Abstract
Peri-urban agriculture is becoming a potential step to promote sustainable and environmental food production systems. Our aim was to study the effect of biochar application at various rates on faba bean growth, cellulose decomposition, nodulation, and selected enzyme activities associated with carbon cycling in clay and sandy soils collected from peri-urban agricultural areas near the city of El-Minia, Egypt. To achieve this aim, incubation and pot experiments were conducted under controlled greenhouse conditions using clay and sandy soil. Among the studied treatments, using biochar at the rate of 3 kg/sq·m was the most effective soil amendment followed by biochar at the rate of 2 kg/sq·m. At 60 days of incubation, the count of cellulose-decomposing microorganisms reached a high level in both clay and sandy soil, and then decreased after 90 days, regardless of the biochar rate. The response of the cellulose-decomposer ratio (Fcd/Bcd) was positively correlated with biochar rates and incubation time. The obtained results showed significant increases in fresh and dry weight in clay soil compared to sandy soil. In any case, the use of biochar as a soil amendment enhanced soil health, soil microbial communities, and increased cellulose-decomposing microorganisms, thus improving faba bean nodulation and growth.
1. Introduction
Urban farming might aid in addressing a city’s social, economic, and environmental problems. By decreasing heat during the summer season and retaining water during heavy rains, plants can enhance the Earth’s climate. Recently, as a result of the intense urban sprawl, notably in Egypt, and encroachment on agricultural lands, it was very important to pay attention to semi-urban agricultural areas. Cities create a lot of organic waste, such as leftovers from recently harvested plants. Compost and biochar made from organic waste can be utilized in urban gardening. Soil organic matter and its biodegradation are very important to agroecosystem productivity; plant wastes afford the main source of this organic matter. Therefore, organic matter decomposition is one of the most important processes occurring in soils [1,2,3].
The most important carbonaceous substance created by higher plants is cellulose, which is likely the most abundant organic compound in nature [2,4]. Since plants in most cases contain up to 60% cellulose [5], cellulose decomposition is a key driver of soil microorganisms, and it is dynamic to energy flows beyond soils and to the cycling of C, N, P, and S (the decomposition of cellulose is usually convoyed by the immobilization of these nutrient elements).
The decomposition of cellulose is essentially a specialized depolymerization process followed by hydrolysis to glucose, which is quickly utilized as an energy source by the majority of heterotrophic soil microorganisms. By recycling vital plant nutrients through the decomposition and mineralization of organic carbonaceous materials, soil microbes serve a variety of roles in soil fertility [1,6,7]. Cellulose, the most prevalent carbohydrate generated by plants, is recycled by certain cellulolytic microorganisms, which play a significant role in the biosphere.
In recent years, attention has been focused on the fast rise in the use of biochar in agriculture. Biochar has been considered a potential approach for sustainable agricultural systems and improving the long-term sustainable use of soils for the growth of plants and soil microorganisms [8,9,10]. Biochar is a carbon-rich substance produced when biomass is burned without oxygen (pyrolysis), and its potential to improve soil fertility has been documented universally [11,12,13,14,15]. Biochar significantly impacts the soil microbial communities directly and indirectly by altering the soil’s physicochemical functions [16,17,18]. Biochar addition improved cellulose decomposition because of enlarged microbial activity with increased moisture content, and it enhanced the decomposition development rather than changing the decomposition pattern [19,20].
The application of biochar to soil has the potential to sequester carbon in the long range because of its high consistency and largescale production possibility [15,20]. Furthermore, research by Bi et al. [21] supported the claim that biochar fertilization enhanced soil organic matter and total nitrogen levels and dissolved organic carbon. They also detected that incorporating biochar into the soil significantly increased crop yield and nitrogen absorption. On the other hand, the biological N2 fixation process is carried out by both nodule-forming and free-living bacteria. All crops benefit from nitrogen sources and from nitrogen fixation. In order to promote sustainable N inputs into agroecosystems, increased nodulation after biochar application is foreseeable [22]. However, biochar technology is still quite novel, and it is yet unclear how various global conditions may affect the long-term fate of biochar in the ecosystem. The potential of biochar amendment in cellulose decomposition has not been well-studied. Although biochar appears to be beneficial for several chemical parameters of soil, the effects of biochar on soil microbes that decompose cellulose have received far less research [23,24,25]. Furthermore, there is hardly any research on how biochar affects microbial communities that were present in the cellulose decomposition process in soils. Therefore, the goals of the current investigation were to study the following: (a) improve our understanding of biochar’s effect on the cellulose decomposition process; (b) analyze the impact of different biochar application rates on cellulose-decomposing microorganisms and cellulose decomposition rates in two different soils collected from peri-urban agricultural regions near El-Minia city; and (c) study the effects of different biochar application rates on plant growth, nodulation, and selected enzyme activities related to the carbon cycle. It was hypothesized that adding biochar using high rates to the soil would encourage cellulose breakdown and vastly increase the number of cellulose-decomposing microorganisms and nodules.
2. Materials and Methods
2.1. Soils Used
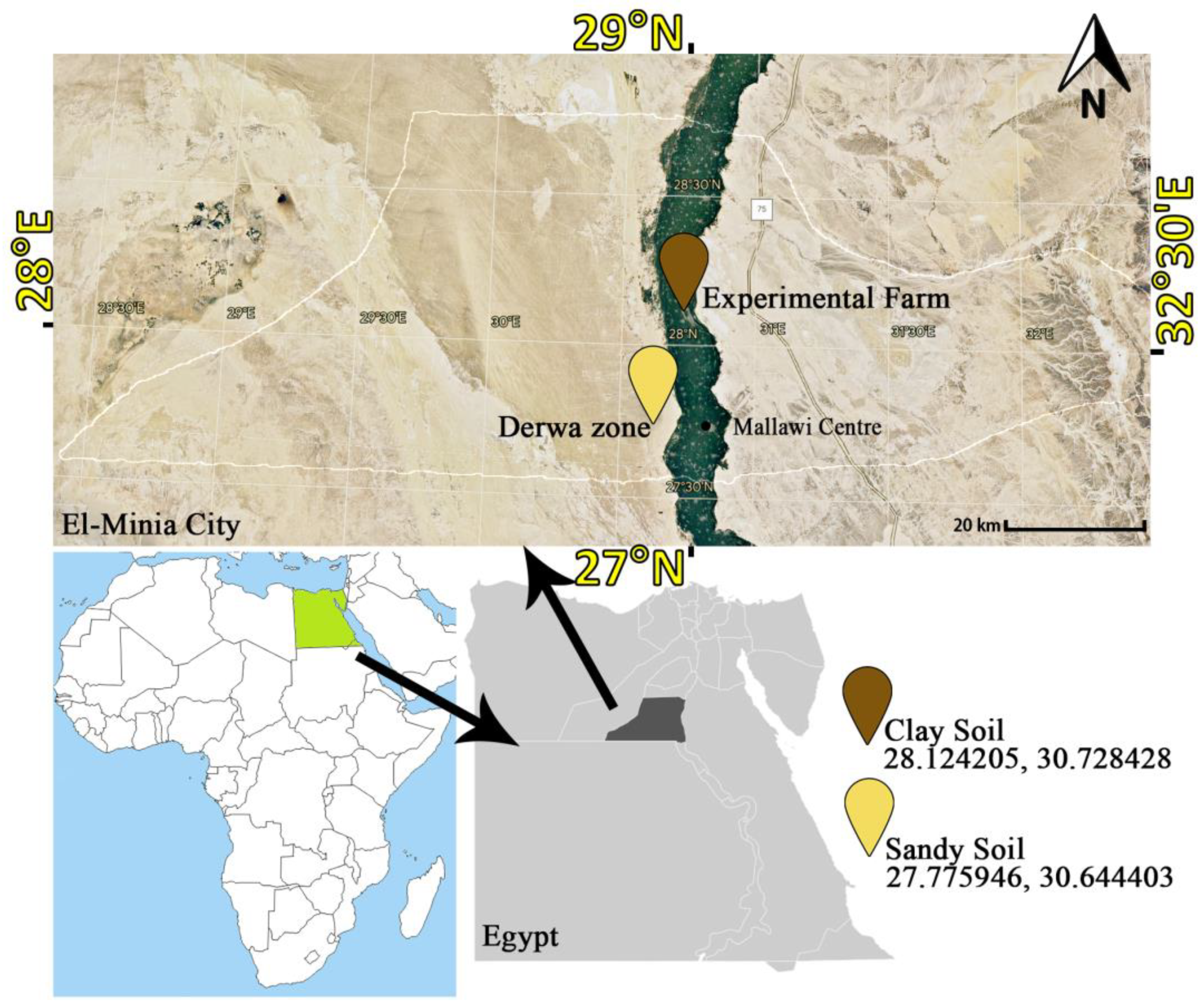
Two soil types were employed, each having distinct properties collected from peri-urban agricultural regions near El-Minia city. The location and source are presented in Figure 1. (a) Clay soil was collected from the Experimental Farm of Minia University, El-Minia, Egypt. (b) Newly reclaimed sandy soil was obtained from the Derwa zone, Mallawi Centre, El-Minia, Egypt. For the incubation experiment, each soil sample was taken from the top 20 cm layer and was air-dried, mixed, and passed through a 2 mm sieve. Some physical and chemical analyses according to Page et al. [26] of both soils used are presented in Table 1.
Figure 1.
Location of soil source used in this study.

Table 1.
The physicochemical properties of soils under study.
2.2. Source and Type of Biochar
After 30 min at 600 °C, the corncobs were slowly pyrolyzed to produce biochar. Before being used for the incubation or cellulose decomposing rate experiments, the biochar was air-dried and then finely powdered to pass through a 2 mm sieve. The actual amount of biochar applied to the incubation and cellulose decomposition rate experiments was equal to the application rates of 0 (control), 10 (B1), 20 (B2), 30 (B3), and 40 t ha−1 (B4), respectively. Some of the physiochemical properties of the subsamples of dried, powdered, and sieved soil and biochar were determined. The majority of the methods were standard methods for soil analysis [26]. Some important characteristics of the studied corncob biochar are provided in Table 2.

Table 2.
Physiochemical properties of the corncob biochar.
2.3. Structure Profiling of Biochar
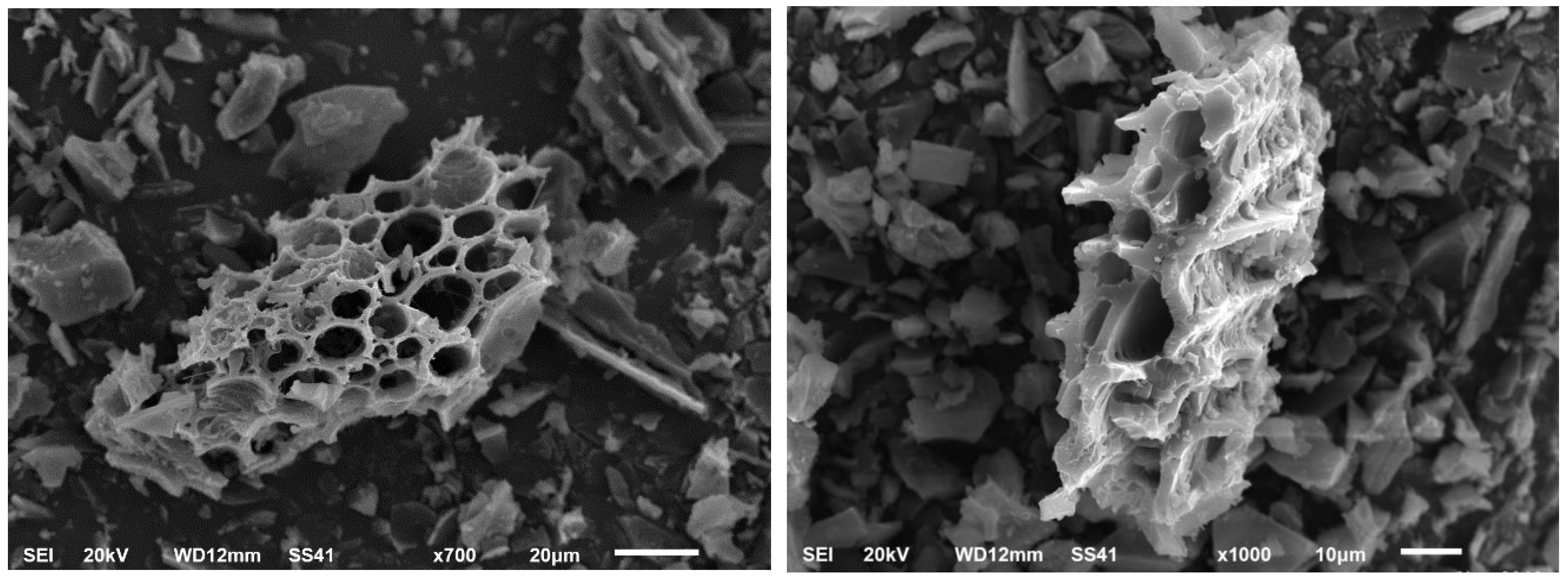
The structure and surface morphology of the biochar sample were determined using a scanning electron microscope (JEOL Model JSM-5510 SEM, Tokyo, Japan) at 20 kV imaging at different degrees of magnification (700 and 1000) (Figure 2).
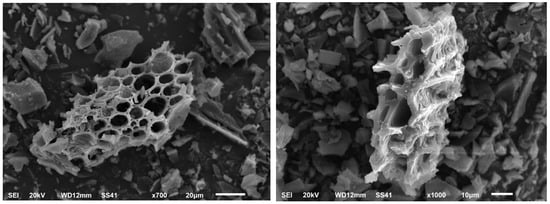
Figure 2.
SEM image of biochar derived from corncob.
2.4. Incubation Experiments
2.4.1. Effect of Biochar Application at Different Rates in Cellulose-Decomposing Bacteria, Fungi, and Actinomycetes
One-kilogram portions of either clay or sandy soil were put in plastic pots with a depth of 15 cm and a diameter of 17 cm to evaluate the impact of biochar (B) on the counts of cellulose-decomposing bacteria, fungi, and actinomycetes. The soil moisture levels for each pot were controlled at 60% of soil water saturation. The following rates of biochar were used: 0 (control), 10 (B1), 20 (B2), 30 (B3), and 40 t ha−1 (B4) which is equivalent to 0, 1, 2, 3, and 4 kg/sq·m, respectively. After combination, the pots were incubated for 13 weeks at 30 °C in the dark. Distilled water was added to the soil to compensate for evaporation losses to maintain a consistent moisture level. For each treatment, three pots were made as replicates, and samples were obtained after 15, 30, 60, and 90 days to count the cellulose-decomposing microorganisms (bacteria, fungi, and actinomycetes).
Nutrient agar medium containing carboxy methyl cellulose (CMC) 0.5 (w/v) was employed to ascertain the counts of cellulose-decomposing bacteria and actinomycetes [27]. Czapek Dox’s agar medium with CMC 0.1% (w/v) was used to count the fungi that decompose cellulose [28]. Plates were incubated for 5 days for growth of colonies and stained with 1% Congo Red dye for 0.5–1 h followed by distaining with 1 M NaCl solution for 15–20 min. Cellulolytic microorganisms on CMC plates were only counted around the colonies that were surrounded by colorless zones. Also, the ratio of fungal/bacterial cellulose decomposers (Fcd/Bcd) was determined to evaluate the sustainability of farming systems [29].
2.4.2. Microbial Biomass Resistance Index (MB-RS)
The microbial biomass resistance index (MB-RS) was calculated according to the counts of cellulose-decomposing bacteria, fungi, and actinomycetes positively or negatively affected by soil amendments with biochar at different rates using the equation proposed by Orwin and Wardle [30]:
where D0 is the difference between control sample (C0) and biochar application rates (P0) after 15, 30, 60, and 90 days of incubation.
2.5. Cellulose Decomposition Rates
This experiment was carried out to compare the existence of cellulose-decomposing microorganisms in soils enriched with biochar at different application rates to soils containing biochar-enriched filter paper as a source of cellulose. Nearly all the structural components of the filter paper (roughly 95%) were composed of cellulose. The methodology outlined by Grossbard and Wingfield [31] and Hiroki and Watanabe [32] was used with a few minor adjustments.
One sheet of 9 cm diameter Whatman No. 1 ashless filter paper was dried overnight at 40 °C, weighed, and put beneath nylon mesh in 9 cm diameter sterilized Petri dishes. Over the nylon mesh in the dish, fifty grammes of either clay or sandy soil mixed with different levels of biochar were applied. Throughout the experiment, the soil moisture was maintained at 60% of its water holding capacity, and plates were incubated at a temperature of 30 ± 2 °C. After 21 days, the filter papers were taken out the soil-filled nylon mesh, washed, dried overnight at 40 °C, and weighed. The percentage of cellulose decomposition was calculated as:
where A is the filter paper’s initial weight and B is the filter paper’s final weight after incubation. Each treatment was carried out five times. In a different experiment, the filter paper itself received the same amounts of biochar treatment (soil was left untreated). Untreated filter paper as a control was involved for contrast.
(A − B)/A × 100
2.6. Greenhouse Experiments
The greenhouse experiments’ purpose was to study the effect of different biochar rates on some plant quality parameters and selected enzyme activity (cellulase and β-glucosidase) of one of the most fundamental leguminous crops [faba bean (Vicia faba)]. A complete randomized design (CRD) pot experiment was conducted under greenhouse conditions with clay and sandy soils. Each soil was well-mixed with biochar using 4 rates [10 (B1), 20 (B2), 30 (B3), and 40 t ha−1 (B4)]. In this experiment, 5 kg of soil was placed in plastic pots (22.5 cm diameter), and the moisture content was regulated to 60% of the WHC using distilled water.
The plants (six seeds pot−1) were cultivated for 90 days before being thinned to three plants after 15 days. The Agricultural Microbiology Department at Minia University in Egypt provided the requisite rhizobium inoculants, including a minimum of 4 × 107 viable cells mL−1, which were applied to the seeds prior to planting. During plant growth, the soil moisture in each pot was maintained at 60% of the field’s capacity by randomly weighing the pots and adding water as needed. Fresh and dry plant weights were measured using a digital balance that can measure masses from 0.001 to 300 g. Results were reported to two significant digits. Nodules were removed from roots and the number was reported. Nodules were imaged and dry weights were reported.
2.7. Enzymatic Hydrolysis
The effect of biochar at different application rates on cellulase and β-glucosidase activity was studied. The activity of cellulase (mg glucose kg−1 soil) and β-glucosidase was determined as described by Deng and Tabatabai [4] and Eivazi and Tabatabai [33]. The results are the average of three replicate analyses calculated on an oven-dried basis. The activities of β-glucosidase were estimated using the methodology described by Tabatabai [34], which involves incubating the soil sample with the colorless specific substrate (1 mL of 50 mM p-nitrophenyl-β_d-glucopyranoside) together with p-nitrophenyl. The quantity of p-nitrophenol produced at the conclusion of the incubation time is then calculated and represented in μg of PNP 1 kg of soil−1 h−1. Analysis was performed after 90 days from planting.
2.8. Statistical Analysis
A complete randomized design (CRD) with five and three replicates was used in the incubation and greenhouse experiments, respectively. The SAS system was used to complete a variance test analysis on the experimental data [35] and differences among means were calculated using least significant difference (LSD) methods at p < 0.05 and p < 0.01. The correlations between cellulose decomposition and biochar application rates were shown in a correlation matrix based on Pearson’s correlation coefficients using p = 0.05 to imply the 95% confidence degrees.
3. Results and Discussion
3.1. Effects of Biochar Amendment at Different Rates on Cellulose Decomposition Rate
3.1.1. Effect on Cellulose-Decomposing Microorganisms
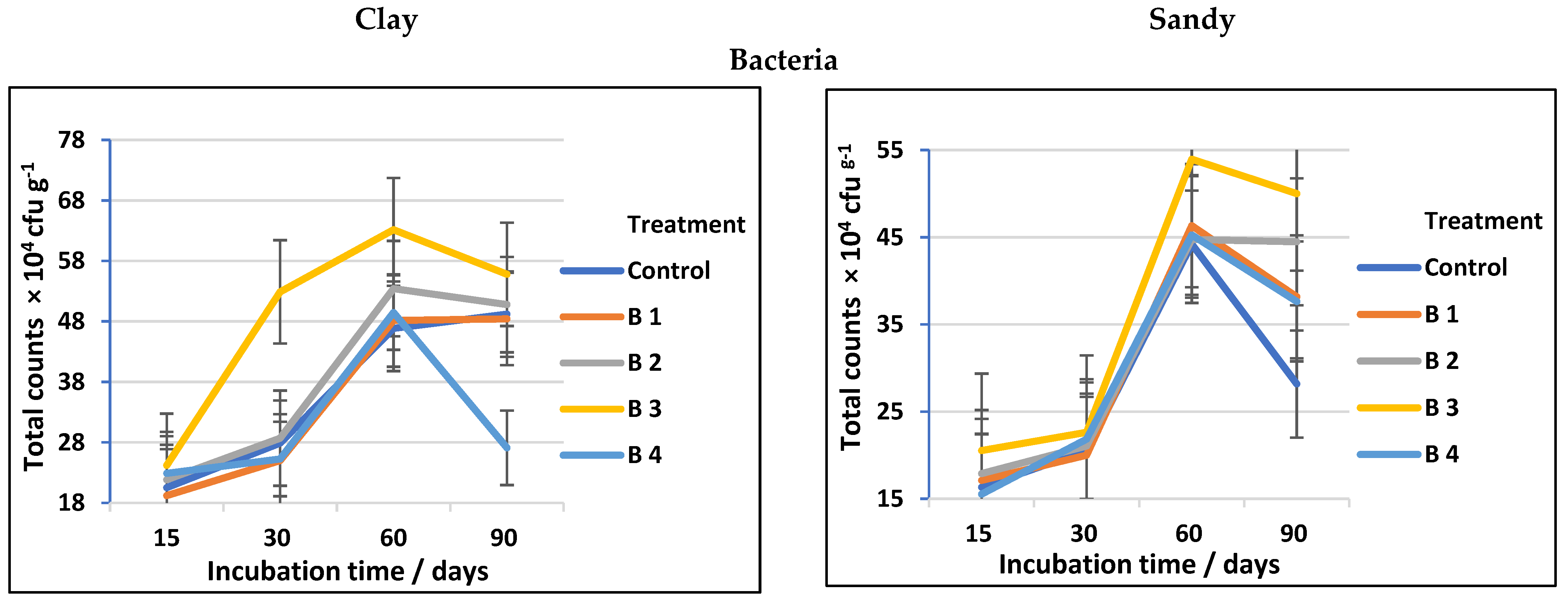
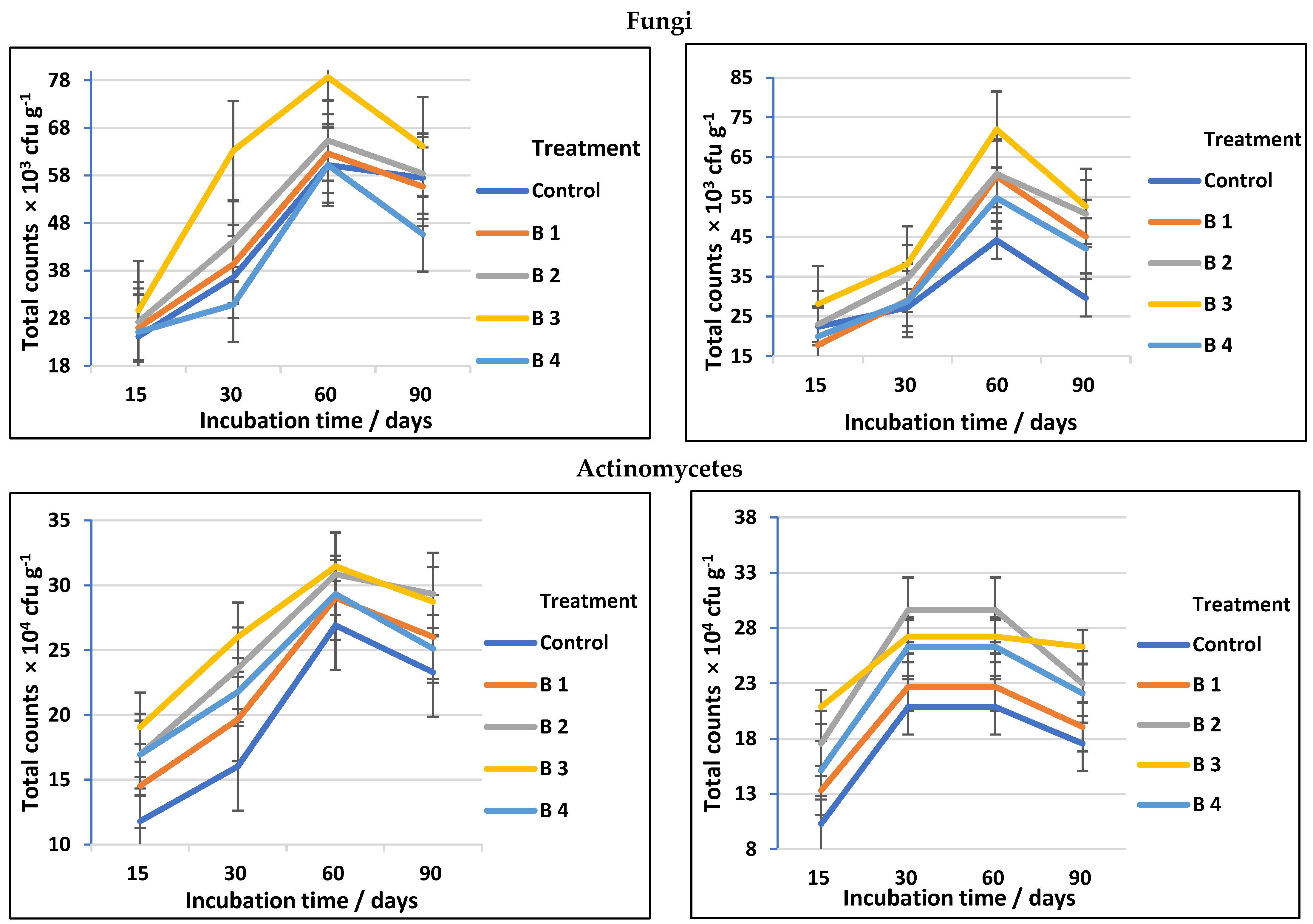
Regardless of the obvious advantages of biochar for altering soil chemical parameters, its impacts on cellulose-decomposing microorganisms have received far less investigation [2]. The data presented in Table 3 and followed by Figure 3 show a significant increase in total counts of cellulose-decomposing bacteria, fungi, and actinomycetes in both clay and sandy soils, using biochar at different rates compared to the control. Among the treatments investigated, using biochar at the rate of 30 t ha−1 (B3) was the most effective soil amendment followed by biochar at the rate of 20 t ha−1 (B2). At 60 days of incubation, the counts of cellulose-decomposing microorganisms reached a high level in both clay and sandy soil, then decreased after 90 days of incubation regardless of biochar addition (Figure 3). This completely matches Han et al. [36], who stated that the soil microbial efficient activities and community assembly might be thought of as useful indicators in assessing the impacts of biochar on soil biological characteristics and that biochar has positive effects on the soil bacterial community.

Table 3.
Analysis of variance (ANOVA) of effects of different rates of biochar on the total counts of selected cellulose-decomposing microorganisms in two different soils.
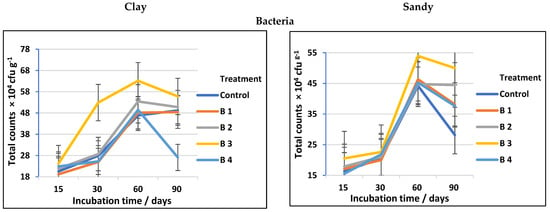
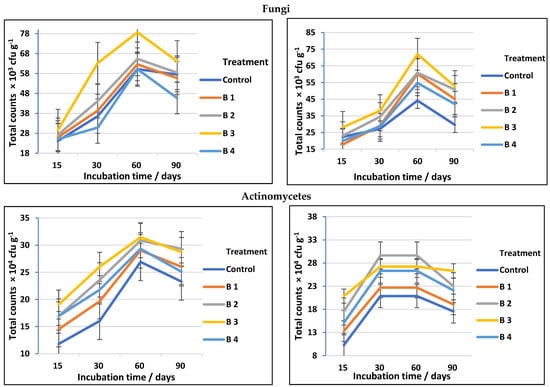
Figure 3.
Total counts of cellulose-decomposing bacteria, fungi, and actinomycetes as affected by biochar at different application rates in clay and sandy soils.
The active soil biota has a significant impact on how quickly organic matter decomposes and turns into humus. When a breakdown of soil organic matter is a naturally occurring biological process, soil microbial biomass is essential; hence, any decline in microbial variety or plenty may negatively affect the availability of nutrients in soils and absorption for plant growth [7]. Over the years, a wide range of cellulolytic microbes, primarily fungus, bacteria, and actinomycetes have been discovered. Different microorganisms create cellulases with varying structures and modes of operation [37]. In soils where a widespread variety of microbes, including bacteria, fungi, algae, and actinomycetes, are implied in the decomposition of cellulose, different groups of organisms can be distinguished. In general, bacteria, fungi, and actinomycetes have been thought of as the main microorganisms for cellulose decomposition.
In general, the counts of cellulose-decomposing microorganisms in soil amended with biochar may vary depending on soil type, biochar rates, and agricultural practices. Cellulose-decomposing microorganisms (bacteria, actinomycetes, and fungi) are prevalent and primary parts of soils; they play considerable roles in the recycling of soil–C and metallic elements, making them accessible to plants [38,39]. The biochar used in our study has a significant effect on the counts of fungal decomposing microorganisms in both clay and sandy soil, which is fully equivalent to the results obtained by Gul et al. [40], who stated that biochar pores may provide a shielding habitat for soil microorganisms, and, because of their greater size, fungi are largely able to colonize biochar macropores, whilst bacteria may access smaller holes and may be more protected from grazing due to their smaller size. Additionally, the well-developed pore structure of biochar may offer microorganisms a dwelling environment. By investigating the pore habitats in biochar, it is believed that both bacteria and fungi will be better protected against predators or rivals [41]. On the other hand, the biochar impact on cellulose decomposer microbes was soil-specific (see Figure 3 and Figure 4). Our results (Table 3) are in agreement with Haddad and Lemanowicz [13], who found that counts of bacteria, fungi, and actinomycetes exhibited a significant enhancement after using different rates of biochar.

Figure 4.
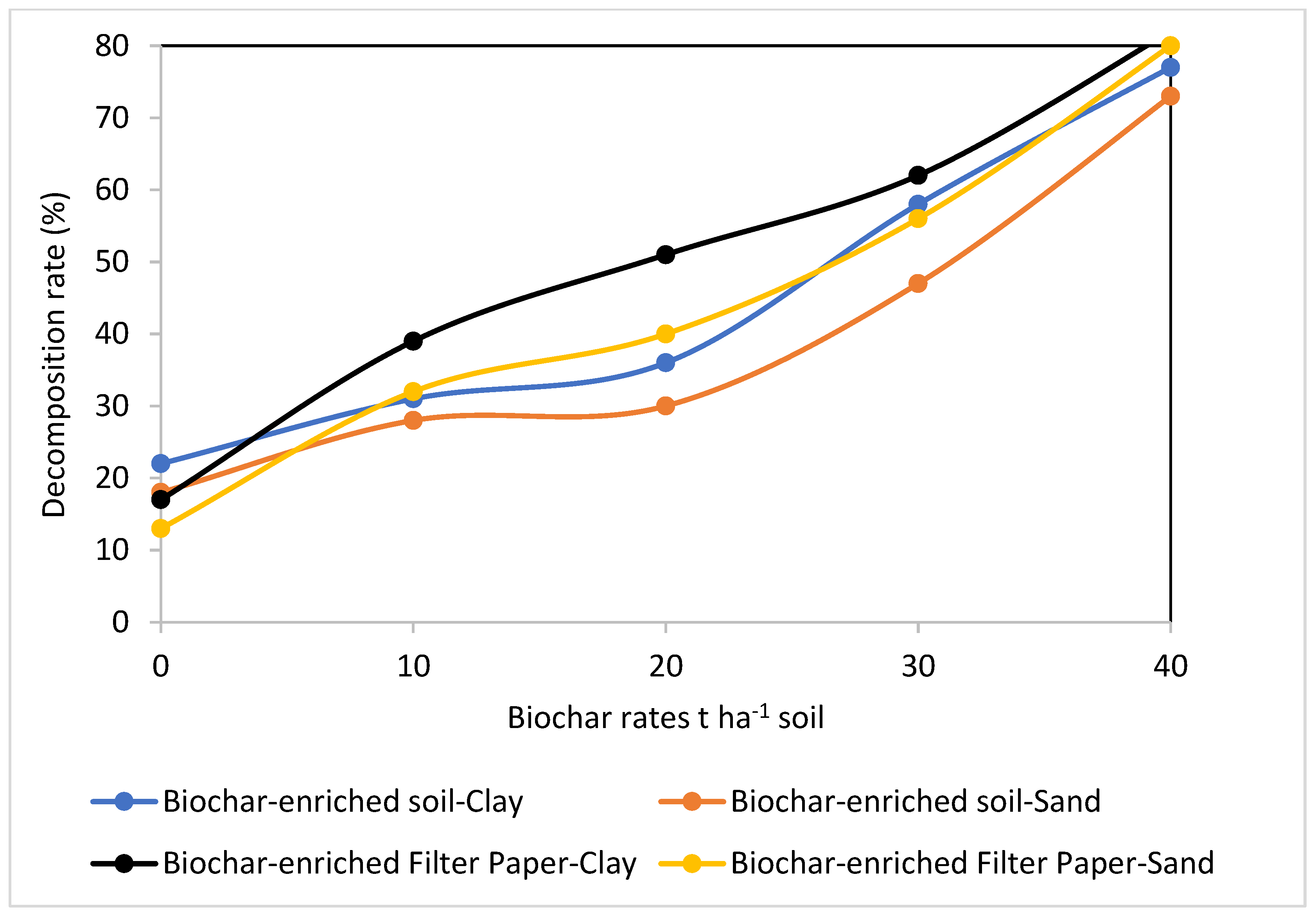
Cellulose decomposition as affected by soils incorporated with biochar at different rates. Control (0), B1 (10), B2 (20), B3 (30), B4 (40)/t ha−1.
Using biochar as a soil amendment would enhance the soil health and increase the cellulose decomposer counts. A scanning electron micrograph (SEM) of biochar samples made from the biomass is represented in Figure 2. Structural differences in terms of porosity in biochar particles can be studied through SEM images after the carbonization process of biomass. The SEM of the sample was found to be highly porous. Microorganisms can find a habitat on the surfaces and pores of biochar, and its addition enhances bulk density, pH, and the circulation of air, water, and nutrients within the soil matrix. These modifications to the soil’s physical and chemical characteristics provide microorganisms with more space and habitats with a wide variety of enlarged niches, which help to encourage their abundance and activity. Through better plant development, the direct positive impact of biochar on soil quality and microorganisms may result in the indirect supply of more habitats and niches for microorganisms [13].
To shield soil microorganisms from predatory soil microarthropods, biochar pores operate as a habitat and haven for species like bacteria (size range: 0.3 to 3 mm), fungi (2–80 mm), and protozoa (7–30 mm). Although biochar also contains micropores (2 nm) and mesopores (2–50 nm) that could store water and dissolved substances that are needed for microbial metabolism, macropores (>200 nm) in biochar are likely to represent most of the protected microbial habitats because they are the right size to accommodate bacteria [42,43].
3.1.2. Fungal/Bacterial Cellulose Decomposer Ratio (Fcd/Bcd)
In this study, the fungal/bacteria cellulose decomposer ratio (Fcd/Bcd) in soils amended with different rates of biochar was determined. The data given in Table 4 and Table 5 show that the Fcd/Bcd ratio significantly increased when using biochar at the rates of 10 t/ha and 20 t/ha (B1, B2) followed by biochar at the rate of 40 t/ha (B4) after 30 days of incubation time, and this decreased significantly after 60 days of incubation.

Table 4.
Fungal/bacterial cellulose decomposer ratio (Fcd/Bcd) of clay soil amended with biochar at different rates during 90 days of incubation.

Table 5.
Fungal/bacterial cellulose decomposer ratio (Fcd/Bcd) of sandy soil amended with biochar at different rates during 90 days of incubation.
For these experimental conditions, the response of the cellulose decomposer ratio (Fcd/Bcd) was positively correlated with biochar rates and incubation time with clay soil (Table 4 and Table 5). On the other hand, the sandy soil cellulose decomposer ratio (Fcd/Bcd) increased significantly when using high rates of biochar along with the incubation time (Table 5). The effects of biochar on fungi are more variable and influenced by factors such as biochar properties, biochar rates, soil type, and microbial community composition. This completely matches several studies which observed that the high rates of biochar (≥5%) in the long-term condition significantly increased soil F/B ratios with preferential stimulation of fungi [13,44,45].
The F/B was frequently used to evaluate the agricultural system’s sustainability [29] with valuable advantages including a more competent crop nutrient uptake mediated by mycorrhizal fungi. The F/B ratio was reduced by expanding the mineral N application rate, primarily due to the destruction of fungal growth. The present results support the previous findings of de Vries et al. [29], who found that adding organic amendments to soils enhanced the F/B ratio by promoting fungal development, whereas applying mineral nitrogen fertilization decreased it. This contrasts with the results obtained by Gomez et al. [46], who stated a significantly lower F:B ratio in four soils (two sandy loam, clayey, and clay loam) amended with biochar after one year of incubation. Moreover, biochar may have negative effects on certain fungal communities, inhibiting their growth and activity [47].
Overall, while biochar has the potential to enhance both bacteria and fungi in the soil, its effects can be complex and milieu-dependent. The specific outcomes may vary based on various factors, and more research is needed to fully understand the interactions between biochar, bacteria, and fungi in different soil environments.
3.1.3. Microbial Biomass Resistance Index (MB-RS)
According to Orwin and Wardle [30], the soil resistance indicator (RS) is a useful tool for gauging how microorganisms react to environmental stress. It may also be used to gauge how well the soil can continue to function in the presence of any source of stress, such as that brought on by increasing the soil carbon amendment [13].
The decreasing values of the (MB-RS) in the current investigation showed and confirmed a considerable impact of employing various rates of biochar on bacteria, fungi, and actinomycetes. Throughout the trial, the microbial biomass resistance index (MB-RS) values for selected cellulolytic microorganisms were positive. However, they varied according to the rates of biochar used, the length of the trial, and the soil texture (Table 6, Table 7 and Table 8). Lower values represent a convinced impact of biochar (low resistance) on microbial activity. This is in complete agreement with the results obtained by Haddad et al. [2], who confirmed that the values of the resistance index (RS) for soil microbes were positive and they differed depending on the dose of the heavy metal applied, incubation time, and soil type.

Table 6.
Microbial biomass resistance index (MB-RS) of bacteria in soils during 90 days of incubation.

Table 7.
Microbial biomass resistance index (MB-RS) of fungi in soils during 90 days of incubation.

Table 8.
Microbial biomass resistance index (MB-RS) of actinomycetes in soils during 90 days of incubation.
In the current study, increasing biochar rates produced a significant decrease in the microbial biomass resistance index (Table 6, Table 7 and Table 8). Lower MB-RS values were noticed in sandy soil compared to clay soil, revealing that sandy soil was less resistant to biochar application rates. The MB-RS values in both sandy and clay soil decreased when biochar was applied at 20 t h−1 soil or more during 90 days of incubation.
3.2. Effect of Biochar at Different Rates on Cellulose Decomposition Rates
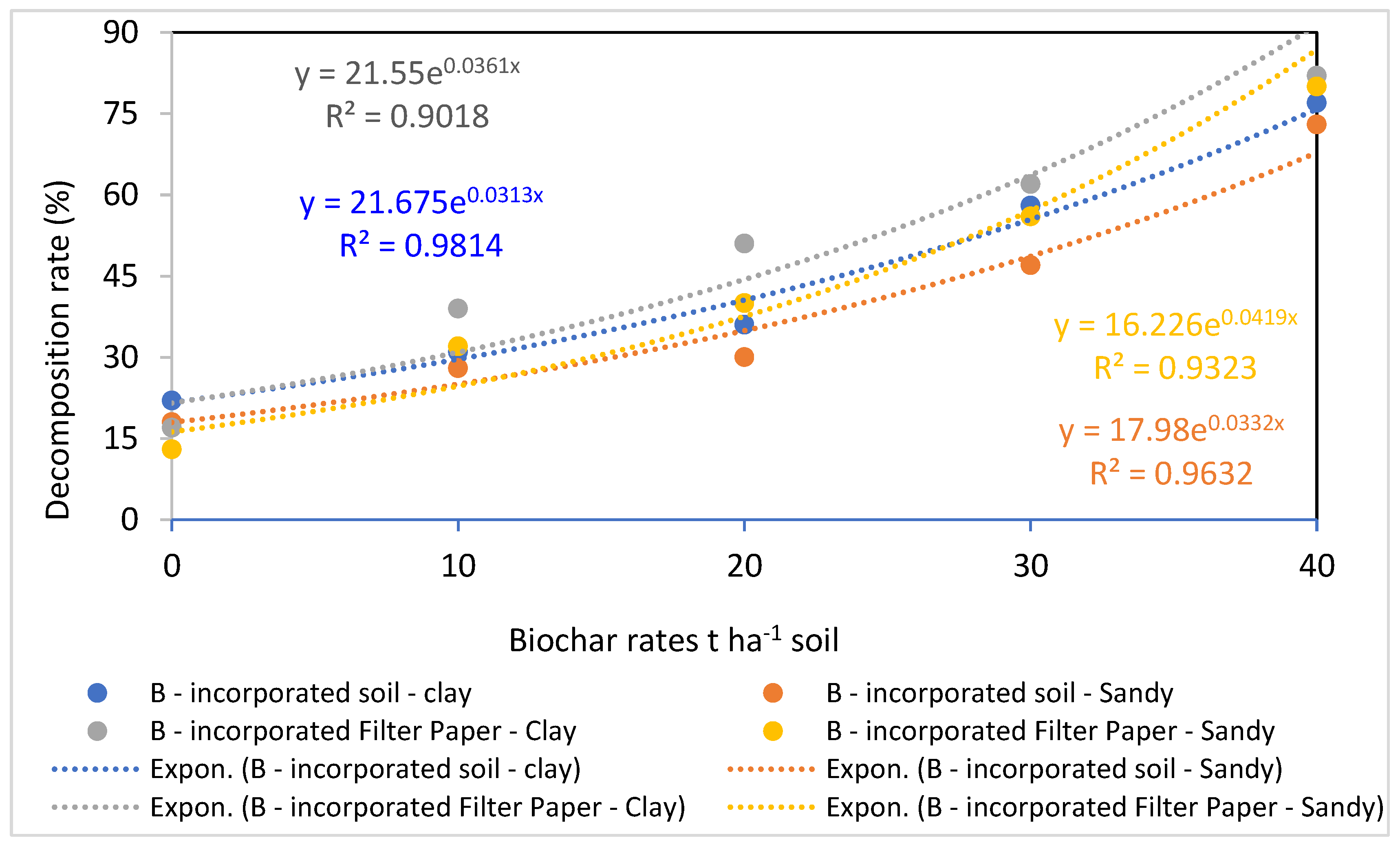
The data presented in Figure 4, Figure 5, Figure 6 and Figure 7 show how biochar affects cellulose decomposition at different application rates. In general, applying biochar at various application rates accelerated the rate of cellulose decomposition and had a considerably favorable impact on soil microbial activity. Compared to sandy soil, the examined clay soil showed a much faster rate of filter paper decomposition. Over a period of 21 days of incubation, significant variations in cellulose decomposition rates were found in clay soil as compared to sandy soil at 10, 20, 30, and 40 t ha−1 for biochar treatments. This indicated that these soils responded positively to the addition of more biochar (Figure 7).

Figure 5.
Cellulose decomposition as affected by filter paper enriched with biochar at different rates. Control (0), B1 (10), B2 (20), B3 (30), B4 (40)/t ha−1.
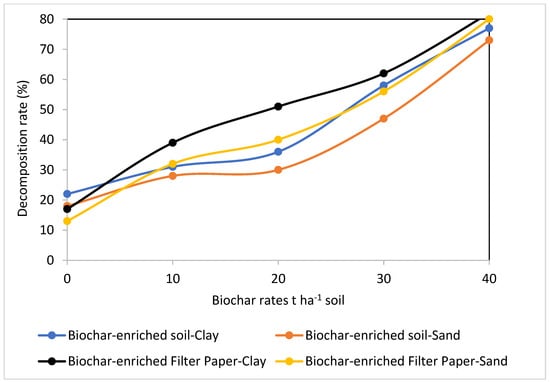
Figure 6.
Effect of biochar at different rates on cellulose decomposition in clay or sandy soils, estimated as percentage loss of the primary weight.
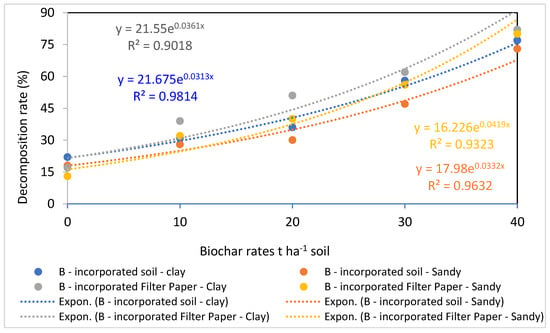
Figure 7.
Correlation between cellulose decomposition rates and biochar at different application rates.
Figure 4 and Figure 5 compare filter paper losses over a period of incubation as a percentage of the original paper weight for filter papers incorporated in clay or sandy soils supplemented with biochar at different rates. This approach of calculating decomposition rates determines the overall impact rather than identifying the specific microorganism involved in the decomposition process. In comparison to the control, filter papers and soils supplemented with biochar showed significant differences in the rates at which cellulose decomposed. In comparison to the biochar-enriched filter paper that was buried beneath soils, losses in biochar-enriched soil samples at all levels were noticeably slower (Figure 4, Figure 5, Figure 6 and Figure 7). This finding was attributed by Kaiser [48] to the higher sorption and adsorption capacities of biochar-enriched soils, where the humified and particulate organic matter fraction is frequently significantly metal-enriched in soils. However, filter papers that have been enriched with biochar are almost entirely composed of the more readily broken cellulose, accounting for 95% of the structural material.
Soil microbes play a critical role in soil fertility by converting organic matter into mineral forms of vital plant nutrients. Numerous studies assess how biochar affects soil microorganisms [8,14,25]; however, there is hardly any research on how biochar affects microbial communities that were present in the cellulose decomposition process in soils. As the decomposition rates increased to more than 80% at the application rate of 40 t ha−1 for both soils, the use of biochar at various rates appears to be the primary factor impacting the microbial decomposition of the filter paper, and consequently, cellulose, in the tested soils. When clay and sandy soils were studied, cellulose breakdown rates and biochar rates showed a strong correlation. This completely matches Haddad et al. [2], who stated that, among the contaminated clay and sandy soils examined, there was a strong correlation between the rates of cellulose degradation and the types and quantities of heavy metals.
In comparison to the lower applications of 10 and 20 t ha−1 of biochar, the higher application rates of biochar applied separately at 30 and 40 t ha−1 considerably boosted the breakdown rate of cellulose in both clay and sandy soils. This demonstrates how biochar increased the populations of useful microorganisms after 21 days of incubation [47]. An increase in cellulose decomposition rates occurred with increasing application rates and reliance on a particular soil in this study, demonstrating a clear correlation between the action of biochar and high application rates. The coefficients of association between the amounts of biochar and the rates of cellulose decomposition (Figure 7) demonstrated that this impact was distinct and dependent on the rate of biochar.
3.3. Effect of Biochar on Plant Growth and Nodulation
The use of biochar in agricultural soils can have a significant positive impact on plant productivity. However, it is still unclear how biochar rates, soil types, and their combinations impact the plant development and nodulation process.
In the current study, faba beans (V. faba) were cultivated in two soil types in the greenhouse at various biochar rates. Fresh weight, dry weight, nodule number, and nodule dry weight were assessed and are presented in Table 9. The results obtained showed significant increases in fresh and dry weight in clay soil compared to sandy when using a biochar rate of 30 t ha−1 (B3) followed by a biochar rate of 40 t ha−1 (B4). Research on biochar’s influence on plant growth is widespread. According to a recent meta-analysis by Jeffery et al. [49] and Dai et al. [50], which included more than 1254 individual studies, the positive impacts of adding biochar to soils outweighed the neutral and adverse effects (just one study found adverse effects). They discovered that biochar additions had on average a 10% impact on crop productivity. This seems to be a slight increase, which might be explained by the large variety of biochar and substrate types in drastically different environments. Regardless of the characteristics of the biochar and the soil conditions, the meta-analysis revealed that the overall effect of biochar on plant production was calculated to be 16.0 ± 1.26%. However, the interaction between the qualities of the biochar and the soil conditions may have a significant impact on how well the biochar improves plant development.

Table 9.
Fresh weight, dry weight, number, and weight of nodules for faba beans (V. faba) grown in two soil types in the greenhouse under different biochar rates.
In comparison to sandy soil, clay soil has a greater number of nodules. In both clay and sandy soil, adding biochar as a soil amendment clearly and favorably increased the number of nodules (Figure 8). This finding matches the results obtained by Egamberdieva et al. [51], who stated that, in order to improve the rate of rhizobium survival and soybean nodulation, biochar was utilized as an inoculant carrier and the ability of biochar to provide additional pore space and air to nodule bacteria which attach to the biochar surface of soil pores. Additionally, research shows that biochar created favorable circumstances for the symbiosis of N2-fixing bacteria, which is advantageous for soil remediation in heavy-metal-contaminated regions since it encourages revegetation by introducing nitrogen through symbiotic fixation [13].

Figure 8.
Vigorous nodules and faba bean plants grown in sandy soils amended at different biochar rates (photos: Abdelaziz Saleh).
As a result, the biochar’s stimulating effect on the number of nodules suggested that the biological nitrogen fixation (BNF) may provide more N to the concentration of nitrogen in the roots and shoots. Accordingly, Rondon et al.’s [52] pot experiment revealed that adding biochar boosted the amount of BNF-derived N in beans from 50% to 72%. Further data indicated that biochar increased the correlation between the number of nodules and the fresh and dry weight (Table 9).
3.4. Effect of Biochar on Cellulase and β-Glucosidase Activity of Soils
In comparison to the unamended soils examined, all biochar-amended soils generally exhibited significantly greater enzyme activity rates (Table 10). B4 was the most successful biochar level evaluated for activating cellulase and β-glucosidase activities, followed by B3. With activation percentages of 23.4 and 26.74% in the clay soil, B1 was the least effective in promoting cellulase and β-glucosidase activity. Sandy soil had activation percentages of 24.2 and 34%, respectively. Cellulase and β-glucosidase activation percentages for clay and sandy soil exceeded 60% when employing the maximum rate of biochar (B4). This agrees with Sun et al. [53], who found that biochar addition to soil increased urease, cellulase, and invertase activity by an average of 58.13%, 53.12%, and 16.54%, respectively.

Table 10.
Effects of biochar with different rates on selected enzyme activity in two different soils.
Improvements in soil physicochemical characteristics due to biochar addition may have boosted soil microbial and enzymatic activity and particularly cellulase functional groups that may also have contributed to soil fertility. This completely matched the results obtained by Haddad et al. [2], who confirmed that the relative inhibitory efficacy of cellulase and β-glucosidase was mostly determined by the type of heavy metals present in the soil and its physiochemical characteristics. The tested soils’ pH levels ranged from 7.7 for clay soil to 8.6 for sandy soil, showing a wide variation. Therefore, changes in soil pH and biochar reactions in soil or cellulose functional groups were the cause of the increased activation of cellulase and β-glucosidase activity in the sandy soil compared to clay soils in the presence of high quantities of biochar. Acosta-Martinez and Tabatabai [54], on the other hand, showed that altering soil pH by liming had a substantial impact on 14 enzyme activities related to the nitrogen, carbon, phosphorus, and sulfur cycles in soils. These findings suggested that the physiochemical characteristics of the soil and the properties and rates of biochar are reliable predictors of the effects of soil amendments on the rates and enzyme activities of cellulose decomposition.
When biochar was used as a soil amendment, the activation rate of soil β-glucosidase was significantly higher than that of cellulase (Table 10). As β-glucosidase is a member of the cellulose enzyme groups, the enzyme responsible for cellobiose hydrolysis and glucose generation, the augmentation of β-glucosidase is partially responsible for the increase in cellulase activity brought on by the addition of biochar. Due to the considerable functional redundancy of β-glucosidase activity, it appears that biochar application greatly increases β-glucosidase activity compared to cellulase. As a result, several bacteria or fungi were able to accelerate enzyme activity.
For a healthy soil ecosystem, microbial enzyme activity in soils conduct critical biochemical tasks. The cellulase enzyme system is crucial for the breakdown of cellulose into reducing sugars as well as serving as a general indication of biological activity in soil [4]. It is preferable to conduct further research on the effects of soil microbial diversity, especially enzymes engaged in the carbon, nitrogen, phosphorus, and sulfur cycles, on soils with high quantities of biochar.
4. Conclusions
Recently, biochar use in agriculture has been considered a potential approach to improve soil health and crop productivity. The results of this research stated that the application of biochar at different rates improved the soil microbial functional activities of bacteria and fungi and increased cellulose-decomposing microorganisms and soil health resulting in enhanced faba bean growth and nodulation. Among studied treatments, using biochar at the rate of 3 kg/sq·m was the most effective soil amendment followed by biochar at the rate of 2 kg/sq·m. Additionally, all biochar-amended soils generally exhibited significantly greater enzyme activity rates and created favorable circumstances for the symbiosis of N2-fixing bacteria in both clay and sandy soils. In any case, the use of biochar as a soil amendment enhanced soil health and soil microbial communities and increased cellulose-decomposing microorganisms, thus improving faba bean nodulation and growth. Further research under field conditions is necessary to confirm these findings, especially under arid conditions.
Author Contributions
Conceptualization, S.A.H., S.A. and O.S.; data curation, S.A.H., M.M.A.E.-A., H.A. and A.S.; formal analysis, S.A.H., H.A. and A.S.; funding acquisition, G.E.E.; investigation, O.S. and S.A.H.; methodology, H.A., A.S. and S.A.H.; project administration, O.S. and S.A.H.; resources, S.A.H. and G.E.E.; software, H.A.; supervision, O.S., S.A. and S.A.H.; validation, M.M.A.E.-A., G.E.E. and J.L.; visualization, O.S. and S.A.H.; writing—original draft, H.A., A.S. and S.A.H.; writing—review and editing, M.M.A.E.-A., J.L. and S.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Researchers Supporting Project Number (RSP2023R161), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to the Agricultural Microbiology Department at Minia University for their support in this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bekier, J.; Jamroz, E.; Walenczak-Bekier, K.; Uściła, M. Soil Organic Matter Composition in Urban Soils: A Study of Wrocław Agglomeration, SW Poland. Sustainability 2023, 15, 2277. [Google Scholar] [CrossRef]
- Haddad, S.A.; Lemanowicz, J.; Abd El-Azeim, M.M. Cellulose Decomposition in Clay and Sandy Soils Contaminated with Heavy Metals. Int. J. Environ. Sci. Technol. 2019, 16, 3275–3290. [Google Scholar] [CrossRef]
- Weber, J.; Kocowicz, A.; Bekier, J.; Jamroz, E.; Tyszka, R.; Debicka, M.; Parylak, D.; Kordas, L. The Effect of a Sandy Soil Amendment with Municipal Solid Waste (MSW) Compost on Nitrogen Uptake Efficiency by Plants. Eur. J. Agron. 2014, 54, 54–60. [Google Scholar] [CrossRef]
- Deng, S.P.; Tabatabai, M.A. Cellulase Activity of Soils; Elsevier: Amsterdam, The Netherlands, 1994; Volume 26. [Google Scholar]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry; Academic Press Inc.: London, UK, 1989. [Google Scholar]
- Haddad, S.A.; Tabatabai, M.A.; Abdel-Moneim, A.M.A.; Loynachan, T.E. Inhibition of Nodulation and Nitrogen Nutrition of Leguminous Crops by Selected Heavy Metals. Air Soil and Water Research 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Xie, Y.; Fan, J.; Zhu, W.; Amombo, E.; Lou, Y.; Chen, L.; Fu, J. Effect of Heavy Metals Pollution on Soil Microbial Diversity and Bermudagrass Genetic Variation. Front. Plant Sci. 2016, 7, 755. [Google Scholar] [CrossRef] [PubMed]
- Hardy, B.; Sleutel, S.; Dufey, J.E.; Cornelis, J.T. The Long-Term Effect of Biochar on Soil Microbial Abundance, Activity and Community Structure Is Overwritten by Land Management. Front. Environ. Sci. 2019, 7, 110. [Google Scholar] [CrossRef]
- Gunarathne, N.; de Alwis, A.; Alahakoon, Y. Challenges Facing Sustainable Urban Mining in the E-Waste Recycling Industry in Sri Lanka. J. Clean. Prod. 2020, 251, 119641. [Google Scholar] [CrossRef]
- Abd El-Azeim, M.M.; Menesi, A.M.; Abd El-Mageed, M.M.; Lemanowicz, J.; Haddad, S.A. Wheat Crop Yield and Changes in Soil Biological and Heavy Metals Status in a Sandy Soil Amended with Biochar and Irrigated with Drainage Water. Agriculture 2022, 12, 1723. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Earthscan: London, UK, 2009. [Google Scholar]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of Biochar on Alleviation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Grown on Cd-Contaminated Saline Soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Haddad, S.A.; Lemanowicz, J. Benefits of Corn-Cob Biochar to the Microbial and Enzymatic Activity of Soybean Plants Grown in Soils Contaminated with Heavy Metals. Energies 2021, 14, 5763. [Google Scholar] [CrossRef]
- Haddad, S.A.; Mowrer, J.; Thapa, B. Biochar and Compost from Cotton Residues Inconsistently Affect Water Use Efficiency, Nodulation, and Growth of Legumes under Arid Conditions. J. Environ. Manag. 2022, 307, 114558. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A.; Tobiasova, E. The Application of Biochar from Waste Biomass to Improve Soil Fertility and Soil Enzyme Activity and Increase Carbon Sequestration. Energies 2023, 16, 380. [Google Scholar] [CrossRef]
- Yue, X.; Liu, X.; Wang, F.; Shen, C.; Zhang, Y. Contrasting Effects of Organic Materials versus Their Derived Biochars on Maize Growth, Soil Properties and Bacterial Community in Two Type Soils. Front. Microbiol. 2023, 14, 1174921. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Effects of Biochar-Based Fertilizer on Soil Bacterial Network Structure in a Karst Mountainous Area. Catena 2021, 206, 105535. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-Based Slow-Release of Fertilizers for Sustainable Agriculture: A Mini Review. Environ. Sci. Ecotechnol. 2022, 10, 100167. [Google Scholar] [CrossRef]
- Minamino, Y.; Fujitake, N.; Suzuki, T.; Yoshitake, S.; Koizumi, H.; Tomotsune, M. Effect of Biochar Addition on Leaf-Litter Decomposition at Soil Surface during Three Years in a Warm-Temperate Secondary Deciduous Forest, Japan. Sci. Rep. 2019, 9, 16961. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Bromm, T.; Glaser, B. Soil Organic Carbon Sequestration after Biochar Application: A Global Meta-Analysis. Agronomy 2021, 11, 2474. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, Q.; Zhan, L.; Xu, X.; Zhang, X.; Dong, Y.; Yan, X.; Xiong, Z. Biochar and Organic Substitution Improved Net Ecosystem Economic Benefit in Intensive Vegetable Production. Biochar 2022, 4, 46. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood Biochar Influences Calcareous Soil Physicochemical and Microbiological Status. J. Environ. Qual. 2014, 43, 681–689. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil. Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting Effects of Biochar versus Manure on Soil Microbial Communities and Enzyme Activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Ok, Y.S.; Awad, Y.M.; Lee, S.S.; Sung, J.K.; Koutsospyros, A.; Moon, D.H. Impacts of Biochar Application on Upland Agriculture: A Review. J. Environ. Manag. 2019, 234, 52–64. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis: Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 2. ISBN 089118 0729.
- Gundi, V.A.K.B.; Viswanath, B.; Chandra, M.S.; Kumar, V.N.; Reddy, B.R. Activities of Cellulase and Amylase in Soils as Influenced by Insecticide Interactions. Ecotoxicol. Environ. Saf. 2007, 68, 278–285. [Google Scholar] [CrossRef]
- Onsori, H.; Raza Zamani, M.; Motallebi, M.; Zarghami, N. Identification of over Producer Strain of Endo-1,4-Glucanase in Aspergillus Species: Characterization of Crude Carboxymethyl Cellulase. Afr. J. Biotechnol. 2005, 4, 26–30. [Google Scholar]
- de Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/Bacterial Ratios in Grasslands with Contrasting Nitrogen Management. Soil. Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef]
- Orwin, K.H.; Wardle, D.A. New Indices for Quantifying the Resistance and Resilience of Soil Biota to Exogenous Disturbances. Soil. Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- Grossbard, E.; GI, W. Techniques for the Assay of Effects of Herbicides on the Soil Microflora. II. The Effect of Herbicides on Cellulose Decomposition. Soc. Appl. Bacteriol. Tech. Ser. 1975, 8, 236–256. [Google Scholar]
- Hiroki, M.; Watanabe, M.M. Microbial Community and Rate of Cellulose Decomposition in Peat Soils in a Mire. Soil. Sci. Plant Nutr. 1996, 42, 893–903. [Google Scholar] [CrossRef]
- Elvazrt, F.; Tabatabai, M.A. Factors Affecting Glucosidase and Galactosidase Activities in Soils; Elsevier: Amsterdam, The Netherlands, 1990; Volume 22. [Google Scholar]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties, 5.2; Chapter 37; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994. [Google Scholar]
- SAS Institute. Base SAS 9.3 Procedures Guide: Statistical Procedures; SAS Institute: Cary, NC, USA, 2011; ISBN 9781607648963. [Google Scholar]
- Han, L.; Zhao, X.; Jin, J.; Gao, B.; Yang, Y.; Sun, K.; Li, F. Using Sequential Extraction and DGT Techniques to Assess the Efficacy of Plant- and Manure-Derived Hydrochar and Pyrochar for Alleviating the Bioavailability of Cd in Soils. Sci. Total Environ. 2019, 678, 543–550. [Google Scholar] [CrossRef]
- Sapkota, R.; Santos, S.; Farias, P.; Krogh, P.H.; Winding, A. Insights into the Earthworm Gut Multi-Kingdom Microbial Communities. Sci. Total Environ. 2020, 727, 138301. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ouyang, Y.; Gu, W.; Yang, W.; Xu, Q. Evaluation of Nutrient Removal Efficiency and Microbial Enzyme Activity in a Baffled Subsurface-Flow Constructed Wetland System. Bioresour. Technol. 2013, 146, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Polifka, S.; Wiedner, K.; Glaser, B. Increased CO2 Fluxes from a Sandy Cambisol under Agricultural Use in the Wendland Region, Northern Germany, Three Years after Biochar Substrates Application. GCB Bioenergy 2018, 10, 432–443. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-Chemical Properties and Microbial Responses in Biochar-Amended Soils: Mechanisms and Future Directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of Biochar: Biochar Properties. In Biochar for Environmental Management Science and Technology; Earthscan: London, 2009; pp. 85–105. ISBN 9781844076581. [Google Scholar]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the “charosphere”—Does Biochar in Agricultural Soil Provide a Significant Habitat for Microorganisms? Soil. Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Biochar-Soil Interactions in Four Agricultural Soils; Elsevier: Amsterdam, The Netherlands, 2015; Volume 25. [Google Scholar]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, Nutrient Availability and Hydrophobicity of Biochars Derived from Manure, Crop Residues, and Municipal Solid Waste for Their Use as Soil Amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial Utilisation of Biochar-Derived Carbon. Sci. Total Environ. 2013, 465, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar Addition Rate Influences Soil Microbial Abundance and Activity in Temperate Soils. Eur. J. Soil. Sci. 2014, 65, 28–39. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Zhao, Y.; Shi, H.; Liu, G. Effect of Biochar on Rhizosphere Soil Microbial Diversity and Metabolism in Tobacco-Growing Soil. Ecologies 2022, 3, 539–556. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. Mineral Surfaces and Soil Organic Matter. Eur. J. Soil. Sci. 2003, 54, 219–236. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined Effects of Biochar Properties and Soil Conditions on Plant Growth: A Meta-Analysis. Sci. Total Environ. 2020, 713, 136635. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Hua, M.; Reckling, M.; Wirth, S.; Bellingrath-Kimura, S.D. Potential Effects of Biochar-Based Microbial Inoculants in Agriculture. Environ. Sustain. 2018, 1, 19–24. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological Nitrogen Fixation by Common Beans (Phaseolus vulgaris L.) Increases with Bio-Char Additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Sun, J.; Lu, X.; Wang, S.; Tian, C.; Chen, G.; Luo, N.; Zhang, Q.; Li, X. Biochar Blended with Nitrogen Fertilizer Promotes Maize Yield by Altering Soil Enzyme Activities and Organic Carbon Content in Black Soil. Int. J. Environ. Res. Public Health 2023, 20, 4939. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Enzyme Activities in a Limed Agricultural Soil; Springer: Berlin/Heidelberg, Germany, 2000; Volume 31. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).