Characterization of Cross-Linking in Guar Gum Hydrogels via the Analysis of Thermal Decomposition Behavior and Water Uptake Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
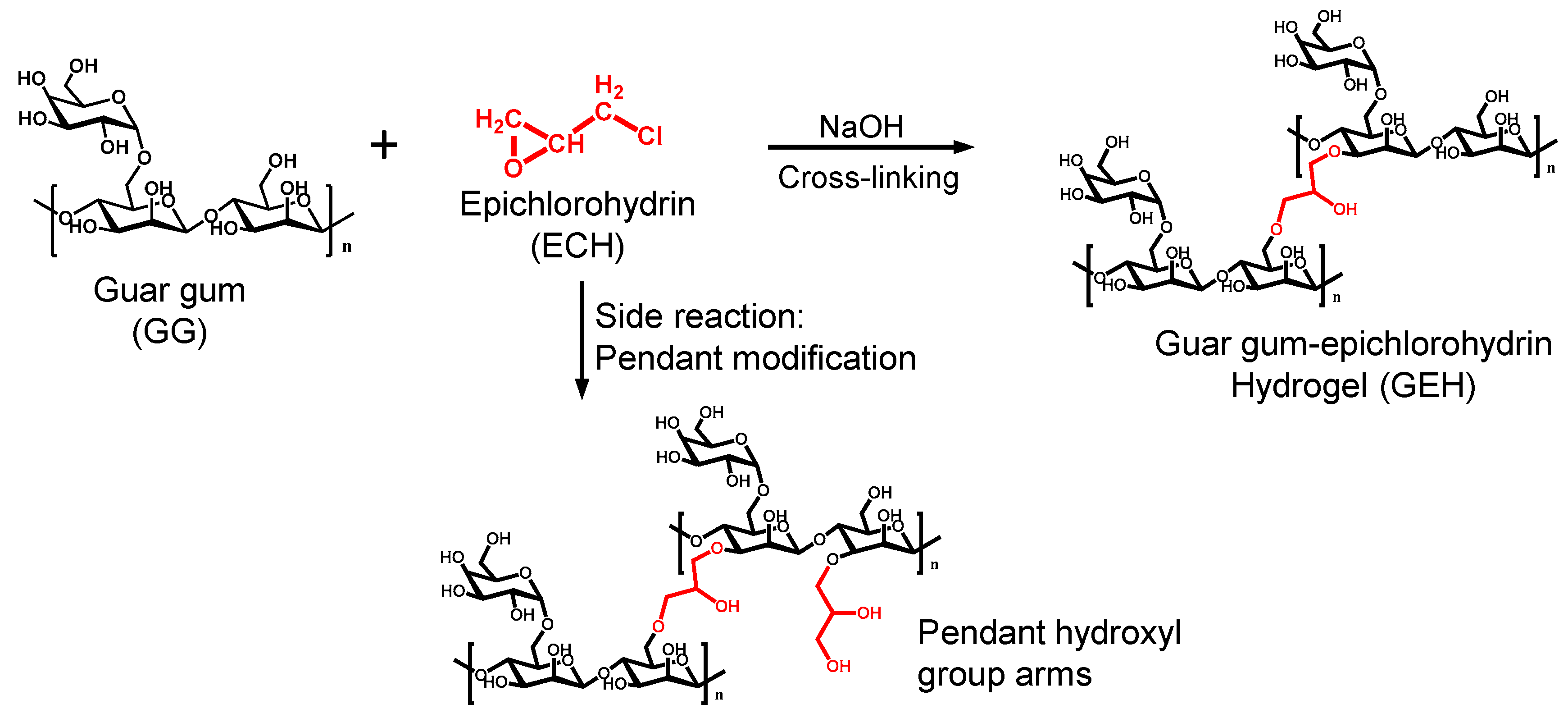
2.2. GEH Preparation
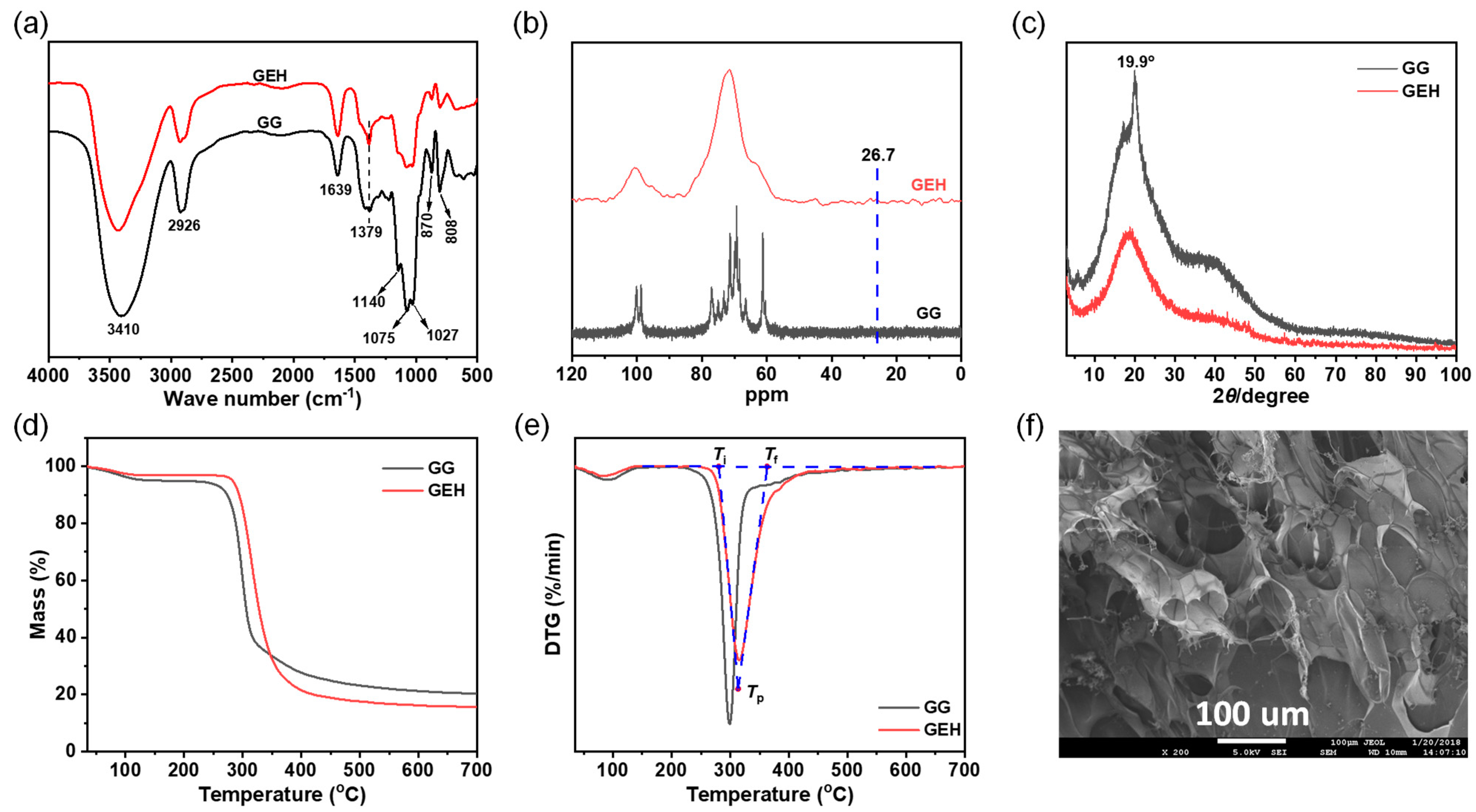
2.3. Characterization
2.4. Mechanical Strength
2.5. Swelling Rate
2.6. Water Uptake
2.7. Degree of Mass Cross-Linking
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Physical and Chemical Properties
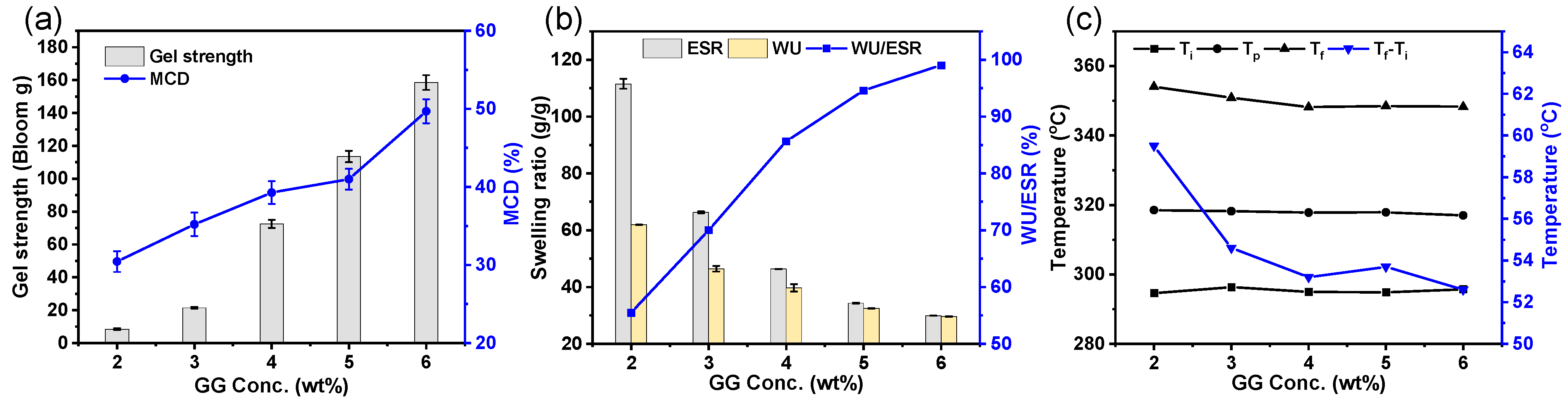
3.2. Influence of GG Concentration on the Cross-Linking Characteristics of the GEHs
3.3. Influence of Cross-Linking Agent Concentration on the Cross-Linking Characteristics of the GEHs
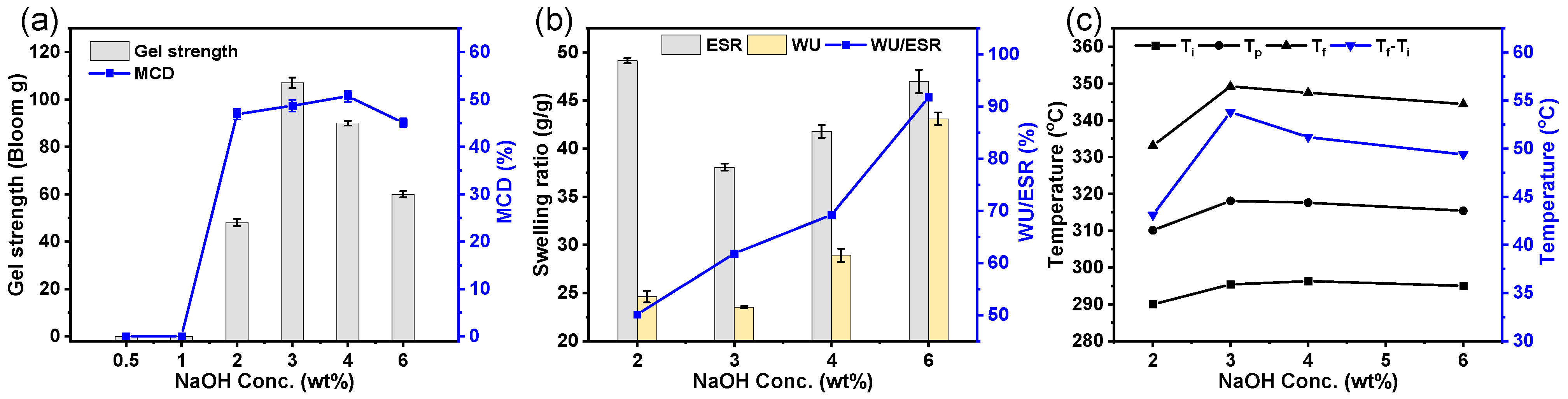
3.4. Influence of Catalyst Concentration on the Cross-Linking Characteristics of the GEHs
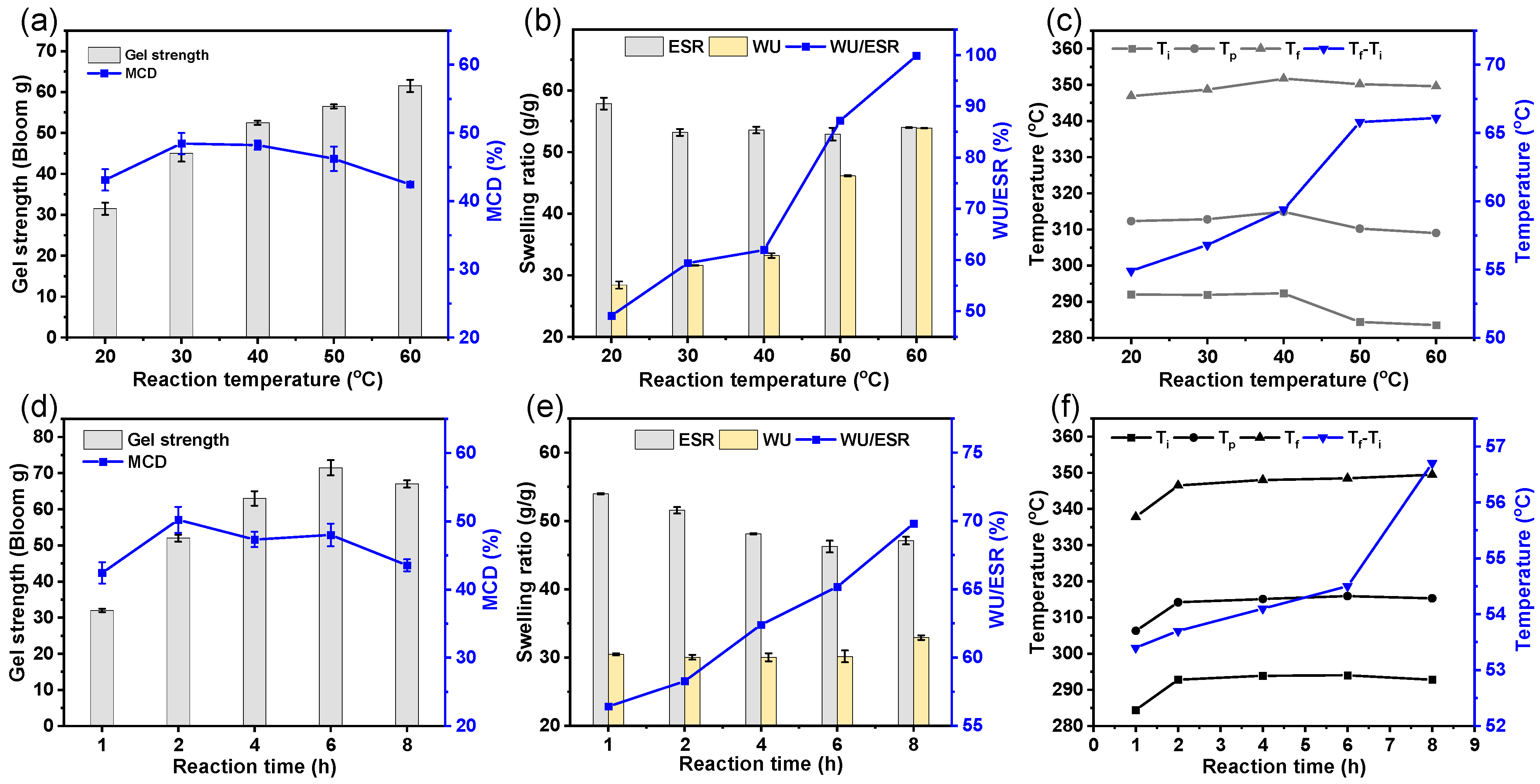
3.5. Influence of Cross-Linking Temperature and Time on the Cross-Linking Characteristics of the GEHs
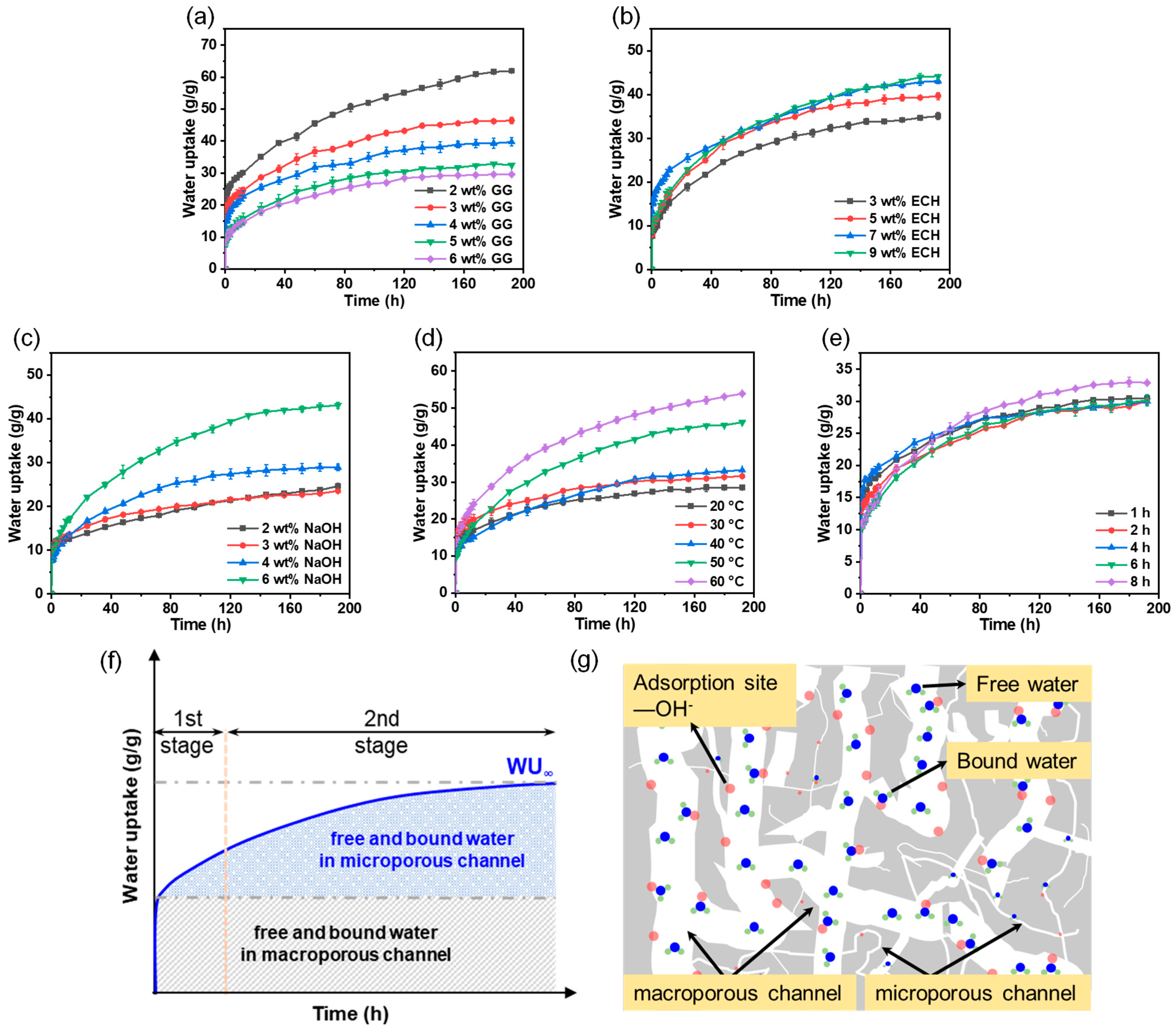
3.6. Relationship between the Water Uptake Kinetics and Cross-Linking Characteristics of the Freeze-Dried Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, H.; Maiti, S. Research Progress in Galactomannan-Based Nanomaterials: Synthesis and Application. Int. J. Biol. Macromol. 2020, 163, 2113–2126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Jian, H.; Cristhian, C.; Zhang, W.; Sun, R. Structural and Thermal Characterization of Galactomannans from Genus Gleditsia Seeds as Potential Food Gum Substitutes. J. Sci. Food Agric. 2011, 91, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Cristhian, C.; Zhang, W.; Jiang, J. Influence of Dehulling Pretreatment on Physicochemical Properties of Gleditsia Sinensis Lam. Gum. Food Hydrocoll. 2011, 25, 1337–1343. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, X.; Gao, Q. Digestibility and Physicochemical Properties of Starch-Galactomannan Complexes by Heat-Moisture Treatment. Food Hydrocoll. 2018, 77, 853–862. [Google Scholar] [CrossRef]
- Rashid, F.; Hussain, S.; Ahmed, Z. Extraction Purification and Characterization of Galactomannan from Fenugreek for Industrial Utilization. Carbohydr. Polym. 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Depoorter, J.; Mourlevat, A.; Sudre, G.; Morfin, I.; Prasad, K.; Serghei, A.; Bernard, J.; Fleury, E.; Charlot, A. Fully Biosourced Materials from Combination of Choline Chloride-Based Deep Eutectic Solvents and Guar Gum. ACS Sustain. Chem. Eng. 2019, 7, 16747–16756. [Google Scholar] [CrossRef]
- Seeli, D.S.; Prabaharan, M. Guar Gum Oleate-Graft-Poly(Methacrylic Acid) Hydrogel as a Colon-Specific Controlled Drug Delivery Carrier. Carbohydr. Polym. 2017, 158, 51–57. [Google Scholar] [CrossRef]
- Pal, P.; Rangra, N.; Samanta, S.; Aryan, A.; Pandey, J.P.; Sen, G. Graft Copolymer of PVP—A Sutureless, Haemostatic Bioadhesive for Wound Healing Application. Polym. Bull. 2020, 77, 5191–5212. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A Review on Latest Innovations in Natural Gums Based Hydrogels: Preparations & Applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar]
- Sharma, G.; Kumar, A.; Devi, K.; Sharma, S.; Naushad, M.; Ghfar, A.A.; Ahamad, T.; Stadler, F.J. Guar Gum-Crosslinked-Soya Lecithin Nanohydrogel Sheets as Effective Adsorbent for the Removal of Thiophanate Methyl Fungicide. Int. J. Biol. Macromol. 2018, 114, 295–305. [Google Scholar] [CrossRef]
- Liu, C.; Lei, F.; Li, P.; Jiang, J.; Wang, K. Borax Crosslinked Fenugreek Galactomannan Hydrogel as Potential Water-Retaining Agent in Agriculture. Carbohydr. Polym. 2020, 236, 116100. [Google Scholar] [CrossRef]
- Chen, W.; Bu, Y.; Li, D.; Liu, Y.; Chen, G.; Wan, X.; Li, N. Development of High-Strength, Tough, and Self-Healing Carboxymethyl Guar Gum-Based Hydrogels for Human Motion Detection. J. Mater. Chem. C 2020, 8, 900–908. [Google Scholar] [CrossRef]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and Development of Guar Gum Based Novel, Superabsorbent and Moisture Retaining Hydrogels for Agricultural Applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Li, Y.; Li, Q.; Liu, X. Hydroxypropylation of Cross-Linked Sesbania Gum, Characterization and Properties. Int. J. Biol. Macromol. 2020, 152, 1010–1019. [Google Scholar] [CrossRef]
- Zhao, N.; Chai, Y.; Wang, T.; Wang, K.; Jiang, J.; Yang, H. Preparation and Physical/Chemical Modification of Galactomannan Film for Food Packaging. Int. J. Biol. Macromol. 2019, 137, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Orsu, P.; Matta, S. Fabrication and Characterization of Carboxymethyl Guar Gum Nanocomposite for Application of Wound Healing. Int. J. Biol. Macromol. 2020, 164, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.; Jabeen, S.; Nisar, N.; Islam, A.; Gull, N.; Iqbal, S.S.; Khan, S.M.; Yameen, B. Controlled Release of Cephradine by Biopolymers Based Target Specific Crosslinked Hydrogels. Int. J. Biol. Macromol. 2019, 121, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Gu, J.; Liu, W.; Wang, K.; Huang, C.; Lai, C.; Yong, Q. Actuating, Shape Reconstruction, and Reinforcement of Galactomannan-Based Hydrogels by Coordination Bonds Induced Metal Ions Capture. Int. J. Biol. Macromol. 2020, 165, 2721–2730. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Tokuyama, H.; Nakahata, Y.; Ban, T. Diffusion Coefficient of Solute in Heterogeneous and Macroporous Hydrogels and Its Correlation with the Effective Crosslinking Density. J. Membr. Sci. 2020, 595, 117533. [Google Scholar] [CrossRef]
- Eelkema, R.; Pich, A. Pros and Cons: Supramolecular or Macromolecular: What Is Best for Functional Hydrogels with Advanced Properties? Adv. Mater. 2020, 32, 1906012. [Google Scholar] [CrossRef]
- Kopač, T.; Ručigaj, A.; Krajnc, M. The Mutual Effect of the Crosslinker and Biopolymer Concentration on the Desired Hydrogel Properties. Int. J. Biol. Macromol. 2020, 159, 557–569. [Google Scholar] [CrossRef]
- Wende, F.J.; Xue, Y.; Nestor, G.; Öhrlund, Å.; Sandström, C. Relaxation and Diffusion of Water Protons in BDDE Cross-Linked Hyaluronic Acid Hydrogels Investigated by NMR Spectroscopy—Comparison with Physicochemical Properties. Carbohydr. Polym. 2020, 248, 116768. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Y.; Zhang, F.; Lei, F.; Wang, K.; Jiang, J. Physicochemical Characterization of Gleditsia Triacanthos Galactomannan during Deposition and Maturation. Int. J. Biol. Macromol. 2020, 144, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, T.; He, J.; Jiang, J.; Lei, F. Synthesis, Characterization, and Selective Dye Adsorption by PH- and Ion-Sensitive Polyelectrolyte Galactomannan-Based Hydrogels. Carbohydr. Polym. 2021, 264, 118009. [Google Scholar] [CrossRef]
- Uhl, F.M.; Levchik, G.F.; Levchik, S.V.; Dick, C.; Liggat, J.J.; Snape, C.E.; Wilkie, C.A. The Thermal Stability of Cross-Linked Polymers: Methyl Methacrylate with Divinylbenzene and Styrene with Dimethacrylates. Polym. Degrad. Stab. 2001, 71, 317–325. [Google Scholar] [CrossRef]
- Li, D.; Yang, N.; Zhang, Y.; Guo, L.; Sang, S.; Jin, Z.; Xu, X. Structural and Physicochemical Changes in Guar Gum by Alcohol–Acid Treatment. Carbohydr. Polym. 2018, 179, 2–9. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. X-ray Diffraction, IR Spectroscopy and Thermal Characterization of Partially Hydrolyzed Guar Gum. Int. J. Biol. Macromol. 2012, 50, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.V.L.; de Castro, E.V.R.; Muri, E.J.B.; Loureiro, B.V.; Costalonga, M.L.; Filgueiras, P.R. Thermal and Spectroscopic Analyses of Guar Gum Degradation Submitted to Turbulent Flow. Int. J. Biol. Macromol. 2019, 131, 43–49. [Google Scholar] [CrossRef]
- Dodi, G.; Pala, A.; Barbu, E.; Peptanariu, D.; Hritcu, D.; Popa, M.I.; Tamba, B.I. Carboxymethyl Guar Gum Nanoparticles for Drug Delivery Applications: Preparation and Preliminary in-Vitro Investigations. Mater. Sci. Eng. C 2016, 63, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Das, A.; Basu, A.; Datta, P.; Gupta, M.; Mukherjee, A. Newer Guar Gum Ester/Chicken Feather Keratin Interact Films for Tissue Engineering. Int. J. Biol. Macromol. 2021, 180, S0141813021005523. [Google Scholar] [CrossRef]
- Mahto, A.; Mishra, S. Design, Development and Validation of Guar Gum Based PH Sensitive Drug Delivery Carrier via Graft Copolymerization Reaction Using Microwave Irradiations. Int. J. Biol. Macromol. 2019, 138, 278–291. [Google Scholar] [CrossRef]
- Su, T.; Wu, L.; Pan, X.; Zhang, C.; Shi, M.; Gao, R.; Qi, X.; Dong, W. Pullulan-Derived Nanocomposite Hydrogels for Wastewater Remediation: Synthesis and Characterization. J. Colloid Interface Sci. 2019, 542, 253–262. [Google Scholar] [CrossRef]
- Uddin, K.M.A.; Ago, M.; Rojas, O.J. Hybrid Films of Chitosan, Cellulose Nanofibrils and Boric Acid: Flame Retardancy, Optical and Thermo-Mechanical Properties. Carbohydr. Polym. 2017, 177, 13–21. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Bian, C.; Zhong, Y.; Jing, X. Effect of Chemical Structure and Cross-Link Density on the Heat Resistance of Phenolic Resin. Polym. Degrad. Stab. 2015, 111, 239–246. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, G.; Shi, M.; Suo, Z. Fracture, Fatigue, and Friction of Polymers in Which Entanglements Greatly Outnumber Cross-Links. Science 2021, 374, 212–216. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Li, Q.; Liu, Y.; Li, J. Ultralight and Highly Flexible Aerogels with Long Cellulose I Nanofibers. Soft Matter 2011, 7, 10360–10368. [Google Scholar] [CrossRef]
- Díez-García, I.; de Costa Lemma, M.R.; Barud, H.S.; Eceiza, A.; Tercjak, A. Hydrogels Based on Waterborne Poly(Urethane-Urea)s by Physically Cross-Linking with Sodium Alginate and Calcium Chloride. Carbohydr. Polym. 2020, 250, 116940. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Simões, J.; Teixeira, J.A.; Domingues, M.R.M.; Coimbra, M.A.; Vicente, A.A. Structural and Thermal Characterization of Galactomannans from Non-Conventional Sources. Carbohydr. Polym. 2011, 83, 179–185. [Google Scholar] [CrossRef]
- Fukata, Y.; Kimura, S.; Iwata, T. Synthesis of α-1,3-Glucan Branched Ester Derivatives with Excellent Thermal Stability and Thermoplasticity. Polym. Degrad. Stab. 2020, 177, 109130. [Google Scholar] [CrossRef]
- Yang, B.; Guo, X.; Zang, H.; Liu, J. Determination of Modification Degree in BDDE-Modified Hyaluronic Acid Hydrogel by SEC/MS. Carbohydr. Polym. 2015, 131, 233–239. [Google Scholar] [CrossRef]
- Ruhr, D.; John, M.; Reiche, A. Determination of the Effective Degree of Cross-Linking of Porous Cellulose Membranes Cross-Linked with Bifunctional Epoxides. Carbohydr. Polym. 2021, 251, 117043. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L.; Zhou, J.; Zhang, L.; Kennedy, J.F. Structure and Properties of Hydrogels Prepared from Cellulose in NaOH/Urea Aqueous Solutions. Carbohydr. Polym. 2010, 82, 122–127. [Google Scholar] [CrossRef]
- Salimi-Kenari, H.; Mollaie, F.; Dashtimoghadam, E.; Imani, M.; Nyström, B. Effects of Chain Length of the Cross-Linking Agent on Rheological and Swelling Characteristics of Dextran Hydrogels. Carbohydr. Polym. 2018, 181, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Cameron, R.E. The Effects of Concentration and Sodium Hydroxide on the Rheological Properties of Potato Starch Gelatinisation. Carbohydr. Polym. 2002, 50, 133–143. [Google Scholar] [CrossRef]
- Berglund, J.; Azhar, S.; Lawoko, M.; Lindström, M.; Vilaplana, F.; Wohlert, J.; Henriksson, G. The Structure of Galactoglucomannan Impacts the Degradation under Alkaline Conditions. Cellulose 2019, 26, 2155–2175. [Google Scholar] [CrossRef]
- Haseeb, M.T.; Hussain, M.A.; Yuk, S.H.; Bashir, S.; Nauman, M. Polysaccharides Based Superabsorbent Hydrogel from Linseed: Dynamic Swelling, Stimuli Responsive on–off Switching and Drug Release. Carbohydr. Polym. 2016, 136, 750–756. [Google Scholar] [CrossRef]
- Patra, P.; Patra, N.; Pal, S. Opposite Swelling Characteristics through Changing the Connectivity in a Biopolymeric Hydrogel Based on Glycogen and Glycine. Polym. Chem. 2020, 11, 2630–2634. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, Drying Process and Medical Application of Polysaccharide-Based Aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Ciftci, D.; Ubeyitogullari, A.; Huerta, R.R.; Ciftci, O.N.; Flores, R.A.; Saldaña, M.D.A. Lupin Hull Cellulose Nanofiber Aerogel Preparation by Supercritical CO2 and Freeze Drying. J. Supercrit. Fluids 2017, 127, 137–145. [Google Scholar] [CrossRef]
- Gharekhani, H.; Olad, A.; Mirmohseni, A.; Bybordi, A. Superabsorbent Hydrogel Made of NaAlg-g-Poly(AA-Co-AAm) and Rice Husk Ash: Synthesis, Characterization, and Swelling Kinetic Studies. Carbohydr. Polym. 2017, 168, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-X.; Wang, Q.; Zhang, L.; Tian, X.; Cao, Q.; Jin, L. Influence of the Degrees of Polymerization of Cellulose on the Water Absorption Performance of Hydrogel and Adsorption Kinetics. Polym. Degrad. Stab. 2019, 168, 108958. [Google Scholar] [CrossRef]
- Tan, T.; Zhou, J.; Gao, X.; Tang, X.; Zhang, H. Synthesis, Characterization and Water-Absorption Behavior of Tartaric Acid-Modified Cellulose Gel Fromcorn Stalk Pith. Ind. Crops Prod. 2021, 169, 113641. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, X.; Zhen, J.; Lei, Z. Preparation of Superabsorbent Resin with Fast Water Absorption Rate Based on Hydroxymethyl Cellulose Sodium and Its Application. Carbohydr. Polym. 2019, 225, 115214. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Pandey, S.; Arotiba, O.A. Development of a Sodium Alginate-Based Organic/Inorganic Superabsorbent Composite Hydrogel for Adsorption of Methylene Blue. Carbohydr. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef] [PubMed]
- da Silva Fernandes, R.; Tanaka, F.N.; de Moura, M.R.; Aouada, F.A. Development of Alginate/Starch-Based Hydrogels Crosslinked with Different Ions: Hydrophilic, Kinetic and Spectroscopic Properties. Mater. Today Commun. 2019, 21, 100636. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Xie, Z.; Wang, T.; Lu, J.; Li, P.; Jiang, J. Characterization of Cross-Linking in Guar Gum Hydrogels via the Analysis of Thermal Decomposition Behavior and Water Uptake Kinetics. Sustainability 2023, 15, 9778. https://doi.org/10.3390/su15129778
Lan Y, Xie Z, Wang T, Lu J, Li P, Jiang J. Characterization of Cross-Linking in Guar Gum Hydrogels via the Analysis of Thermal Decomposition Behavior and Water Uptake Kinetics. Sustainability. 2023; 15(12):9778. https://doi.org/10.3390/su15129778
Chicago/Turabian StyleLan, Yanjiao, Zhoujian Xie, Ting Wang, Jianfang Lu, Pengfei Li, and Jianxin Jiang. 2023. "Characterization of Cross-Linking in Guar Gum Hydrogels via the Analysis of Thermal Decomposition Behavior and Water Uptake Kinetics" Sustainability 15, no. 12: 9778. https://doi.org/10.3390/su15129778
APA StyleLan, Y., Xie, Z., Wang, T., Lu, J., Li, P., & Jiang, J. (2023). Characterization of Cross-Linking in Guar Gum Hydrogels via the Analysis of Thermal Decomposition Behavior and Water Uptake Kinetics. Sustainability, 15(12), 9778. https://doi.org/10.3390/su15129778






