6, 90–102. [Google Scholar] [CrossRef]
- Alshammari, S.O.; Abd El Aty, A.A.; Hegazy, M.E.F. New sesquiterpene lactone via fungal transformation of Rhizopus oryzaeKX685359: Antimicrobial In-Vitro and In-Silico Study. Catal. Lett. 2022. [Google Scholar] [CrossRef]



| Compound | Reference | Compound | Reference |
|---|---|---|---|
| Acetylbetacarboline | Hemamalini et al. [8] | Myristicacid | Myristicacid |
| Arachidonicacid | Bai et al. [21] | Neophytadiene | Neophytadiene |
| Apigenin | Malviya et al. [22] | Nonane,5-(2-methylpropyl)- | Nonane,5-(2-methylpropyl)- |
| Catechin | Malviya et al. [22] | Octadecane,3-ethyl-5-(2-ethylbutyl)- | Octadecane,3-ethyl-5-(2-ethylbutyl)- |
| Cedrane-8,13-diol | Bai et al. [21] | Oxirane,hexadecyl- | Oxirane,hexadecyl- |
| Cinnamicacid, 3-hydroxy-4-methoxy | Bai et al. [21] | Palmiticacid | Palmiticacid |
| Cystine | Malviya et al. [22] | Palmitoylchloride | Palmitoylchloride |
| Cyanidin | Malviya et al. [22] | Pelargonaldehyde | Pelargonaldehyde |
| Decane,3,7-dimethyl- | Bai et al. [21] | Pentadecane | Pentadecane |
| Decylsulfide | Bai et al. [21] | Phthalicacid | Phthalicacid |
| Dihydrocitronellol | Bai et al. [21] | Phthalicacid,butyloctylester | Phthalicacid,butyloctylester |
| Dipalmitin | Bai et al. [21] | Stearicacid | Stearicacid |
| Dotriacontane | Bai et al. [21] | Stearicacidethylester | Stearicacidethylester |
| Eicosane | Bai et al. [21] | 3-picoline-2-nitro | 3-picoline-2-nitro |
| Fumaricacid, ethyl2-methylallylester | Bai et al. [21] | Pyrocatechol | Pyrocatechol |
| Gallicacid | Malviya et al. [22] | Propionicacid-2-chloro,ethylester | Propionicacid-2-chloro,ethylester |
| D-Glucoronicacid | Hemamalini et al. [23] | Tetracosane | Tetracosane |
| Heptadecane | Bai et al. [21] | Tetrapentacontane | Tetrapentacontane |
| Hexadecane | Bai et al. [21] | Transdecalone | Transdecalone |
| Hexatriacontane | Bai et al. [21] | Threonine | Threonine |
| Hydroxycitronellal | Hemamalini et al. [8] | Tryptophan | Tryptophan |
| IsopropylPalmitate | Bai et al. [21] | Undecane | Undecane |
| Kaempfrol | Malviya et al. [22] | 1,11-Hexadecadiyne | 1,11-Hexadecadiyne |
| Lavandulylacetate | Hemamalini et al. [23] | 1-Chlorohexadecane | 1-Chlorohexadecane |
| Lariciresinol | Bai et al. [21] | 2-Methylresorcinol,acetate | 2-Methylresorcinol,acetate |
| Linolenicacid | Bai et al. [21] | 3,4,7-trimethylquercetin | 3,4,7-trimethylquercetin |
| Linolenicacid,methylester | Bai et al. [21] | 3′,5′-Dimethoxyacetophenone | 3′,5′-Dimethoxyacetophenone |
| Lysine | Malviya et al. [22] | 6-dimethylamine-saccharin | 6-dimethylamine-saccharin |
| Megastigmatrienone | Bai et al. [21] | δ-5-Avenasterol | δ-5-Avenasterol |
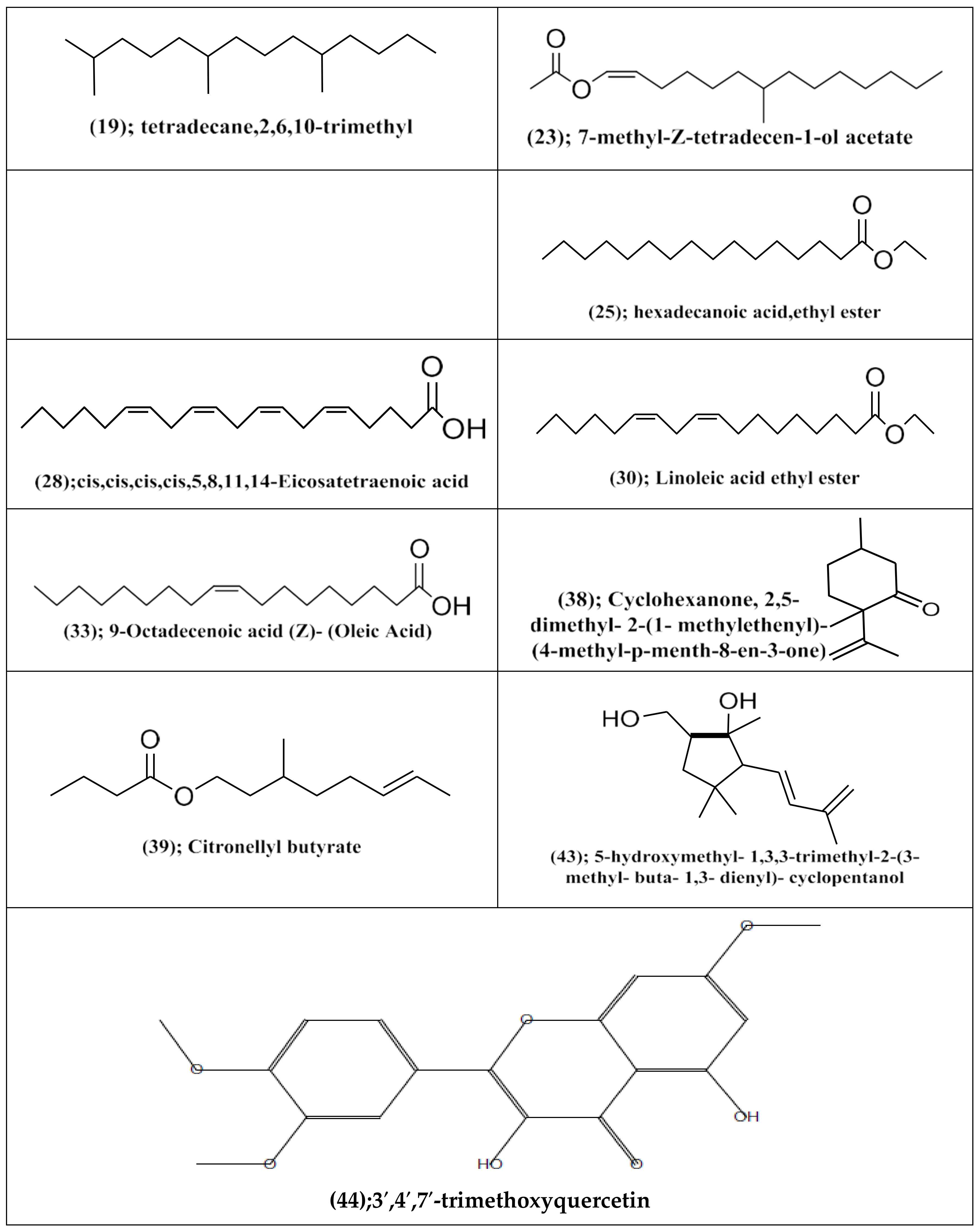
| Rt (min) | Compound No. | M.F | Area (%) | MS Data: m/z [Identity] (Rel. Int. %) |
|---|---|---|---|---|
| 24.82 | (38) | C11H18O | 4.29 | 166[M]+(57),151[M-CH3]+(6),123[151- C2H4]+(72),109(50),95[123-C2H4]+ (70),81(100),67[95-CO]+(81),53(45). |
| 24.88 | (39) | C14H26O2 | 6.31 | 166[M+-C2H4O]+(21),151[166- CH3]+(7),123[151-C2H4]+(51),81[123- CH3]+(100),67(77),55(45). |
| 33.62 | (43) | C14H24O2 | 0.90 | 224[M]+(4),181[M-C3H7]+(3), 163[181- H2O]+(17),125(23),96(27),83(52), 67(36),55(79),43(100). |
| 34.57 | (23) | C17H32O2 | 0.96 | 253[M-CH3]+(7),226[253-C2H3]+(5), 181[226-C2H5O]+(6),162(13),135(13), 99(25),85(94),73(16),41(100). |
| 35.93 | (19) | C17H36 | 1.4 | 195(5),155(10),135(17),113(25), 71(100),57(80). |
| 39.66 | (33) | C18H34O2 | 0.83 | 268(3),228(2),215(8),180(7),163(11), 135(20),115(13),73(42),57(59), 43(100). |
| 39.98 | (24) | C17H34O2 | 7.94 | 270[M]+(3),241(2),199(1), 171(2), 143(8),101(9),87(63),74(100),55(43). |
| 41.57 | (25) | C18H36O2 | 3.58 | 284[M]+(3),241[M-C3H7]+(9),207(5), 175(35),129(8),101(100),71(33), 57(58). |
| 44.51 | (28) | C21H34O2 | 5.30 | 318[M]+(1),268(1),245(3), 207(3), 135(26),106(19),95(50),79(100), 67(93),55(73). |
| 45.82 | (30) | C20H36O2 | 2.64 | 308[M]+(2),281[M-C2H3]+(5),253[281- C2H4]+(5),209(5),179(8),150(7), 135(28),123(10),108(27),95(57), 67(100),55(82). |
| 56.37 | (44) | C16H16O7 | 0.89 | 344[M]+(1),305(1),281(9), 267(2), 253(14),221(2),207(29),191(5), 147(32),135(23),91(13),73(100), 55(14). |
| Compound No. | MS Data: m/z [Identity] (Rel. Int. %) |
|---|---|
| 2-hydroxybicyclo [2,2,1] hept-5-ene 2-carboxylic acid | 111(0.4), 81(0.5), 66(100). |
| 5-hydroxynicotinic acid | 127(0.4), 84(100), 64(8). |
| L-pipecolic acid | 84[M-C2H5O]+(100), 64(8). |
| Eicosane | 282[M]+(13), 239[M-C3H7]+(6), 224[239-CH3]+(11), 197[224-C2H3]+(11), 141(18), 127(21), 113(26), 99(36), 85(83), 71(100), 57(96). |
| 4-methyl-p-menth-8-en-3-one | 166[M]+(57), 151[M-CH3]+(6), 123[151-C2H4]+ (72), 109(50), 95[123-C2H4]+ (70), 81(100), 67 [95-CO]+ (81), 53(45). |
| citronellyl butyrate | 166[M+-C2H4O]+(21), 151[166-CH3]+(7), 123[151-C2H4]+(51), 81[123-CH3]+(100), 67(77), 55(45). |
| Docosane | 310[M]+(18), 253[M-C4H9]+(11), 220(41), 197(13), 175(23), 135(21), 135(21), 127(25), 99(40), 85(99), 71(100), 57(88). |
| β-oplopenone | 205[M-CH3]+(18), 177[205-C2H4]+ (100), 149[177-C2H4]+ (65),135(76), 121 [149-CO]+ (36), 105(41), 91[121-CH2O]+(63), 77(45), 67(65), 57(50). |
| Daucol | 238[M]+(1), 221[M-OH]+(10), 193[221-C2H4]+ (10), 165[193-C2H4]+ (100), 137 [165-CO]+ (34), 111(23), 81(16), 57(79). |
| aromadendrene oxide | 220[M]+(15), 177[M-C3H7]+ (17), 145[177-CH4O]+(100), 133(42), 77(50), 57(58). |
| 5-hydroxymethyl-1,3,3-trimethyl-2-(3-methylbuta-1,3-dienyl)-cyclopentanol | 224[M]+(4), 181[M-C3H7]+ (3), 163[181-H2O]+ (17), 125(23), 96(27), 83(52), 67(36), 55(79), 43(100). |
| 7-methyl-Z-tetradecen-1-ol acetate | 253[M-CH3]+(7), 226[253-C2H3]+(5), 181[226-C2H5O]+(6), 162(13), 135(13), 99(25), 85(94), 73(16), 41(100). |
| 2,6,10-trimethyl-tetradecane | 195(5), 155(10), 135(17), 113(25), 71(100), 57(80). |
| (Z)-9-octadecenoic acid (oleic Acid) | 268(3), 228(2), 215(8), 180(7), 163(11), 135(20), 115(13), 73(42), 57(59), 43(100). |
| 14-methylpentadecanoic acidmethyl ester | 270[M]+(3), 241(2), 199(1), 171(2), 143(8), 101(9), 87(63), 74(100), 55(43). |
| 1-eicosanol | 280[M-H2O]+ (5), 252[280-C2H4]+(4), 207(4), 168(7), 148(19), 125(27), 111(72), 83(92), 69(76), 56(96), 43(100). |
| 2-methyl heneicosane | 281(7), 260(2), 207(4), 147(7), 125(19), 111(37), 84(25), 71(56), 55(79), 43(100). |
| hexadecanoic acid, ethyl ester | 284[M]+(3), 241[M-C3H7]+ (9), 207(5), 175(35), 129(8), 101(100), 71(33), 57(58). |
| (Z,Z)-9,12-octadecadienoic acid methyl ester | 294[M]+(4), 262[M-CH4O]+ (4), 220 [262-C3H6]+ (2), 150(5), 123(10), 110(15), 95(34), 81(86), 55(100). |
| 5,8,11,14-eicosatetraenoic acid | 318[M]+(1), 268(1), 245(3), 207(3), 135(26), 106(19), 95(50), 79(100), 67(93), 55(73). |
| octadecanoic acid methyl ester (Methyl stearate) | 298[M]+(26), 280[M-H2O]+ (5), 221 [280-C2H3O2]+(9), 199(19), 185(9), 147 (21),121(7),97(23),87(100),73(80),55(77) |
| 3-ethyl-5-(2-ethylbutyl)-octadecane | 329(2), 280(9), 253(8), 224(4), 197(8), 155(8), 141(21), 113(7), 97(40), 85(71), 57(89), 43(100). |
| ethyl oleate | 310[M]+(8), 283[M-C2H3]+(3), 265[283-H2O]+ (10), 222[265-C3H7]+ (13), 193(3), 149(25), 124(26), 111(33), 96(58), 85(52), 67(100). |
| linoleic acid ethyl ester | 308[M]+(2), 281[M-C2H3]+(5), 253[281-C2H4]+(5), 209(5), 179(8), 150(7), 135(28),123(10), 108(27), 95(57), 67(100), 55(82). |
| 1-heneicosyl formate | 309[M-CH3O]+ (1), 280(2), 207(2), 167(3), 167(3), 139(10), 125(29), 112(15), 98(21), 83(84), 71(52). 57(100). |
| octadecanoic acid, ethyl ester | 312[M]+(10), 282[M-CH2O]+ (2), 239[282-C3H7]+ (3), 213(4), 185(6), 157(16), 141(6), 113(14), 97(27), 84(9), 71(43), 57(51), 43(100). |
| 1,3,5-trimethyl-2-cyclohexane, octadecylcyclohexane | 376[M]+(2), 327(5), 282(5), 252(7), 225(4), 207(18), 171(18), 147(15), 112(18), 97(42), 69(28), 57(100). |
| 3′,4′,7-trimethoxyquercetin | 344[M]+(1), 305(1), 281(9), 267(2), 253(14), 221(2), 207(29), 191(5), 147(32), 135(23), 91(13), 73(100), 55(14). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamel, R.M.E.; Haroun, S.A.; Alkhateeb, O.A.; Soliman, E.A.; Tanash, A.B.; Sherief, A.-D.A.; Abdel-Mogib, M.; Abdou, A.H.; Ali, H.S.A.M.; Al-Harbi, N.A.; et al. Role of Biotransformation of Acacia nilotica Metabolites by Aspergillus subolivaceus in Boosting Lupinus termis Yield: A Promising Approach to Sustainable Agriculture. Sustainability 2023, 15, 9509. https://doi.org/10.3390/su15129509
Gamel RME, Haroun SA, Alkhateeb OA, Soliman EA, Tanash AB, Sherief A-DA, Abdel-Mogib M, Abdou AH, Ali HSAM, Al-Harbi NA, et al. Role of Biotransformation of Acacia nilotica Metabolites by Aspergillus subolivaceus in Boosting Lupinus termis Yield: A Promising Approach to Sustainable Agriculture. Sustainability. 2023; 15(12):9509. https://doi.org/10.3390/su15129509
Chicago/Turabian StyleGamel, Rasha M. E., Samia A. Haroun, Omar Abdullah Alkhateeb, Eman A. Soliman, Arafat B. Tanash, Abdel-Dayem A. Sherief, Mamdoh Abdel-Mogib, Ahmed Hassan Abdou, Howayda Said Ahmed Mohamed Ali, Nadi Awad Al-Harbi, and et al. 2023. "Role of Biotransformation of Acacia nilotica Metabolites by Aspergillus subolivaceus in Boosting Lupinus termis Yield: A Promising Approach to Sustainable Agriculture" Sustainability 15, no. 12: 9509. https://doi.org/10.3390/su15129509
APA StyleGamel, R. M. E., Haroun, S. A., Alkhateeb, O. A., Soliman, E. A., Tanash, A. B., Sherief, A.-D. A., Abdel-Mogib, M., Abdou, A. H., Ali, H. S. A. M., Al-Harbi, N. A., Abdelaal, K., & Kazamel, A. M. (2023). Role of Biotransformation of Acacia nilotica Metabolites by Aspergillus subolivaceus in Boosting Lupinus termis Yield: A Promising Approach to Sustainable Agriculture. Sustainability, 15(12), 9509. https://doi.org/10.3390/su15129509










