Microplastic Contamination in Different Marine Species of Bintaro Fish Market, Indonesia
Abstract
1. Introduction
2. Results and Discussion
2.1. Total MPs Detected in Edible Tissue of Fish Species
2.2. Characterization of MPs
2.3. Chemical Determination of MPs with FTIR
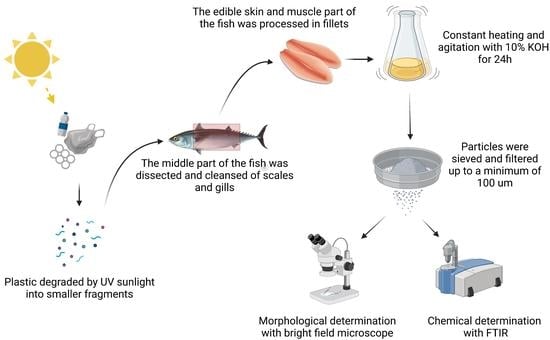
3. Materials and Methods
3.1. Fish Collection
3.2. Isolation of MPs
3.3. Microplastic Characterization
3.4. Quality Assurance and Control
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic Pollution in the Marine Environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding Plastic Degradation and Microplastic Formation in the Environment: A Review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef] [PubMed]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An Overview on Separation, Identification and Characterization of Microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in Fish and Fishmeal: An Emerging Environmental Challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Alberghini, L.; Truant, A.; Santonicola, S.; Colavita, G.; Giaccone, V. Microplastics in Fish and Fishery Products and Risks for Human Health: A Review. Int. J. Environ. Res. Public Health 2022, 20, 789. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sobhan, F.; Uddin, M.N.; Sharifuzzaman, S.M.; Chowdhury, S.R.; Sarker, S.; Chowdhury, M.S.N. Microplastics in Fishes from the Northern Bay of Bengal. Sci. Total Environ. 2019, 690, 821–830. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Ranking of Potential Hazards from Microplastics Polymers in the Marine Environment. J. Hazard. Mater. 2022, 429, 128399. [Google Scholar] [CrossRef]
- Cverenkárová, K.; Valachovičová, M.; Mackuľak, T.; Žemlička, L.; Bírošová, L. Microplastics in the Food Chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Yang, H.; Chen, G.; Wang, J. Microplastics in the Marine Environment: Sources, Fates, Impacts and Microbial Degradation. Toxics 2021, 9, 41. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Tariq, T.; Fatima, B.; Sahar, A.; Tariq, F.; Munir, S.; Khan, S.; Nawaz Ranjha, M.M.A.; Sameen, A.; Zeng, X.-A.; et al. An Insight into Bisphenol A, Food Exposure and Its Adverse Effects on Health: A Review. Front Nutr 2022, 9, 1047827. [Google Scholar] [CrossRef] [PubMed]
- Total Produksi. Available online: https://statistik.kkp.go.id/home.php?m=total&i=2 (accessed on 19 January 2023).
- Fisheries Country Profile: Indonesia. SEAFDEC. Available online: http://www.seafdec.org (accessed on 15 December 2022).
- Ni’am, A.C.; Hassan, F.; Shiu, R.-F.; Jiang, J.-J. Microplastics in Sediments of East Surabaya, Indonesia: Regional Characteristics and Potential Risks. Int. J. Environ. Res. Public Health 2022, 19, 12348. [Google Scholar] [CrossRef]
- Lestari, P.; Trihadiningrum, Y.; Wijaya, B.A.; Yunus, K.A.; Firdaus, M. Distribution of Microplastics in Surabaya River, Indonesia. Sci. Total Environ. 2020, 726, 138560. [Google Scholar] [CrossRef]
- Cordova, M.R.; Purwiyanto, A.I.S.; Suteja, Y. Abundance and Characteristics of Microplastics in the Northern Coastal Waters of Surabaya, Indonesia. Mar. Pollut. Bull. 2019, 142, 183–188. [Google Scholar] [CrossRef]
- Fajaruddin Natsir, M.; Selomo, M.; Ibrahim, E.; Arsin, A.A.; Alni, N.C. Analysis on Microplastics in Dug Wells around Tamangapa Landfills, Makassar City, Indonesia. Gac. Sanit. 2021, 35 (Suppl. S1), S87–S89. [Google Scholar] [CrossRef]
- Rauf, A.U.; Mallongi, A.; Lee, K.; Daud, A.; Hatta, M.; Al Madhoun, W.; Astuti, R.D.P. Potentially Toxic Element Levels in Atmospheric Particulates and Health Risk Estimation around Industrial Areas of Maros, Indonesia. Toxics 2021, 9, 328. [Google Scholar] [CrossRef]
- Widya, L.K.; Hsu, C.-Y.; Lee, H.-Y.; Jaelani, L.M.; Lung, S.-C.C.; Su, H.-J.; Wu, C.-D. Comparison of Spatial Modelling Approaches on PM10 and NO2 Concentration Variations: A Case Study in Surabaya City, Indonesia. Int. J. Environ. Res. Public Health 2020, 17, 8883. [Google Scholar] [CrossRef]
- Mostarda, E.; Campo, D.; Castriota, L.; Esposito, V.; Scarabello, M.P.; Andaloro, F. Feeding Habits of the Bullet Tuna Auxis Rochei in the Southern Tyrrhenian Sea. J. Mar. Biol. Assoc. U. K. 2007, 87, 1007–1012. [Google Scholar] [CrossRef]
- Steer, M.; Cole, M.; Thompson, R.C.; Lindeque, P.K. Microplastic Ingestion in Fish Larvae in the Western English Channel. Environ. Pollut. 2017, 226, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and Seafood: Lower Trophic Organisms at Highest Risk of Contamination. Ecotoxicol. Environ. Saf. 2020, 190, 110066. [Google Scholar] [CrossRef] [PubMed]
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic Fibers—Underestimated Threat to Aquatic Organisms? Sci. Total Environ. 2021, 777, 146045. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhang, C.; Wang, S.; Sun, D.; Zhou, A.; Xie, S.; Xu, G.; Zou, J. Occurrence of Microplastics in the Gastrointestinal Tract and Gills of Fish from Guangdong, South China. J. Mar. Sci. Eng. 2021, 9, 981. [Google Scholar] [CrossRef]
- Karbalaei, S.; Golieskardi, A.; Hamzah, H.B.; Abdulwahid, S.; Hanachi, P.; Walker, T.; Karami, A. Abundance and Characteristics of Microplastics in Commercially Sold Fishes from Cebu Island, Philippines. Int. J. Aquat. Biol. 2021, 8, 424–433. [Google Scholar] [CrossRef]
- Chen, J.-C.; Fang, C.; Zheng, R.-H.; Hong, F.-K.; Jiang, Y.-L.; Zhang, M.; Li, Y.; Hamid, F.S.; Bo, J.; Lin, L.-S. Microplastic Pollution in Wild Commercial Nekton from the South China Sea and Indian Ocean, and Its Implication to Human Health. Mar. Environ. Res. 2021, 167, 105295. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Morote, E.; Gil, C.; Ramos-Miras, J.J.; Torrijos, M.; Rodríguez Martin, J.A. Mercury Contents in Relation to Biometrics and Proximal Composition and Nutritional Levels of Fish Eaten from the Western Mediterranean Sea (Almería Bay). Mar. Pollut. Bull. 2018, 135, 783–789. [Google Scholar] [CrossRef]
- Blackburn, K.; Green, D. The Potential Effects of Microplastics on Human Health: What Is Known and What Is Unknown. Ambio 2022, 51, 518–530. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and Effects of Orally Ingested Polystyrene Microplastic Particles in Vitro and in Vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Lee, Y.-H.; Hsu, Y.-H.; Chiu, I.-J.; Huang, C.C.-Y.; Huang, C.-C.; Chia, Z.-C.; Lee, C.-P.; Lin, Y.-F.; Chiu, H.-W. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice. Environ. Health Perspect. 2021, 129, 057003. [Google Scholar] [CrossRef]
- Arias, A.H.; Ronda, A.C.; Oliva, A.L.; Marcovecchio, J.E. Evidence of Microplastic Ingestion by Fish from the Bahía Blanca Estuary in Argentina, South America. Bull. Environ. Contam. Toxicol. 2019, 102, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ye, C.; Pan, Y. Abundance and Characteristics of Microplastics in Municipal Wastewater Treatment Plant Effluent: A Case Study of Guangzhou, China. Environ. Sci. Pollut. Res. Int. 2021, 28, 11572–11585. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.; Aleixo, M.; De Pablo, H.; Lopes, C.; Raimundo, J. Microplastics in Wastewater: Microfiber Emissions from Common Household Laundry. Environ. Sci. Pollut. Res. Int. 2020, 27, 26643–26649. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J.; Kwon, M. The Effect of the Physical and Chemical Properties of Synthetic Fabrics on the Release of Microplastics during Washing and Drying. Polymers 2022, 14, 3384. [Google Scholar] [CrossRef]
- Su, L.; Sharp, S.M.; Pettigrove, V.J.; Craig, N.J.; Nan, B.; Du, F.; Shi, H. Superimposed Microplastic Pollution in a Coastal Metropolis. Water Res. 2020, 168, 115140. [Google Scholar] [CrossRef] [PubMed]
- Herzke, D.; Ghaffari, P.; Sundet, J.H.; Tranang, C.A.; Halsband, C. Microplastic Fiber Emissions from Wastewater Effluents: Abundance, Transport Behavior and Exposure Risk for Biota in an Arctic Fjord. Front. Environ. Sci. 2021, 9, 662168. [Google Scholar] [CrossRef]
- Salvador Cesa, F.; Turra, A.; Baruque-Ramos, J. Synthetic Fibers as Microplastics in the Marine Environment: A Review from Textile Perspective with a Focus on Domestic Washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Rudiarto, I.; Handayani, W.; Sih Setyono, J. A Regional Perspective on Urbanization and Climate-Related Disasters in the Northern Coastal Region of Central Java, Indonesia. Land 2018, 7, 34. [Google Scholar] [CrossRef]
- Vriend, P.; Hidayat, H.; van Leeuwen, J.; Cordova, M.R.; Purba, N.P.; Löhr, A.J.; Faizal, I.; Ningsih, N.S.; Agustina, K.; Husrin, S.; et al. Plastic Pollution Research in Indonesia: State of Science and Future Research Directions to Reduce Impacts. Front. Environ. Sci. 2021, 9, 187. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-Levels of Microplastic Pollution in a Large, Remote, Mountain Lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Kozioł, A.; Paso, K.G.; Kuciel, S. Properties and Recyclability of Abandoned Fishing Net-Based Plastic Debris. Catalysts 2022, 12, 948. [Google Scholar] [CrossRef]
- Gilman, E.; Musyl, M.; Suuronen, P.; Chaloupka, M.; Gorgin, S.; Wilson, J.; Kuczenski, B. Highest Risk Abandoned, Lost and Discarded Fishing Gear. Sci. Rep. 2021, 11, 7195. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.; Cicala, G.; Acierno, D. Eco-Sustainability of the Textile Production: Waste Recovery and Current Recycling in the Composites World. Polymers 2020, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, M.-J.; Rashidi, A. Evaluation of the Disintegration of Linen Fabric under Composting Conditions. Environ. Sci. Pollut. Res. Int. 2018, 25, 29070–29077. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H. Microplastic Fragments and Microbeads in Digestive Tracts of Planktivorous Fish from Urban Coastal Waters. Sci. Rep. 2016, 6, 34351. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, K.; Soefihara, M.D.A.; Nababan, D.C.; Kim, S. Current Status of the Recycling of E-Waste in Indonesia. Geosystem Eng. 2022, 25, 1–12. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Karkanorachaki, K.; Kalogerakis, G.C.; Triantafyllidi, E.I.; Gotsis, A.D.; Partsinevelos, P.; Fava, F. Microplastics Generation: Onset of Fragmentation of Polyethylene Films in Marine Environment Mesocosms. Front. Mar. Sci. 2017, 4, 84. [Google Scholar] [CrossRef]
- Damar, A.; Hariyadi, S. Roles and Interrelation between Variables: A Study Case of Plastic Waste Management in Jakarta Bay. J. Coast. Conserv. 2022, 26, 41. [Google Scholar] [CrossRef]
- Mahendradhata, Y.; Andayani, N.L.P.E.; Hasri, E.T.; Arifi, M.D.; Siahaan, R.G.M.; Solikha, D.A.; Ali, P.B. The Capacity of the Indonesian Healthcare System to Respond to COVID-19. Front. Public Health 2021, 9, 649819. [Google Scholar] [CrossRef]
- Veerasingam, S.; Saha, M.; Suneel, V.; Vethamony, P.; Rodrigues, A.C.; Bhattacharyya, S.; Naik, B.G. Characteristics, Seasonal Distribution and Surface Degradation Features of Microplastic Pellets along the Goa Coast, India. Chemosphere 2016, 159, 496–505. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhang, K.; Yang, R.; Li, R.; Li, Y. Characterization, Source, and Retention of Microplastic in Sandy Beaches and Mangrove Wetlands of the Qinzhou Bay, China. Mar. Pollut. Bull. 2018, 136, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Cordova, M.R.; Nurhati, I.S. Major Sources and Monthly Variations in the Release of Land-Derived Marine Debris from the Greater Jakarta Area, Indonesia. Sci. Rep. 2019, 9, 18730. [Google Scholar] [CrossRef] [PubMed]
- Balestra, V.; Bellopede, R. Microplastic Pollution in Show Cave Sediments: First Evidence and Detection Technique. Environ. Pollut. 2022, 292, 118261. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Pérez, C.; Amezcua, F.; Rosales-Valencia, A.; Green, L.; Pollorena-Melendrez, J.E.; Sarmiento-Martínez, M.A.; Tomita Ramírez, I.; Gil-Manrique, B.D.; Hernandez-Lozano, M.Y.; Muro-Torres, V.M.; et al. First Insight into Plastics Ingestion by Fish in the Gulf of California, Mexico. Mar. Pollut. Bull. 2021, 171, 112705. [Google Scholar] [CrossRef] [PubMed]
- JRC Publications Repository—Guidance on Monitoring of Marine Litter in European Seas. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC83985 (accessed on 19 January 2023).
- Isobe, A.; Uchida, K.; Tokai, T.; Iwasaki, S. East Asian Seas: A Hot Spot of Pelagic Microplastics. Mar. Pollut. Bull. 2015, 101, 618–623. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Ocaya, H.; Pabire, W.G. Microplastic Pollution in Surface Water of Lake Victoria. Sci. Total Environ. 2020, 741, 140201. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential Human Health Risks Due to Environmental Exposure to Nano- and Microplastics and Knowledge Gaps: A Scoping Review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene Translocation and Fetal Deposition after Acute Lung Exposure during Late-Stage Pregnancy. Part. Fibre. Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-Endpoint Toxicological Assessment of Polystyrene Nano- and Microparticles in Different Biological Models in Vitro. Toxicol. Vitr. 2019, 61, 104610. [Google Scholar] [CrossRef]
- Cunsolo, S.; Williams, J.; Hale, M.; Read, D.S.; Couceiro, F. Optimising Sample Preparation for FTIR-Based Microplastic Analysis in Wastewater and Sludge Samples: Multiple Digestions. Anal. Bioanal. Chem. 2021, 413, 3789–3799. [Google Scholar] [CrossRef]
- De Frond, H.; Cowger, W.; Renick, V.; Brander, S.; Primpke, S.; Sukumaran, S.; Elkhatib, D.; Barnett, S.; Navas-Moreno, M.; Rickabaugh, K.; et al. What Determines Accuracy of Chemical Identification When Using Microspectroscopy for the Analysis of Microplastics? Chemosphere 2023, 313, 137300. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.R.M.; Ying, P.X.; Zainuddin, A.H.; Razak, M.R.; Aris, A.Z. Occurrence, Abundance, and Distribution of Microplastics Pollution: An Evidence in Surface Tropical Water of Klang River Estuary, Malaysia. Environ. Geochem. Health 2021, 43, 3733–3748. [Google Scholar] [CrossRef] [PubMed]
- Uurasjärvi, E.; Hartikainen, S.; Setälä, O.; Lehtiniemi, M.; Koistinen, A. Microplastic Concentrations, Size Distribution, and Polymer Types in the Surface Waters of a Northern European Lake. Water Environ. Res. 2020, 92, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, E.; Minor, E.C.; Schreiner, K. Microplastic Abundance and Composition in Western Lake Superior As Determined via Microscopy, Pyr-GC/MS, and FTIR. Environ. Sci. Technol. 2018, 52, 1787–1796. [Google Scholar] [CrossRef]
- Yan, M.; Nie, H.; Xu, K.; He, Y.; Hu, Y.; Huang, Y.; Wang, J. Microplastic Abundance, Distribution and Composition in the Pearl River along Guangzhou City and Pearl River Estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef]
- Argeswara, J.; Hendrawan, I.G.; Dharma, I.G.B.S.; Germanov, E. What’s in the Soup? Visual Characterization and Polymer Analysis of Microplastics from an Indonesian Manta Ray Feeding Ground. Mar. Pollut. Bull. 2021, 168, 112427. [Google Scholar] [CrossRef]
- Sulistyowati, L.; Nurhasanah; Riani, E.; Cordova, M.R. The Occurrence and Abundance of Microplastics in Surface Water of the Midstream and Downstream of the Cisadane River, Indonesia. Chemosphere 2022, 291, 133071. [Google Scholar] [CrossRef]
- Šaravanja, A.; Pušić, T.; Dekanić, T. Microplastics in Wastewater by Washing Polyester Fabrics. Materials 2022, 15, 2683. [Google Scholar] [CrossRef]
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.-K. Quantifying Shedding of Synthetic Fibers from Textiles; a Source of Microplastics Released into the Environment. Environ. Sci. Pollut. Res. Int. 2018, 25, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Khosrovyan, A.; Doria, H.B.; Kahru, A.; Pfenninger, M. Polyamide Microplastic Exposure Elicits Rapid, Strong and Genome-Wide Evolutionary Response in the Freshwater Non-Biting Midge Chironomus Riparius. Chemosphere 2022, 299, 134452. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human Health Concerns Regarding Microplastics in the Aquatic Environment—From Marine to Food Systems. Sci. Total Environ. 2022, 823, 153730. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Manisha, A.; Roy, P.D.; Venkatramanan, S.; Chung, S.Y.; Muthukumar, P.; Jesuraja, K.; Elgorban, A.M.; Ahmed, B.; Elzain, H.E. Microplastics and Trace Metals in Fish Species of the Gulf of Mannar (Indian Ocean) and Evaluation of Human Health. Environ. Pollut. 2021, 291, 118089. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the Environment: Challenges in Analytical Chemistry—A Review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Weimer, R. Comparison of Nylon, Polyester, and Olefin Fibers Using FTIR and Melting. JASTEE 2015, 6, 1. [Google Scholar]
- Wahyuni, S.; Safira, A.; Pramesti, M. Investigating the Impact of Growth Mindset on Empowerment, Life Satisfaction and Turn over Intention: Comparison between Indonesia and Vietnam. Heliyon 2023, 9, e12741. [Google Scholar] [CrossRef]
- Polyamide Fabric in Indonesia|OEC. Available online: https://oec.world/en/profile/bilateral-product/polyamide-fabric/reporter/idn (accessed on 18 March 2023).
- Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.-S. Possibility Routes for Textile Recycling Technology. Polymers 2021, 13, 3834. [Google Scholar] [CrossRef]
- Yin, X.; Wu, J.; Liu, Y.; Chen, X.; Xie, C.; Liang, Y.; Li, J.; Jiang, Z. Accumulation of Microplastics in Fish Guts and Gills from a Large Natural Lake: Selective or Non-Selective? Environ. Pollut. 2022, 309, 119785. [Google Scholar] [CrossRef]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N.; Thomson, K.T. Microplastics in the Edible Tissues of Shellfishes Sold for Human Consumption. Chemosphere 2021, 264, 128554. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Hossini, H.; Nazmara, Z.; Mansouri, K.; Pirsaheb, M. Occurrence and Exposure Analysis of Microplastic in the Gut and Muscle Tissue of Riverine Fish in Kermanshah Province of Iran. Mar. Pollut. Bull. 2021, 173, 112915. [Google Scholar] [CrossRef]
- ACIAR Market Fishes of Indonesia/Jenis-Jenis Ikan Di Indonesia [Bilingual Publication: English/Indonesian]. Available online: https://www.aciar.gov.au/publication/books-and-manuals/market-fishes-indonesia-jenis-jenis-ikan-di-indonesia-bilingual-publication-english (accessed on 19 January 2023).
- Carpenter, K.E.; Krupp, F.; Jones, D.A.; Zajonz, U. The Living Marine Resources of Kuwait, Eastern Saudi Arabia, Bahrain, Qatar, and the United Arab Emirates. In FAO Species Identification Field Guide for Fishery Purposes; FAO: Hot Springs, VA, USA, 1997; ISSN 1020-4547. [Google Scholar]
- Mele, S.; Saber, S.; Gómez-Vives, M.J.; Garippa, G.; Alemany, F.; Macías, D.; Merella, P. Metazoan Parasites in the Head Region of the Bullet Tuna Auxis Rochei (Osteichthyes: Scombridae) from the Western Mediterranean Sea. J. Helminthol. 2015, 89, 734–739. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Du, F. The Complete Mitochondrial Genome of Bullet Tuna (Auxis Rochei) from South China Sea. Mitochondrial. DNA Part B 2019, 4, 1526–1527. [Google Scholar] [CrossRef]
- Baeck, G.W.; Quinitio, G.F.; Vergara, C.J.; Kim, H.J.; Jeong, J.M. English Diet Composition of Bullet Mackerel, Auxis rochei (Risso, 1810) in the Coastal Waters of Iloilo, Philippines. Korean J. Ichthyol. 2014, 26, 349–354. [Google Scholar]
- The Living Marine Resources of Somalia. Available online: https://www.fao.org/3/v8730e/v8730e00.htm (accessed on 19 January 2023).
- Cardona, L.; Álvarez de Quevedo, I.; Borrell, A.; Aguilar, A. Massive Consumption of Gelatinous Plankton by Mediterranean Apex Predators. PLoS ONE 2012, 7, e31329. [Google Scholar] [CrossRef]
- Rummer, J.L.; Binning, S.A.; Roche, D.G.; Johansen, J.L. Methods Matter: Considering Locomotory Mode and Respirometry Technique When Estimating Metabolic Rates of Fishes. Conserv. Physiol. 2016, 4, cow008. [Google Scholar] [CrossRef]
- Ollé-Vilanova, J.; Pérez-Bielsa, N.; Araguas, R.M.; Sanz, N.; Saber, S.; Macías, D.; Viñas, J. Larval Retention and Homing Behaviour Shape the Genetic Structure of the Bullet Tuna (Auxis Rochei) in the Mediterranean Sea. Fishes 2022, 7, 300. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase; World Wide Web Electronic Publication. 2023. Available online: www.fishbase.org (accessed on 15 January 2023).
- Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. Available online: https://repository.library.noaa.gov/view/noaa/10296 (accessed on 19 January 2023).
- Lopes, C.; Fernández-González, V.; Muniategui-Lorenzo, S.; Caetano, M.; Raimundo, J. Improved Methodology for Microplastic Extraction from Gastrointestinal Tracts of Fat Fish Species. Mar. Pollut. Bull. 2022, 181, 113911. [Google Scholar] [CrossRef]
- Savino, I.; Campanale, C.; Trotti, P.; Massarelli, C.; Corriero, G.; Uricchio, V.F. Effects and Impacts of Different Oxidative Digestion Treatments on Virgin and Aged Microplastic Particles. Polymers 2022, 14, 1958. [Google Scholar] [CrossRef]
- Valente, T.; Ventura, D.; Matiddi, M.; Sbrana, A.; Silvestri, C.; Piermarini, R.; Jacomini, C.; Costantini, M.L. Image Processing Tools in the Study of Environmental Contamination by Microplastics: Reliability and Perspectives. Environ. Sci. Pollut. Res. Int. 2023, 30, 298–309. [Google Scholar] [CrossRef]
- Hebner, T.S.; Maurer-Jones, M.A. Characterizing Microplastic Size and Morphology of Photodegraded Polymers Placed in Simulated Moving Water Conditions. Environ. Sci. Process Impacts 2020, 22, 398–407. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Fang, J.K.-H. Effects of Temperature and Particle Concentration on Aggregation of Nanoplastics in Freshwater and Seawater. Sci. Total Environ. 2022, 817, 152562. [Google Scholar] [CrossRef] [PubMed]





| Assignment | Wavenumbers/cm−1 | ||
|---|---|---|---|
| N. thynnoides | A. rochei | C. teres | |
| N-H stretching | 3447 | 3434 | 3422 |
| CH2 stretching (asymmetric) | 2926 | 2966 | 2976 |
| CH2 stretching (symmetric) | 2856 | 2925 | - |
| Amide I stretching | 1645 | 1641 | 1644 |
| Amide II stretching | 1585 | 1581 | 1580 |
| N-H deformation and CH2 wagging | 1470 and 1419 | 1464 and 1409 | 1451 and 1409 |
| C-C stretching | 1052 | 1034 | 1036 |
| aromatic rings | 874 | 872 and 775 | 874 and 780 |
| CC bending and deformation | 604 and 567 | 603 and 566 | 577 |
| Species | Marine Habitats | Feeding Habitats | Body Length (cm) | Wet Weight (g) |
|---|---|---|---|---|
| Naso thynnoides | Pelagic | Zooplanktons, algae | 27.43 ± 0.60 | 274.70 ± 6.74 |
| Auxis rochei | Pelagic | Zooplanktons, small fish, crustaceans | 23.33 ± 0.76 | 149.53 ± 10.76 |
| Caesio teres | Midwater | Zooplanktons | 28.33 ± 2.31 | 342.53 ± 5.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyastuti, S.; Abidin, A.S.; Hikmaturrohmi, H.; Ilhami, B.T.K.; Kurniawan, N.S.H.; Jupri, A.; Candri, D.A.; Frediansyah, A.; Prasedya, E.S. Microplastic Contamination in Different Marine Species of Bintaro Fish Market, Indonesia. Sustainability 2023, 15, 9836. https://doi.org/10.3390/su15129836
Widyastuti S, Abidin AS, Hikmaturrohmi H, Ilhami BTK, Kurniawan NSH, Jupri A, Candri DA, Frediansyah A, Prasedya ES. Microplastic Contamination in Different Marine Species of Bintaro Fish Market, Indonesia. Sustainability. 2023; 15(12):9836. https://doi.org/10.3390/su15129836
Chicago/Turabian StyleWidyastuti, Sri, Angga Susmana Abidin, Hikmaturrohmi Hikmaturrohmi, Bq Tri Khairina Ilhami, Nanda Sofian Hadi Kurniawan, Ahmad Jupri, Dining Aidil Candri, Andri Frediansyah, and Eka Sunarwidhi Prasedya. 2023. "Microplastic Contamination in Different Marine Species of Bintaro Fish Market, Indonesia" Sustainability 15, no. 12: 9836. https://doi.org/10.3390/su15129836
APA StyleWidyastuti, S., Abidin, A. S., Hikmaturrohmi, H., Ilhami, B. T. K., Kurniawan, N. S. H., Jupri, A., Candri, D. A., Frediansyah, A., & Prasedya, E. S. (2023). Microplastic Contamination in Different Marine Species of Bintaro Fish Market, Indonesia. Sustainability, 15(12), 9836. https://doi.org/10.3390/su15129836