Phytohormones Promote the Growth, Pigment Biosynthesis and Productivity of Green Gram [Vigna radiata (L.) R. Wilczek]
Abstract
:1. Introduction
2. Materials and Methods
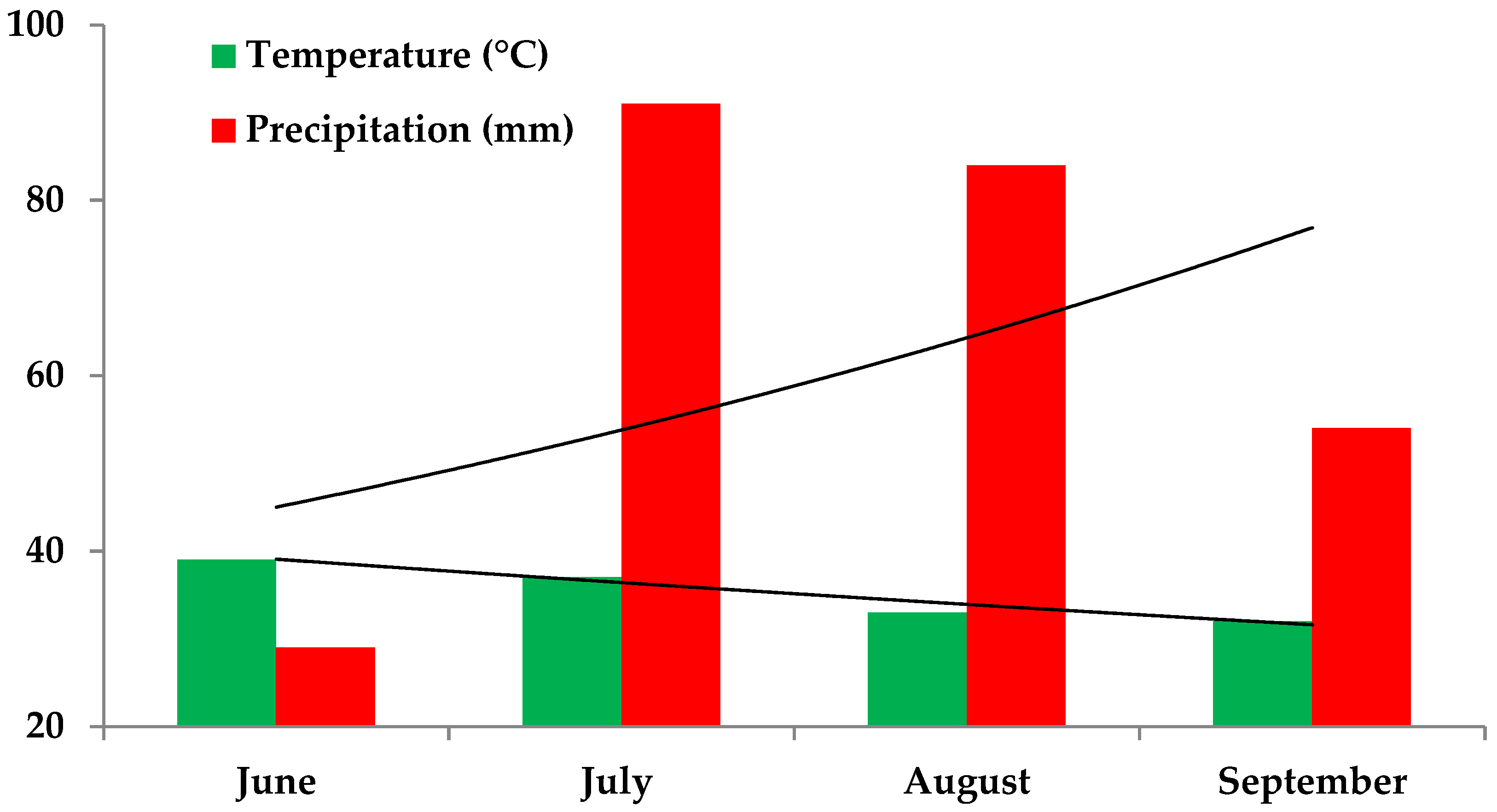
2.1. Experimental Site’s Meteorological Features and Physicochemical Description
2.2. Details of Treatments and Experiment’s Execution
2.3. Response Variable Recordings
Plot area (m2)
2.4. Statistical Analyses
3. Results
3.1. Crop Growth and Net Assimilation Rates
3.2. Yield Attributes
3.3. Grain and Biological Yields and Harvest Index
3.4. Chlorophyll a, Chlorophyll b, Total Chlorophyll, Carotenoid and Protein Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CGR | crop growth rate |
| NAR | net assimilation rate |
| PH | plant height |
| PBs | pod-bearing branches |
| NP | number of pods |
| PL | pod length |
| SP | seeds per pod |
| GY | grain yield |
| BY | biological yield |
| SA | salicylic acid |
| GA | gibberellic acid |
| P | Protein |
References
- Mahmood, S.; Daur, I.; Yasir, M.; Waqas, M.; Hirt, H. Synergistic Practicing of Rhizobacteria and Silicon Improve Salt Tolerance: Implications from Boosted Oxidative Metabolism, Nutrient Uptake, Growth and Grain Yield in Mung Bean. Plants 2022, 11, 1980. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Chattha, M.U.; Khan, I.; Mahmood, A.; Hassan, M.U.; Al-Huqail, A.A.; Salem, M.Z.; Ali, H.M.; Hano, C.; El-Esawi, M.A. Exogenously applied trehalose augments cadmium stress tolerance and yield of mung bean (Vigna radiata L.) grown in soil and hydroponic systems through reducing cd uptake and enhancing photosynthetic efficiency and antioxidant defense systems. Plants 2022, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A. Physiological aspects of mungbean plant (Vigna radiata L. Wilczek) in response to salt stress and gibberellic acid treatment. Res. J. Agr. Biol. Sci. 2007, 3, 200–213. [Google Scholar]
- Zhao, T.; Meng, X.; Chen, C.; Wang, L.; Cheng, X.; Xue, W. Agronomic Traits, Fresh Food Processing Characteristics and Sensory Quality of 26 Mung Bean (Vigna radiata L.) Cultivars (Fabaceae) in China. Foods 2022, 11, 1687. [Google Scholar] [CrossRef]
- Abdallah, Y.; Hussien, M.; Omar, M.O.; Elashmony, R.M.; Alkhalifah, D.H.M.; Hozzein, W.N. Mung bean (Vigna radiata) treated with magnesium nanoparticles and its impact on soilborne Fusarium solani and Fusarium oxysporum in clay soil. Plants 2022, 11, 1514. [Google Scholar] [CrossRef]
- Nair, R.M.; Yang, R.Y.; Easdown, W.J.; Thavarajah, D.; Thavarajah, P.; Hughes, J.d.A.; Keatinge, J. Biofortification of mungbean (Vigna radiata) as a whole food to enhance human health. J. Sci. Food Agric. 2013, 93, 1805–1813. [Google Scholar] [CrossRef]
- Pataczek, L.; Zahir, Z.A.; Ahmad, M.; Rani, S.; Nair, R.; Schafleitner, R.; Cadisch, G.; Hilger, T. Beans with benefits—The role of Mungbean (Vigna radiate) in a changing environment. Am. J. Plant Sci. 2018, 9, 1577. [Google Scholar] [CrossRef] [Green Version]
- Kumara, P.; Pakeerathan, K.; Deepani, L.P. Assessment of Yield Loss in Green Gram (Vigna radiata (L.) R. Wilczek) Cultivation and Estimation of Weed-Free Period for Eco-Friendly Weed Management. Biol. Life Sci. Forum 2021, 3, 22. [Google Scholar]
- Kaur, G.; Brar, H.; Singh, G. Effect of weed management on weeds, nutrient uptake, nodulation, growth and yield of summer mungbean (Vigna radiata). Indian J. 2010, 42, 114–119. [Google Scholar]
- Wang, L.; Wang, S.; Luo, G.; Zhang, J.; Chen, Y.; Chen, H.; Cheng, X. Evaluation of the production potential of mung bean cultivar “Zhonglv 5”. Agronomy 2022, 12, 707. [Google Scholar] [CrossRef]
- Ghotbi, V.; Mahrokh, A.; Tehrani, A.M.; Asadi, H. Evaluation of Forage Yield and Quality of Cowpea, Guar, and Mung Bean under Drought Stress Conditions. Chem. Proc. 2022, 10, 62. [Google Scholar]
- Azooz, M.M.; Youssef, A.M.; Ahmad, P. Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Int. J. Plant Physiol. Biochem. 2011, 3, 253–264. [Google Scholar]
- Sharhrtash, M.; Mohsenzadeh, S.; Mohabatkar, H. Salicylic acid alleviates paraquat oxidative damage in maize seedling. Asian J. Exp. Biol. Sci 2011, 2, 377–382. [Google Scholar]
- Horváth, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Pan, Q.; Zhan, J.; Liu, H.; Zhang, J.; Chen, J.; Wen, P.; Huang, W. Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Sci. 2006, 171, 226–233. [Google Scholar] [CrossRef]
- Naz, S.; Bilal, A.; Saddiq, B.; Ejaz, S.; Ali, S.; Ain Haider, S.T.; Sardar, H.; Nasir, B.; Ahmad, I.; Tiwari, R.K. Foliar Application of Salicylic Acid Improved Growth, Yield, Quality and Photosynthesis of Pea (Pisum sativum L.) by Improving Antioxidant Defense Mechanism under Saline Conditions. Sustainability 2022, 14, 14180. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mohamed, H.I.; Mahmoud, A.; Elkelish, A.; Misra, A.N.; Guy, K.M.; Kamran, M.; Ai, S.; Zhang, M. Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 2020, 9, 724. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [Green Version]
- Shamili, M.; Esfandiari Ghalati, R.; Samari, F. The impact of foliar salicylic acid in salt-exposed guava (Psidium Guajava L.) seedlings. Int. J. Fruit Sci. 2021, 21, 323–333. [Google Scholar] [CrossRef]
- Salehi, S.; Khajehzadeh, A.; Khorsandi, F. Growth of tomato as affected by foliar application of salicylic acid and salinity. Am.-Eurasian J. Agric. Environ. Sci. 2011, 11, 564–567. [Google Scholar]
- Chen, N.; Hu, M.; Yang, S. The effects of inducing treatments on phenolic metabolism of melon leaves. Acta Hortic. Sin. 2010, 37, 1759–1766. [Google Scholar]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J. Soil Sci. Plant Nutr. 2016, 16, 662–676. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Pandey, N.; Pandey-Rai, S. Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasma 2018, 255, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The role of salicylic acid in plants exposed to heavy metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [Green Version]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Khalil, N.; Elhady, S.S.; Diri, R.M.; Fekry, M.I.; Bishr, M.; Salama, O.; El-Zalabani, S.M. Salicylic Acid Spraying Affects Secondary Metabolites and Radical Scavenging Capacity of Drought-Stressed Eriocephalus africanus L. Agronomy 2022, 12, 2278. [Google Scholar] [CrossRef]
- Knörzer, O.C.; Lederer, B.; Durner, J.; Böger, P. Antioxidative defense activation in soybean cells. Physiol. Plant. 1999, 107, 294–302. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, A.; Alyemeni, M.N. Salicylic Acid: Plant Growth and Development; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Mardani, H.; Bayat, H.; Saeidnejad, A.H.; Rezaie, E.E. Assessment of salicylic acid impacts on seedling characteristic of cucumber (Cucumis sativus L.) under water stress. Not. Sci. Biol. 2012, 4, 112–115. [Google Scholar] [CrossRef] [Green Version]
- Bayat, H.; Alirezaie, M.; Neamati, H. Impact of exogenous salicylic acid on growth and ornamental characteristics of calendula (Calendula officinalis L.) under salinity stress. J. Stress Physiol. Biochem. 2012, 8, 258–267. [Google Scholar]
- Wang, K.; Shen, Y.; Wang, H.; He, S.; Kim, W.S.; Shang, W.; Wang, Z.; Shi, L. Effects of exogenous salicylic acid (SA), 6-benzylaminopurine (6-BA), or abscisic acid (ABA) on the physiology of Rosa hybrida ‘Carolla’under high-temperature stress. Horticulturae 2022, 8, 851. [Google Scholar] [CrossRef]
- Kaur, P.; Gupta, R.; Dey, A.; Malik, T.; Pandey, D.K. Optimization of salicylic acid and chitosan treatment for bitter secoiridoid and xanthone glycosides production in shoot cultures of Swertia paniculata using response surface methodology and artificial neural network. BMC Plant Biol. 2020, 20, 225. [Google Scholar] [CrossRef]
- Gorni, P.H.; Pacheco, A.C. Growth promotion and elicitor activity of salicylic acid in Achillea millefolium L. Afric. J. Biotechnol. 2016, 15, 657–665. [Google Scholar]
- Pacheco, A.C.; Gorni, P.H. Elicitation with Salicylic Acid as a Tool for Enhance Bioactive Compounds in Plants. In Salicylic Acid-A Versatile Plant Growth Regulatori; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–15. [Google Scholar]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [Green Version]
- Nawalagatti, C.; Ashwini, G.; Doddamani, M.; Chetti, M.; Hiremath, S. Influence of organics, nutrients and plant growth regulators on growth, yield and yield components in french bean. Int. J. Plant Sci. 2009, 4, 367–372. [Google Scholar]
- Rafique, M.; Naveed, M.; Mustafa, A.; Akhtar, S.; Munawar, M.; Kaukab, S.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z. The combined effects of gibberellic acid and rhizobium on growth, yield and nutritional status in chickpea (Cicer arietinum L.). Agronomy 2021, 11, 105. [Google Scholar] [CrossRef]
- Marciniak, K.; Przedniczek, K. Gibberellin Signaling Repressor LlDELLA1 Controls the flower and pod development of yellow lupine (Lupinus luteus L.). Int. J. Mol. Sci. 2020, 21, 1815. [Google Scholar] [CrossRef] [Green Version]
- Wilmowicz, E.; Frankowski, K.; Glazińska, P.; Sidłowska, M.; Marciniak, K.; Kopcewicz, J. The role of gibberellins in the regulation of flowering in plants. Kosmos 2011, 60, 129–140. [Google Scholar]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magome, H.; Yamaguchi, S.; Hanada, A.; Kamiya, Y.; Oda, K. Dwarf and delayed-flowering 1, a novel Arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J. 2004, 37, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, K.; Kućko, A.; Wilmowicz, E.; Świdziński, M.; Kęsy, J.; Kopcewicz, J. Photoperiodic flower induction in Ipomoea nil is accompanied by decreasing content of gibberellins. Plant Growth Regul. 2018, 84, 395–400. [Google Scholar] [CrossRef] [Green Version]
- De Jong, M.; Mariani, C.; Vriezen, W.H. The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 2009, 60, 1523–1532. [Google Scholar] [CrossRef] [Green Version]
- Gubler, F.; Chandler, P.M.; White, R.G.; Llewellyn, D.J.; Jacobsen, J.V. Gibberellin signaling in barley aleurone cells. Control of SLN1 and GAMYB expression. Plant Physiol. 2002, 129, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Rahman, M.M.; Mohi-Ud-Din, M.; Akter, M.; Zaman, E.; Keya, S.S.; Hasan, M.; Hasanuzzaman, M. Cytokinin and gibberellic acid-mediated waterlogging tolerance of mungbean (Vigna radiata L. Wilczek). PeerJ 2022, 10, e12862. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, G.; Chen, Y.; Gao, J.; Sun, Y.-R.; Sun, M.-F.; Chen, J.-P. Exogenous application of gibberellic acid and ascorbic acid improved tolerance of okra seedlings to NaCl stress. Acta Physiol. Plant. 2019, 41, 93. [Google Scholar] [CrossRef]
- Tasnim, S.; Alam, M.J.; Rahman, M.M.; Islam, M.S.; Sikdar, M.S.I. Response of mungbean growth and yield to GA3 rate and time of application. Asian J. Crop Soil Sci. Plant Nutr. 2019, 1, 28–36. [Google Scholar]
- Parmar, V.; Dudhatra, M.; Thesiya, N. Effect of growth regulators on yield of summer greengram. Legume Res. Int. J. 2011, 34, 65–67. [Google Scholar]
- Sadiq, R.; Maqbool, N.; Hussain, M.; Tehseen, S.; Naseer, M.; Rafique, T.; Zikrea, A.; Naqve, M.; Mahmood, A.; Javaid, A. Boosting antioxidant defense mechanism of mungbean with foliar application of gibberellic acid to alleviate cadmium toxicity. Plant Physiol. Rep. 2021, 26, 741–748. [Google Scholar] [CrossRef]
- Chakrabarti, N.; Mukherji, S. Effect of phytohormone pretreatment on metabolic changes in Vigna radiata under salt stress. J. Environ. Biol. 2002, 23, 295–300. [Google Scholar]
- Abu-Zahra, T.R. Berry size of Thompson seedless as influenced by the application of gibberellic acid and cane girdling. Pak. J. Bot. 2010, 42, 1755–1760. [Google Scholar]
- Sajid, G.M.; Kaukab, M.; Ahmad, Z. Foliar application of plant growth regulators (PGRs) and nutrients for improvement of lily flowers. Pak. J. Bot. 2009, 41, 233–237. [Google Scholar]
- Copur, O.; Demirel, U.; Karakuş, M. Effects of several plant growth regulators on the yield and fiber quality of cotton (Gossypium hirsutum L.). Not. Bot. Hort. Agro. Cluj-Nap. 2010, 38, 104–110. [Google Scholar]
- Bora, R.; Sarma, C. Effect of gibberellic acid and cycocel on growth, yield and protein content of pea. Asian J. Plant Sci. 2006, 5, 224–230. [Google Scholar]
- Chauhan, J.; Tomar, Y.; Singh, N.I.; Ali, S.; Debarati, A. Effect of growth hormones on seed germination and seedling growth of black gram and horse gram. J. Am. Sci. 2009, 5, 79–84. [Google Scholar]
- Maske, V.; Dotale, R.; Sorte, P.; Tale, B.; Chore, C. Germination, root and shoot studies in soybean as influenced by GA3 and NAA. J. Soil Crops 1997, 7, 147–149. [Google Scholar]
- Aldesuquy, H.; Gaber, A. Effect of growth regulators on Vicia faba plants irrigated by sea water. Leaf area, pigment content and photosynthetic activity. Biol. Plant. 1993, 35, 519–527. [Google Scholar] [CrossRef]
- Aldesuquy, H.; Ibrahim, A. Interactive effect of seawater and growth bioregulators on water relations, abscisic acid concentration and yield of wheat plants. J. Agron. Crop Sci. 2001, 187, 185–193. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, D.-Q. Stimulation effect of gibberellic acid short-term treatment on leaf photosynthesis related to the increase in Rubisco content in broad bean and soybean. Photosynth. Res. 2001, 68, 39–47. [Google Scholar] [CrossRef]
- Salisbury, F.B.; Ross, C.W. Plant Physiology, 6th ed.; Wadsworth: Belmont, CA, USA, 1996. [Google Scholar]
- Taiz, L.; Zeiger, E. Gibberellins: Regulators of plant height and seed germination. In Plant Physiology, Sinauer Associates, Sunderland; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; pp. 583–619. [Google Scholar]
- Black, C. Methods of Soil Analysis Part 2, Amer; Society of Agronomy Inc.: Madisson, WI, USA, 1965; pp. 1372–1376. [Google Scholar]
- Sparks, D.; Page, A.; Helmke, P.; Leoppert, R.; Soltanpour, P.; Tabatabai, M.; Johnston, C.; Sumner, M. Methods of Soil Analysis; Soil Science Society of America Series; American Society of Agronomy, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Naresh, R.; Tomar, S.; Kumar, D.; Singh, S.; Dwivedi, A.; Kumar, V. Experiences with rice grown on permanent raised beds: Effect of crop establishment techniques on water use, productivity, profitability and soil physical properties. Rice Sci. 2014, 21, 170–180. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 2003. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Press Adelaide: Adelaide, Australia, 1950. [Google Scholar]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual; ICARDA: Beirut, Lebanon, 2001. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef] [Green Version]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a determination: Improvements in methodology. Oikos 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Yadava, U.L. A rapid and nondestructive method to determine chlorophyll in intact leaves. HortScience 1986, 21, 1449–1450. [Google Scholar] [CrossRef]
- Steel, R.; Torrie, J.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill Kogakusha, Ltd.: New York, NY, USA, 1997. [Google Scholar]
- Huang, C.; Liao, J.; Huang, W.; Qin, N. Salicylic Acid Protects Sweet Potato Seedlings from Drought Stress by Mediating Abscisic Acid-Related Gene Expression and Enhancing the Antioxidant Defense System. Int. J. Mol. Sci. 2022, 23, 14819. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Schubert, B.; Pieper, K.; Ihmig, S.; Hilgenberg, W. Glucosinolate content in susceptible and resistant Chinese cabbage varieties during development of clubroot disease. Phytochemistry 1997, 44, 407–414. [Google Scholar] [CrossRef]
- Kundu, S.; Chakraborty, D.; Pal, A. Proteomic analysis of salicylic acid induced resistance to Mungbean Yellow Mosaic India Virus in Vigna mungo. J. Proteom. 2011, 74, 337–349. [Google Scholar] [CrossRef]
- Hoque, M.M.; Haque, M.S. Effects of gibberellic acid (GA) on physiological contributing characters of mungbean (Vigna radiata). Pak. J. Biol. Sci 2002, 5, 401–403. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Hasan, M.; Akter, N.; Akhtar, S. Cytokinin and gibberellic acid alleviate the effect of waterlogging in mungbean (Vigna radiata L. wilczek). J. Clean WAS 2021, 5, 21–26. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Du, T.; Shu, Y.; Tan, F.; Wang, J. Effects of Salicylic Acid Concentration and Post-Treatment Time on the Direct and Systemic Chemical Defense Responses in Maize (Zea mays L.) Following Exogenous Foliar Application. Molecules 2022, 27, 6917. [Google Scholar] [CrossRef]
- Abdel, C.G.; Al-Rawi, I.M.T. Response of mungbean (Vigna radiata L., Wilczek) to gibberellic acid (GA3) rates and varying irrigation frequencies. Int. J. Biosci. 2011, 1, 85–92. [Google Scholar]
- Keykha, M.; Ganjali, H.R.; Mobasser, H.R. Effect of salicylic acid and gibberellic acid on some characteristics in mungbean (Vigna radiata). Int. J. Biosci 2014, 5, 70–75. [Google Scholar]
- Islam, M.; Prodhan, A.; Islam, M.; Uddin, M. Effect of plant growth regulator (GABA) on morphological characters and yield of black gram (Vigna mungo L.). J. Agric. Res 2010, 48, 76–77. [Google Scholar]
- Iqbal, H.; Tahir, A.; Khalid, M.; Haq, I.; Ahmad, A. Response of chickpea growth towards foliar application of Gibberellic acid at different growth stages. Pak. J. Biol. Sci. 2001, 4, 433–434. [Google Scholar]
- Abdelgadir, H.A.; Jäger, A.; Johnson, S.; Van Staden, J. Influence of plant growth regulators on flowering, fruiting, seed oil content, and oil quality of Jatropha curcas. S. Afr. J. Bot. 2010, 76, 440–446. [Google Scholar] [CrossRef]
- Gad, M.; Abdul-Hafeez, E.; Ibrahim, O. Foliar application of salicylic acid and gibberellic acid enhances growth and flowering of Ixora coccinea L. plants. J. Plant Prod. 2016, 7, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.S.; Sakthivel, N.; Subramanian, E.; Kalpana, R.; Janaki, P.; Rajesh, P. Influence of foliar spray of nutrients and plant growth regulators on physiological attributes and yield of finger millet (Eleusine coracana (L.) Gaertn.). Inter. J. Chem. Studies 2018, 6, 2876–2879. [Google Scholar]
- Martínez, C.; Pons, E.; Prats, G.; León, J. Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. 2004, 37, 209–217. [Google Scholar] [CrossRef]
- Ilias, I.; Ouzounidou, G.; Giannakoula, A.; Papadopoulou, P. Effects of gibberellic acid and prohexadione-calcium on growth, chlorophyll fluorescence and quality of okra plant. Biol. Plant. 2007, 51, 575–578. [Google Scholar] [CrossRef]
- Ross, J.J.; O’Neill, D.P.; Rathbone, D.A. Auxin-gibberellin interactions in pea: Integrating the old with the new. J. Plant Growth Regul. 2003, 22, 99–108. [Google Scholar] [CrossRef]
- Bano, R.; Khan, M.H.; Khan, R.S.; Rashid, H.; Swati, Z.A. Development of an efficient regeneration protocol for three genotypes of Brassica juncea. Pak. J. Bot. 2010, 42, 963–969. [Google Scholar]
- Inglese, P.; Chessa, I.; La Mantia, T.; Nieddu, G. Evolution of endogenous gibberellins at different stages of flowering in relation to return bloom of cactus pear (Opuntia ficus-indica L. Miller). Sci. Hortic. 1998, 73, 45–91. [Google Scholar] [CrossRef]
- Ancha, S.; Morgan, D.G. Growth and development of the pod wall in spring rape (Brassica napus) as related to the presence of seeds and exogenous phytohormones. J. Agric. Sci. 2009, 127, 487–500. [Google Scholar]
- Singh, A.P.; Dixit, G.; Mishra, S.; Dwivedi, S.; Tiwari, M.; Mallick, S.; Pandey, V.; Trivedi, P.K.; Chakrabarty, D.; Tripathi, R.D. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 340. [Google Scholar] [PubMed] [Green Version]
- Emongor, V. Effects of gibberellic acid on postharvest quality and vaselife life of gerbera cut flowers (Gerbera jamesonii). J. Agron. 2004, 3, 191–193. [Google Scholar] [CrossRef]
- Emongor, V. Gibberellic Acid (GA~3) Influence on Vegetative Growth, Nodulation and Yield of Cowpea (Vigna unguiculata (L.) Walp. J. Agron. 2007, 6, 509. [Google Scholar]
- El-Shraiy, A.M.; Hegazi, A.M. Effect of acetylsalicylic acid, indole-3-bytric acid and gibberellic acid on plant growth and yield of Pea (Pisum Sativum L.). Aust. J. Basic Appl. Sci. 2009, 3, 3514–3523. [Google Scholar]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar]
- Islam, M.S.; Hasan, M.K.; Islam, B.; Renu, N.A.; Hakim, M.A.; Islam, M.R.; Chowdhury, M.K.; Ueda, A.; Saneoka, H.; Ali Raza, M. Responses of water and pigments status, dry matter partitioning, seed production, and traits of yield and quality to foliar application of GA3 in Mungbean (Vigna radiata L.). Front. Agron. 2021, 2, 596850. [Google Scholar]
- Rastogi, A.; Siddiqui, A.; Mishra, B.K.; Srivastava, M.; Pandey, R.; Misra, P.; Singh, M.; Shukla, S. Effect of auxin and gibberellic acid on growth and yield components of linseed (Linum usitatissimum L.). Crop Breed. Appl. Biotechnol. 2013, 13, 136–143. [Google Scholar]
- Gupta, N.K.; Gupta, S. (Eds.) Growth regulators. In Plant Physiology; Oxford and IBH Publishing: New Delhi, India, 2005; pp. 286–349. [Google Scholar]
- Roy, R.; Nasiruddin, K. Effect of different level of GA 3 on growth and yield of cabbage. J. Environ. Sci. Nat. Resour. 2011, 4, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Basuchaudhuri, P. Influences of plant growth regulators on yield of soybean. Growth 2016, 8, 25–38. [Google Scholar]
- Abdel-Mouty, M.M.; El-Greadly, N.H. The productivity of two okra cultivars as affected by gibberilic acid, organic N, rock phosphate and feldspar applications. J. Appl. Sci. Res 2008, 4, 627–636. [Google Scholar]
- Swain, S.M.; Reid, J.B.; Kamiya, Y. Gibberellins are required for embryo growth and seed development in pea. Plant J. 1997, 12, 1329–1338. [Google Scholar] [CrossRef]
- Faizanullah, A.; Bano, A.; Nosheen, A. Role of plant growth regulators on oil yield and biodiesel production of linseed (Linum usitatissimum L.). J. Chem. Soc. Pak. 2010, 32, 568–671. [Google Scholar]
- Rahimi, M.M.; Zarei, M.A.; Arminian, A. Selection criteria of flax (Linum usitatissimum L.) for seed yield, yield components and biochemical compositions under various planting dates and nitrogen. Afric. J. Agric. Res. 2011, 6, 3167–3175. [Google Scholar]
- Ahmad, T.; Tahir, M.; Saleem, M.A.; Zafar, M.A. Response of soil application of boron to improve the growth, yield and quality of wheat (Triticum aestivum L.). J. Environ. Agric 2018, 3, 313–318. [Google Scholar]
- Deotale, R.; Mask, V.; Sorte, N.; Chimurkar, B.; Yerne, A. Effect of GA3 and IAA on morpho-physiological parameters of soybean. J. Soils Crops 1998, 8, 91–94. [Google Scholar]
- Azab, E. Seed Pre-soaking on Gibberellic Acid (GA3) Enhance Growth, Histological and Physiological Traits of Sugar Beet (Beta vulgaris L) under Water Stress. Egypt. J. Agron. 2018, 40, 119–132. [Google Scholar]
- Iftikhar, A.; Rizwan, M.; Adrees, M.; Ali, S.; ur Rehman, M.Z.; Qayyum, M.F.; Hussain, A. Effect of gibberellic acid on growth, biomass, and antioxidant defense system of wheat (Triticum aestivum L.) under cerium oxide nanoparticle stress. Environ. Sci. Pollut. Res. 2020, 27, 33809–33820. [Google Scholar] [CrossRef]
- Ullah, S.; Anwar, S.; Rehman, M.; Khan, S.; Zafar, S.; Liu, L.; Peng, D. Interactive effect of gibberellic acid and NPK fertilizer combinations on ramie yield and bast fibre quality. Sci. Rep. 2017, 7, 10647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, H.; Zeng, J.; Yan, F.; Wang, X.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zhou, Y. Effect of exogenous salicylic acid on manganese toxicity, mineral nutrients translocation and antioxidative system in polish wheat (Triticum polonicum L.). Acta Physiol. Plant. 2015, 37, 32. [Google Scholar] [CrossRef]
- Khatun, S.; Roy, T.S.; Haque, M.N.; Alamgir, B. Effect of plant growth regulators and their time of application on yield attributes and quality of soybean. Int. J. Plant Soil Sci. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Beall, F.D.; Yeung, E.C.; Pharis, R.P. Far-red light stimulates internode elongation, cell division, cell elongation, and gibberellin levels in bean. Can. J. Bot. 1996, 74, 743–752. [Google Scholar] [CrossRef]
- Rahman, M.; Khan, A.; Hasan, M.; Banu, L.; Howlader, M. Effect of foliar application of gibberellic acid on different growth contributing characters of mungbean. Progress. Agric. 2018, 29, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Verma, U.; Abhishek, K.; Tripathi, D. Yield performance of mungbean variety following application of growth regulators. Biochem. Cell. Arch. 2016, 16, 157–161. [Google Scholar]
- Nagar, S.; Singh, V.; Arora, A.; Dhakar, R.; Singh, N.; Singh, G.; Meena, S.; Kumar, S.; Shiv Ramakrishnan, R. Understanding the role of gibberellic acid and paclobutrazol in terminal heat stress tolerance in wheat. Front. Plant Sci. 2021, 12, 692252. [Google Scholar] [CrossRef]
- Talaat, N.B.; Hanafy, A. Plant Growth Stimulators Improve Two Wheat Cultivars Salt-Tolerance: Insights into Their Physiological and Nutritional Responses. Plants 2022, 11, 3198. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, T. Effect of GA3, NAA and 2, 4-D on growth and yield of cowpea (Vigna unguiculata (L.) walf) variety arka garima. Flora Fauna Jhansi 1996, 2, 5–6. [Google Scholar]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Gacnik, S.; Veberič, R.; Hudina, M.; Marinovic, S.; Halbwirth, H.; Mikulič-Petkovšek, M. Salicylic and methyl salicylic acid affect quality and phenolic profile of apple fruits three weeks before the harvest. Plants 2021, 10, 1807. [Google Scholar] [CrossRef]
- Xu, L.; Chen, H.; Zhang, T.; Deng, Y.; Yan, J.; Wang, L. Salicylic acid improves the salt tolerance capacity of Saponaria officinalis by modulating its photosynthetic rate, osmoprotectants, antioxidant levels, and ion homeostasis. Agronomy 2022, 12, 1443. [Google Scholar] [CrossRef]
- Farhadi, N.; Ghassemi-Golezani, K. Physiological changes of Mentha pulegium in response to exogenous salicylic acid under salinity. Sci. Hortic. 2020, 267, 109325. [Google Scholar] [CrossRef]
- Bose, S.K.; Yadav, R.K.; Mishra, S.; Sangwan, R.S.; Singh, A.; Mishra, B.; Srivastava, A.; Sangwan, N.S. Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol. Biochem. 2013, 66, 150–158. [Google Scholar] [CrossRef]
- Khan, N.A.; Syeed, S.; Masood, A.; Nazar, R.; Iqbal, N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 2010, 1, e1. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2011, 6, 1787–1792. [Google Scholar] [CrossRef] [Green Version]
- Rashad, R.T.; Hussien, R.A. A comparison study on the effect of some growth regulators on the nutrients content of maize plant under salinity conditions. Ann. Agric. Sci. 2014, 59, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Yadav, R.; Sharma, N.; Yadav, A.; Nehal, N. Influence of plant growth regulators on biochemical changes of mungbean (Vigna radiata L. Wilczek). J. Pharmacog. Phytochem. 2018, 7, 386–389. [Google Scholar]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef]
| Treatments | Crop Growth Rate (gm−2 d−1) | Net Assimilation Rate (gm−2 d−1) |
|---|---|---|
| Salicylic acid levels | ||
| S1 (0 ppm) | 0.45 b | 113.83 b |
| S2 (75 ppm) | 0.58 a | 126.98 a |
| CV (%) | 8.247 | 11.378 |
| Gibberellic acid levels (G) | ||
| G1 (0 ppm) | 0.42 e | 111.50 e |
| G2 (30 ppm) | 0.48 d | 117.47 d |
| G3 (60 ppm) | 0.53 c | 119.08 c |
| G4 (90 ppm) | 0.57 b | 126.75 a |
| G5 (120 ppm) | 0.59 a | 127.23 a |
| CV (%) | 6.574 | 17.235 |
| S × G interaction effects | ||
| S1G1 | 0.34 j | 103.93 f |
| S1G2 | 0.39 i | 108.70 e |
| S1G3 | 0.47 h | 107.90 e |
| S1G4 | 0.55 f | 124.00 c |
| S1G5 | 0.55 e | 124.00 c |
| S2G1 | 0.51 g | 119.07 d |
| S2G2 | 0.57 d | 127.05 b |
| S2G3 | 0.59 c | 129.16 a |
| S2G4 | 0.61 b | 129.60 a |
| S2G5 | 0.63 a | 130.02 a |
| CV (%) | 10.287 | 14.973 |
| Treatments | Plant Height (cm) | Number of Pod-Bearing Branches | Number of Pods per Plant-1 | Pod Length (cm) | Number of Seeds per Pod |
|---|---|---|---|---|---|
| Salicylic acid levels | |||||
| S1 (0 ppm) | 74.05 b | 6.45 b | 27.10 b | 8.62 b | 8.23 b |
| S2 (75 ppm) | 87.18 a | 9.38 a | 32.15 a | 10.21 a | 10.98 a |
| CV (%) | 12.548 | 10.981 | 8.417 | 6.954 | 11.684 |
| Gibberellic acid levels (G) | |||||
| G1 (0 ppm) | 71.02 e | 5.64 e | 25.47 e | 8.29 e | 7.81 e |
| G2 (30 ppm) | 76.99 d | 7.30 d | 28.62 d | 8.96 d | 8.90 d |
| G3 (60 ppm) | 80.43 c | 7.95 c | 29.82 c | 9.52 c | 9.52 c |
| G4 (90 ppm) | 86.32 b | 9.14 b | 31.71 b | 9.96 b | 10.65 b |
| G5 (120 ppm) | 88.32 a | 9.55 a | 32.52 a | 10.35 a | 11.16 a |
| CV (%) | 11.249 | 16.584 | 9.640 | 12.729 | 7.624 |
| S × G interaction effects | |||||
| S1G1 | 62.67 i | 3.37 h | 23.42 i | 7.67 h | 6.47 g |
| S1G2 | 70.43 h | 5.53 g | 26.67 h | 8.21 g | 7.33 f |
| S1G3 | 73.42 g | 6.57 f | 27.17 gh | 8.52 f | 7.82 f |
| S1G4 | 81.23 e | 8.12 de | 28.86 f | 9.23 d | 9.57 de |
| S1G5 | 82.51 d | 8.67 cd | 29.51 e | 9.51 cd | 10.00 cd |
| S2G1 | 79.37 f | 7.91 e | 27.53 g | 8.92 e | 9.15 e |
| S2G2 | 83.54 d | 9.08 bc | 30.67 d | 9.71 c | 10.47 c |
| S2G3 | 87.45 c | 9.33 b | 32.47 c | 10.50 b | 11.21 b |
| S2G4 | 91.42 b | 10.17 a | 34.56 b | 10.70 b | 11.74 b |
| S2G5 | 94.13 a | 10.43 a | 35.53 a | 11.20 a | 12.33 a |
| CV (%) | 8.861 | 11.337 | 17.249 | 10.684 | 15.294 |
| Treatments | 1000-Seed Weight (g) | Grain Yield (t ha−1) | Biological Yield (t ha−1) | Harvest Index (%) |
|---|---|---|---|---|
| Salicylic acid levels | ||||
| S1 (0 ppm) | 44.13 b | 1.38 b | 3.83 b | 35.87 b |
| S2 (75 ppm) | 48.19 a | 1.94 a | 4.94 a | 39.20 a |
| CV (5%) | 6.289 | 9.105 | 10.824 | 12.774 |
| Gibberellic acid levels (G) | ||||
| G1 (0 ppm) | 42.78 e | 1.28 e | 3.60 e | 35.41 d |
| G2 (30 ppm) | 45.07 d | 1.53 d | 4.12 d | 36.83 c |
| G3 (60 ppm) | 46.72 c | 1.68 c | 4.39 c | 37.77 b |
| G4 (90 ppm) | 47.56 b | 1.85 b | 4.82 b | 38.35 b |
| G5 (120 ppm) | 48.67 a | 1.97 a | 5.00 a | 39.32 a |
| CV (%) | 12.665 | 10.579 | 14.371 | 12.690 |
| S × G interaction effects | ||||
| S1G1 | 40.44 g | 1.10 h | 3.19 j | 34.51 g |
| S1G2 | 42.85 f | 1.22 g | 3.46 i | 35.24 fg |
| S1G3 | 44.59 e | 1.28 g | 3.58 f | 35.97 ef |
| S1G4 | 45.85 d | 1.59 e | 4.35 h | 36.67 de |
| S1G5 | 46.92 c | 1.70 d | 4.60 e | 37.12 d |
| S2G1 | 45.12 e | 1.45 f | 4.02 g | 36.15 de |
| S2G2 | 47.29 c | 1.85 c | 4.8 d | 38.59 c |
| S2G3 | 48.85 b | 2.08 b | 5.22 c | 39.86 b |
| S2G4 | 49.28 b | 2.12 b | 5.31 b | 40.05 b |
| S2G5 | 50.42 a | 2.25 a | 5.4 a | 41.63 a |
| CV (%) | 12.658 | 10.420 | 14.982 | 11.128 |
| Treatments | Chlorophyll a (mg g−1 F. Wt.) | Chlorophyll b (mg g−1 F. Wt.) | Total Chlorophyll (mg g−1 F. Wt.) | Carotenoids (mg g−1 F. Wt.) | Protein (%) |
|---|---|---|---|---|---|
| Salicylic acid levels | |||||
| S1 (ppm) | 1.81 b | 0.86 b | 2.17 b | 2.04 b | 21.61 b |
| S2 (ppm) | 2.43 a | 0.56 a | 2.72 a | 2.66 a | 24.06 a |
| CV (%) | 9.854 | 12.501 | 14.879 | 13.248 | 10.251 |
| Gibberellic acid levels | |||||
| G1 (ppm) | 1.66 e | 0.41 e | 1.85 e | 1.95 e | 21.28 e |
| G2 (ppm) | 1.99 d | 0.66 d | 2.34 d | 2.20 d | 22.08 d |
| G3 (ppm) | 2.14 c | 0.76 c | 2.60 c | 2.28 c | 22.87 c |
| G4 (ppm) | 2.37 b | 0.83 b | 2.69 b | 2.64 b | 23.75 b |
| G5 (ppm) | 2.46 a | 0.87 a | 2.75 a | 2.71 a | 24.19 a |
| CV (%) | 8.615 | 9.348 | 12.440 | 12.054 | 13.570 |
| S × G interaction effects | |||||
| S1G1 | 1.11 i | 0.09 j | 1.14 i | 1.51 j | 20.06 j |
| S1G2 | 1.61 h | 0.48 i | 1.98 h | 1.84 i | 20.53 i |
| S1G3 | 1.84 g | 0.66 h | 2.44 g | 1.91 h | 21.68 h |
| S1G4 | 2.23 f | 0.76 f | 2.61 e | 2.45 f | 22.79 f |
| S1G5 | 2.29 e | 0.79 e | 2.66 d | 2.51 e | 22.99 e |
| S2G1 | 2.22 f | 0.73 g | 2.56 f | 2.38 g | 22.50 g |
| S2G2 | 2.37 d | 0.83 d | 2.69 c | 2.56 d | 23.64 d |
| S2G3 | 2.45 c | 0.86 c | 2.75 b | 2.65 c | 24.06 c |
| S2G4 | 2.51 b | 0.91 b | 2.78 b | 2.83 b | 24.70 b |
| S2G5 | 2.63 a | 0.95 a | 2.84 a | 2.92 a | 25.39 a |
| CV (%) | 8.641 | 11.224 | 10.993 | 12.504 | 13.856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.; Iqbal, M.A.; Akram, I.; Saleem, M.A.; Abbas, R.N.; Alqahtani, M.D.; Ahmed, R.; Rahim, J. Phytohormones Promote the Growth, Pigment Biosynthesis and Productivity of Green Gram [Vigna radiata (L.) R. Wilczek]. Sustainability 2023, 15, 9548. https://doi.org/10.3390/su15129548
Iqbal A, Iqbal MA, Akram I, Saleem MA, Abbas RN, Alqahtani MD, Ahmed R, Rahim J. Phytohormones Promote the Growth, Pigment Biosynthesis and Productivity of Green Gram [Vigna radiata (L.) R. Wilczek]. Sustainability. 2023; 15(12):9548. https://doi.org/10.3390/su15129548
Chicago/Turabian StyleIqbal, Asif, Muhammad Aamir Iqbal, Iqra Akram, Muhammad Abdullah Saleem, Rana Nadeem Abbas, Mashael Daghash Alqahtani, Raees Ahmed, and Junaid Rahim. 2023. "Phytohormones Promote the Growth, Pigment Biosynthesis and Productivity of Green Gram [Vigna radiata (L.) R. Wilczek]" Sustainability 15, no. 12: 9548. https://doi.org/10.3390/su15129548
APA StyleIqbal, A., Iqbal, M. A., Akram, I., Saleem, M. A., Abbas, R. N., Alqahtani, M. D., Ahmed, R., & Rahim, J. (2023). Phytohormones Promote the Growth, Pigment Biosynthesis and Productivity of Green Gram [Vigna radiata (L.) R. Wilczek]. Sustainability, 15(12), 9548. https://doi.org/10.3390/su15129548










