Characteristics of Humic Acids in Drained Floodplain Soils in Temperate Climates: A Spectroscopic Study
Abstract
:1. Introduction
2. Materials and Methods
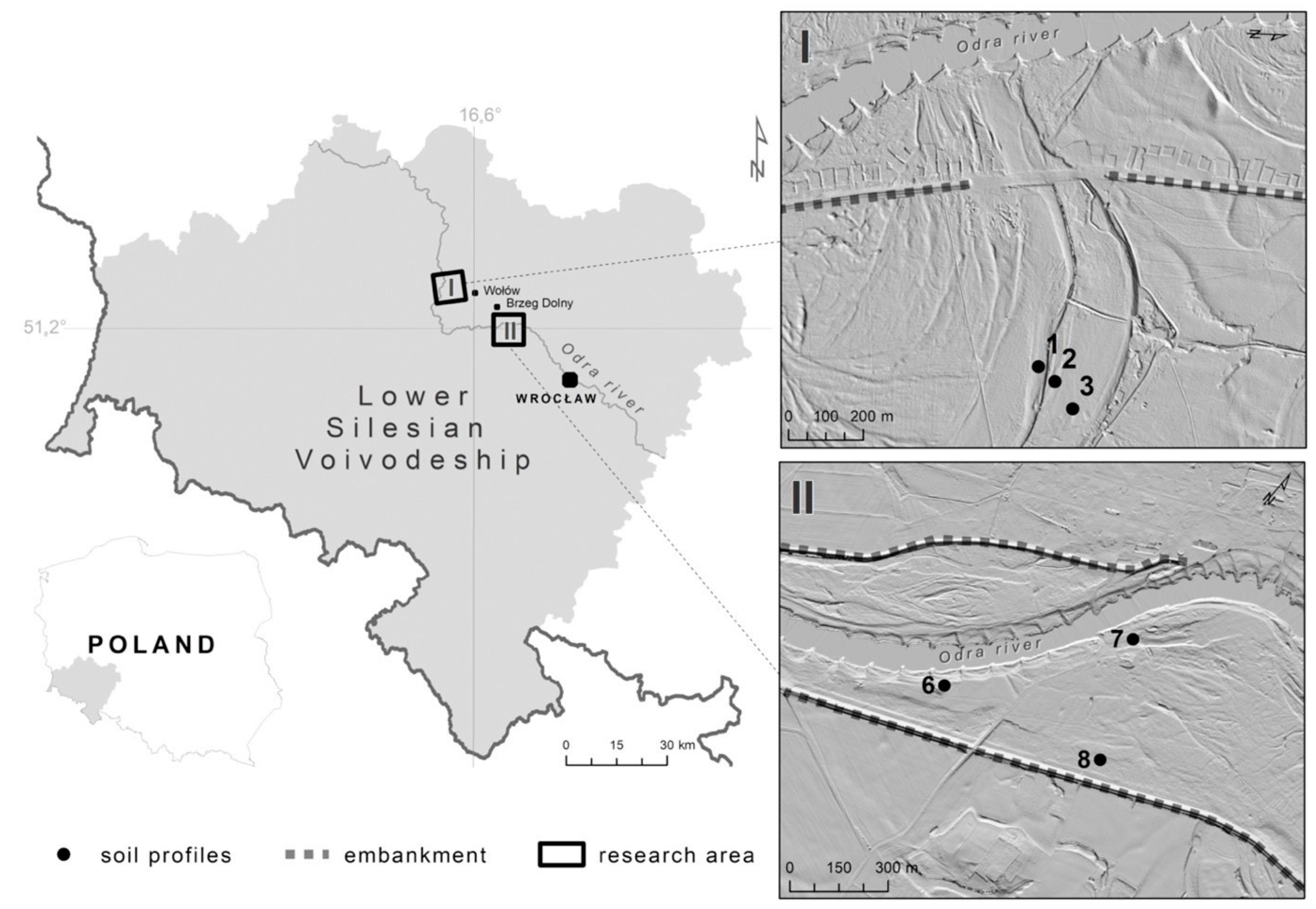
2.1. Site Location, Soil Characteristics and Sampling
2.2. Content of Total Organic Carbon and C/N Ratio
2.3. Chemical Fractionation of the Humic Material
2.4. Extraction and Purification Procedure of Humic Acids
2.5. Spectroscopic Analysis of HA
2.5.1. E4/E6
2.5.2. FTIR
2.5.3. 1H NMR
2.5.4. EPR
2.6. Statistical Analysis
3. Results and Discussion
3.1. Total Organic Carbon and Nitrogen
3.2. Content of Humic Substances
3.3. E4/E6 Ratio Results
3.4. Elemental Composition of and Atomic Ratio of HAs
3.5. FTIR and EPR Analysis
3.6. 1H NMR Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlesinger, W.H. An overview of the C cycle. In Soils and Global Change; Lal, R., Kimble, J., Levin, J., Stewart, B.A., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1995; pp. 9–26. [Google Scholar]
- Wolters, V. Invertebrate control of soil organic matter stability. Biol. Fertil. Soils 2000, 31, 1–19. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Lajtha, K.; Bowden, R.D.; Crow, S.; Fekete, I.; Kotroczó, Z.; Plante, A.; Simpson, M.J.; Nadelhoffer, K.J. The detrital input and removal treatment (DIRT) network: Insights into soil carbon stabilization. Sci. Total Environ. 2018, 640, 1112–1120. [Google Scholar] [CrossRef]
- Jamroz, E.; Kocowicz, A.; Bekier, J.; Weber, J. Properties of soil organic matter in Podzols under mountain dwarf pine (Pinus mugo Turra.) and Norway spruce (Picea abies (L.) Karst.) in various stages of dieback in the East Sudety Mountains, Poland. For. Ecol. Manag. 2014, 330, 261–270. [Google Scholar] [CrossRef]
- De Nobili, M.; Bravo, C.; Chen, Y. The spontaneous secondary synthesis of soil organic matter components: A critical examination of the soil continuum model theory. Appl. Soil Ecol. 2000, 154, 103655. [Google Scholar] [CrossRef]
- Loffredo, E.; Senesi, N. The role of humic substances in the fate of anthropogenic organic pollutants in soil with emphasis on endocrine disruptor compounds. In Soil and Water Pollution Monitoring, Protection and Remediation; Springer: Dordrecht, The Netherlands, 2006; pp. 3–23. [Google Scholar] [CrossRef]
- Martin-Neto, L.; Rosell, R.; Sposito, G. Correlation of spectroscopic indicators of humification with mean annual rainfall along a temperate grassland climosequence. Geoderma 1998, 81, 305–311. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; De Nobili, M.; Chen, Y.; McKnight, D.M.; Wells, M.J.M.; Weber, J. Using Humic Fractions to Understand Natural Organic Matter Processes in Soil and Water: Selected Studies and Applications. J. Environ. Qual. 2019, 48, 1633–1643. [Google Scholar] [CrossRef] [Green Version]
- Polláková, N.; Šimanský, V.; Kravka, M. The influence of soil organic matter fractions on aggregates stabilization in agricultural and forest soils of selected Slovak and Czech hilly lands. J. Soils Sediments 2018, 18, 2790–2800. [Google Scholar] [CrossRef]
- Senesi, N.; D’Orazio, V.; Ricca, G. Humic acids in the first generation of EUROSOILS. Geoderma 2003, 116, 325–344. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994; p. 496. [Google Scholar]
- Weber, J.; Chen, Y.; Jamroz, E.; Miano, T. Preface: Humic substances in the environment. J. Soils Sediments 2018, 18, 2665–2667. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.H.B.; Mylotte, R.; Swift, R.S. Humin: Its Composition and Importance in Soil Organic Matter. Adv. Agron. 2017, 143, 47–138. [Google Scholar]
- Weber, J.; Jamroz, E.; Kocowicz, A.; Debicka, M.; Bekier, J.; Ćwieląg-Piasecka, I.; Ukalska-Jaruga, A.; Mielnik, L.; Bejger, R.; Jerzykiewicz, M. Optimized isolation method of humin fraction from mineral soil material. Environ. Geochem. Health 2022, 44, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Ukalska-Jaruga, A.; Bejger, R.; Debaene, G.; Smreczak, B. Characterization of soil organic matter individual fractions (Fulvic acids, humic acids, and humins) by spectroscopic and electrochemical techniques in agricultural soils. Agronomy 2021, 11, 1067. [Google Scholar] [CrossRef]
- De Mastro, F.; Cocozza, C.; Traversa, A.; Savy, D.; Abdelrahman, H.M.; Brunetti, G. Influence of crop rotation, tillage and fertilization on chemical and spectroscopic characteristics of humic acids. PLoS ONE 2019, 14, e0219099. [Google Scholar] [CrossRef]
- Visser, S.A. Application of Van Krevelen’s Graphical-Statistical Method for the Study of Aquatic Humic Material Environ. Sci. Technol. 1983, 17, 412–417. [Google Scholar] [CrossRef]
- Ćwieląg-Piasecka, I.; Medyńska-Juraszek, A.; Jerzykiewicz, M.; Dębicka, M.; Bekier, J.; Jamroz, E.; Kawałko, D. Humic Acid and Biochar as Specific Sorbents of Pesticides. J. Soils Sediments 2018, 18, 2692–2702. [Google Scholar] [CrossRef] [Green Version]
- Debska, B.; Spychaj-Fabisiak, E.; Szulc, W.; Gaj, R.; Banach-Szott, M. EPR Spectroscopy as a Tool to Characterize the Maturity Degree of Humic Acids. Materials 2021, 14, 3410. [Google Scholar] [CrossRef]
- Machado, W.; Franchini, J.C.; de Fátima Guimarães, M.; Filho, J.T. Spectroscopic Characterization of Humic and Fulvic Acids in Soil Aggregates, Brazil. Heliyon 2020, 6, e04078. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrié, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef] [Green Version]
- Długosz, J.; Kalisz, B.; Łachacz, A. Mineral matter composition of drained floodplain soils in north-eastern Poland. Soil Sci. Ann. 2018, 69, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Gonzáles, E.; Cabezas, A.; Corenblit, D.; Steiger, J. Autoch- thonous versus allochthonous organic matter in recent soil C accumulation along a floodplain biogeomorphic gradient: An exploratory study. J. Environ. Geogr. 2014, 7, 29–38. [Google Scholar] [CrossRef]
- Gmitrowicz-Iwan, J.; Ligęza, S.; Pranagal, J.; Smal, H. Morphometric and location factors shaping sediment texture in small floodplain reservoirs. J. Soils Sediments 2021, 21, 1243–1255. [Google Scholar] [CrossRef]
- Šimanský, V. Can soil properties of Fluvisols be influenced by river flow gradient. Acta Fytotech. Zootech. 2018, 21, 63–76. [Google Scholar] [CrossRef]
- Schnitzler, A. River dynamics as a forest process: Interaction between fluvial systems and alluvial forests in large European river plains. Bot. Rev. 1997, 63, 40–64. [Google Scholar] [CrossRef]
- Cieśla, A. Effect of hydrotechnical constructions on the Oder river on the phytosociological diversity of riparian habitats in the Prawików forest. For. Res. Work. 2009, 70, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.Y.; Zhao, W.W.; Daryanto, S.; Wang, L.X.; Fan, H.; Feng, Q.; Wang, Y.P. The spatial distribution and temporal variation of desert riparian forests and their influencing factors in the downstream Heihe River basin, China. Hydrol. Earth Syst. Sci. 2017, 21, 2405–2419. [Google Scholar] [CrossRef] [Green Version]
- Głuchowska, B.; Pływaczyk, L. Groundwater level in the Odra river valley downstream the Brzeg Dolny. Contemp. Probl. Environ. Eng. 2008, 5, 1–109. [Google Scholar]
- Kawałko, D.; Kaszubkiewicz, J.; Jezierski, P. Morphology and selected properties of alluvial soils in the Odra River valley, SW Poland. Soil Sci. Ann. 2022, 73, 156062. [Google Scholar] [CrossRef]
- Katou, H.; Nakaya, N.; Maeda, K. Changes in sediment volume, liquid limit, and plastic limit of alluvial soils upon drying. Soil Sci. Plant Nutr. 1984, 31, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Kawalko, D.; Jezierski, P.; Kabała, C. Morphology and physicochemical properties of alluvial soils in riparian forests after river regulation. Forests 2021, 12, 329. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kleinschmit, B.; Kowarik, I.; Graf, M.; Lang, F. Organic matter distribution in floodplains can be predicted using spatial and vegetation structure data. River Res. Appl. 2011, 27, 1048–1057. [Google Scholar] [CrossRef]
- Łachacz, A.; Kalisz, B.; Sowiński, P.; Smreczak, B.; Niedźwiecki, J. Transformation of Organic Soils Due to Artificial Drainage and Agricultural Use in Poland. Agriculture 2023, 13, 634. [Google Scholar] [CrossRef]
- Wohl, E.; Pfeiffer, A. Organic carbon storage in floodplain soils of the U.S. prairies. River Res. Appl. 2018, 34, 406–416. [Google Scholar] [CrossRef]
- Brunke, M.; Hoehn, E.; Gonser, T. Patchiness of river-groundwater interactions within two floodplain landscapes and diversity of aquatic invertebrate communities. Ecosystems 2003, 6, 707–722. [Google Scholar] [CrossRef]
- Doulatyari, B.; Basso, S.; Schirmer, M.; Botter, G. River flow regimes and vegetation dynamics along a river transect. Adv. Water Resour. 2014, 73, 30–43. [Google Scholar] [CrossRef]
- Kardol, P.; Bezemer, T.M.; Putten, W.H.V.D. Temporal variation in plant-soil feedback controls succession. Ecol. Lett. 2006, 9, 1080–1088. [Google Scholar] [CrossRef]
- Charlton, R. Fundamentals of Fluvial Geomorfology; Routledge: London, UK, 2008; p. 201. [Google Scholar]
- Matuszkiewicz, J.M. Forest Associations in Poland; PWN: Warsaw, Poland, 2001; p. 376. [Google Scholar]
- Naiman, R.J.; Decamps, H.; McClain, M.E. Riparia: Ecology, Conservation, and Management of Streamside Communities; Elsevier: Amsterdam, The Netherlands, 2010; p. 448. [Google Scholar]
- Nilsson, C.; Berggren, K. Alterations of riparian ecosystems caused by river regulation: Dam operations have caused global-scale ecological changes in riparian ecosystems. How to protect river environments and human needs of rivers remains one of the most important questions of our time. BioScience 2000, 50, 783–792. [Google Scholar]
- Celentano, D.; Rousseau, G.X.; Engel, V.L.; Zelarayán, M.; Oliveira, E.C. Degradation of riparian forest affects soil properties and ecosystem services provision in eastern amazon of Brazil. Land Degrad. Dev. 2017, 28, 482–493. [Google Scholar] [CrossRef]
- Marks, L. Quaternary glaciations in Poland. In Developments in Quaternary Sciences; Elsevier: Amsterdam, The Netherlands, 2011; Volume 15, pp. 299–303. [Google Scholar]
- Kabała, C. (Ed.) Soils of Lower Silesia. Origins, diversity and protection. In Proceedings of the 29th Congress of the Polish Society of Soil Science and International Year of Soil (IYS2015), Wrocław, Poland, 31 August–5 September 2015; Available online: http://www.org.up.wroc.pl/igosr/PTG29/monografia.pdf (accessed on 1 June 2023).
- Pływaczyk, L. The impact of river damming on valley on the example of Brzeg Dolny. Monogr. Wrocław Agric. Univ. 1997, 11, 47. [Google Scholar]
- FAO. Word Reference Base for Soil Resources; Word Soil Resources Report, No. 106; FAO: Rome, Italy, 2015; Available online: http://www.fao.org/soils-portal/data-hub/soil-classification/world-reference-base/en/ (accessed on 1 June 2023).
- Kawałko, D.; Halarewicz, A.; Pruchniewicz, D. Vegetation condition in the Odra river riparian forests in the area of Wołów. Sylwan 2015, 159, 220–226. [Google Scholar]
- Kawałko, D.; Halarewicz, A.; Kaszubkiewicz, J.; Jezierski, J. Decomposition rate of the litter fall in the course of riparian habitat changes. Sylwan 2017, 161, 565–572. [Google Scholar]
- Jahn, R.; Blume, H.P.; Asio, V.B.; Spaargaren, O.; Schad, P. Guidelines for Soil Description; FAO: Rome, Italy, 2006. [Google Scholar]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 1996; pp. 1011–1069. [Google Scholar]
- Senesi, N. Composted materials as organic fertilizers. Sci. Total Environ. 1989, 81, 521–542. [Google Scholar] [CrossRef]
- Ciavatta, C.; Centemero, M.; Toselli, M.; Zaccone, C.; Senesi, N. Compost production, analysis and applications in agriculture. In Multi-Scale Biogeochemical Processes in Soil Ecosystems: Critical Reactions and Resilience to Climate Changes; Yang, Y., Keiluweit, M., Senesi, N., Xing, B., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 297–321. ISBN 9781119480419. [Google Scholar] [CrossRef]
- Drozd, J.; Jamroz, E.; Licznar, M.; Weber, J. Changes of humification indexes of differing maturity composts from municipal solid wastes. In Understanding & Managing Organic Matter in Soils, Sediments & Waters; Swift, R.S., Spark, K.M., Eds.; International Humic Substances Society, Hyde Park Press: Adelaide, Australia, 2021; pp. 181–186. [Google Scholar]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 439. [Google Scholar] [CrossRef]
- Jezierski, A.; Czechowski, F.; Jerzykiewicz, M.; Chen, Y.; Drozd, J. Electron parametric resonance (EPR) studies on stable and transient radicals in humic acids from compost, soil, peat and brown coal. Spectrochim. Acta 2000, 56, 379–385. [Google Scholar] [CrossRef]
- Banach-Szott, M.; Kondratowicz-Maciejewska, K.; Kobierski, M. Humic substances in Fluvisols of the Lower Vistula floodplain, North Poland. Environ. Sci. Pollut. Res. 2018, 25, 23992–24002. [Google Scholar] [CrossRef] [PubMed]
- Kercheva, M.; Sokołowska, Z.; Hajnos, M.; Skic, K.; Shishkov, T. Physical parameters of Fluvisols on flooded and non-flooded terraces. Int. Agrophys. 2017, 31, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Kałuża-Haładyn, A.; Jamroz, E.; Bekier, J. Humic substances of differently matured composts produced from municipal solid wastes and biomass of energetic plants. Soil Sci. Ann. 2019, 70, 292–297. [Google Scholar] [CrossRef]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information Provided on Humic Substances by E4/E6 Ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Zalba, P.; Amiotti, N.M.; Galantini, J.A.; Pistola, S. Soil Humic and Fulvic Acids from Different Land-Use Systems Evaluated By E4/E6 Ratios. Commun. Soil Sci. Plant Anal. 2016, 47, 1675–1679. [Google Scholar] [CrossRef]
- Cunha, T.; Novotny, E.; Madari, B.; Martin-Neto, L.; de O Rezende, M.; Canelas, L.; de M Benites, V. Spectroscopy Characterization of Humic Acids Isolated from Amazonian Dark Earth Soils (Terra Preta De Índio). In Amazonian Dark Earths: Wim Sombroek’s Vision; Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., WinklerPrins, A., Rebellato, L., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 363–372. ISBN 978-1-4020-9031-8. [Google Scholar]
- Traversa, A.; Said-Pullicino, D.; D’Orazio, V.; Gigliotti, G.; Senesi, N. Properties of Humic Acids in Mediterranean Forest Soils (Southern Italy): Influence of Different Plant Covering. Eur. J. For. Res. 2011, 130, 1045–1054. [Google Scholar] [CrossRef]
- Canellas, L.P.; Façanha, A.R. Chemical nature of soil humified fractions and their bioactivity. Pesqui. Agropecu. Bras. 2004, 39, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3extraction procedures. J. Plant. Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Traversa, A.; D’Orazio, V.; Senesi, N. Properties of dissolved organic matter in forest soils: Influence of different plant covering. For. Ecol. Manag. 2008, 256, 2018–2028. [Google Scholar] [CrossRef]
- Provenzano, M.R.; Iannuzzi, G.; Fabbri, C.; Senesi, N. Qualitative characterization and differentiation of digestates from different biowastes using FTIR and fluorescence spectroscopies. J. Environ. Prot. 2011, 2, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Rosa, E.; Debska, B.; Banach-Szott, M.; Tobiasova, E. Use of HPLC, Py-GCMS, FTIR methods in the studies of the composition of soil dissolved organic matter. Pol. J. Soil Sci. 2015, 48, 101–110. [Google Scholar] [CrossRef]
- Witwicki, M.; Jaszewski, A.; Jezierska, J.; Jerzykiewicz, M.; Jezierski, A. The pH-induced shift in the g-tensor components of semiquinone-type radicals in humic acids—DFT and EPR studies. Chem. Phys. Lett. 2008, 462, 300–306. [Google Scholar] [CrossRef]
- Jerzykiewicz, M.; Jezierski, A.; Czechowski, F.; Drozd, J. Influence of metal ions binding on free radical concentration in humic acids. A quantitative Electron Paramagnetic Resonance study. Org. Geochem. 2002, 33, 265–268. [Google Scholar] [CrossRef]
- Jerzykiewicz, M. Humic and hymatomelanic acids interaction with lanthanide ions. Spectrochim. Acta 2012, 96, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Jezierski, A.; Czechowski, F.; Jerzykiewicz, M.; Drozd, J. EPR investigations of humic acids structure from compost, soil, peat and soft brown coal upon oxidation and metal uptake. Appl. Magn. Reson. 2000, 18, 127–136. [Google Scholar] [CrossRef]
- Jezierski, A.; Czechowski, F.; Jerzykiewicz, M.; Golonka, I.; Drozd, J.; Bylińska, E.; Chen, Y.; Seaward, M.R. Quantitative EPR study on free radicals in the natural polyphenols interacting with metal ions and other environmental pollutants. Spectrochim. Acta 2002, 58, 1293–1300. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Un, S.; Deligiannakis, Y. High-Field 285 GHz Electron Paramagnetic Resonance study of indigenous radicals of humic acids. J. Phys. Chem. 2007, 111, 11860–11866. [Google Scholar] [CrossRef]
- Kawałko, D.; Karczewska, A. Profile Distributions of Potentially Toxic Metal(loid)s in Soils of the Middle Odra Floodplain (SW Poland). Int. J. Environ. Res. Public Health 2023, 20, 4196. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. Monitoring changes in the structure and properties of humic substances following ozonation using UV–Vis, FTIR and 1H NMR techniques. Sci. Total Environ. 2016, 541, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Grinhut, T.; Hertkorn, N.; Schnitt-Kopplin, P.; Hadar, Y.; Chen, Y. Mechanisms of humic acids degradation by white rot fungi explored using 1H NMR spectroscopy and FTICR mass spectroscopy. Environ. Sci. Technol. 2011, 45, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Hertkorn, N.; Ruecker, C.; Meringer, M.; Gugisch, R.; Frommberger, M.; Perdue, E.M.; Witt, M.; Schnitt-Kopplin, P. High-precision frequency measurements: Indispensable tools at the core of the molecular-level analysis of complex systems. Ann. Bioanal Chem. 2007, 389, 1311–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamroz, E.; Jerzykiewicz, M. Humic fractions as indicators of soil organic matter responses to clear-cutting in mountain and lowland conditions of southwestern Poland. Land Degrad. Dev. 2022, 33, 368–378. [Google Scholar] [CrossRef]
- Mao, J.D.; Xing, B.; Schmidt-Rohr, K. New structural information on a humic acid from two-dimensional 1H-13C correlation solid-state nuclear magnetic resonance. Environ. Sci. Technol. 2001, 35, 1928–1934. [Google Scholar] [CrossRef]
- Lu, Q.; Jia, L.; Awashti, M.K.; Jing, G.; Wang, Y.; He, L.; Zhao, N.; Chen, Z.; Shi, X. Variations in lignin monomer contents and stable hydrogen isotope ratios in methoxy groups during the biodegradation of garden biomass. Sci. Rep 2022, 12, 8734. [Google Scholar] [CrossRef]





| Object | Distance from River (m) | pH 1M KCl | TOC g.kg−1 | Nt g.kg−1 | C/N | CEC cmol(+).kg−1 | Silt Content (%) | Clay Content (%) |
|---|---|---|---|---|---|---|---|---|
| Rp1a | 900 | 4.5 | 39.7 | 2.9 | 14 | 24.33 | 52.7 | 0 |
| Rp2a | 950 | 4.9 | 26.0 | 3.7 | 7 | 25.41 | 67.2 | 20.7 |
| Rp3a | 1050 | 4.9 | 39.9 | 3.1 | 13 | 17.68 | 41.4 | 9.4 |
| Mw6a | 120 | 4.7 | 45.8 | 3.9 | 12 | 18.20 | 62.0 | 25 |
| Mw7a | 150 | 4.8 | 56.9 | 2.8 | 20 | 16.28 | 62.0 | 14 |
| Mw8a | 200 | 4.6 | 54.2 | 2.8 | 19 | 16.98 | 60.0 | 16 |
| Factor | Distance from River (m) | TOC | CAC | TEC | CHA | CFA | CR |
|---|---|---|---|---|---|---|---|
| g.kg−1 | |||||||
| Mw6a | 120 | 45.8 | 1.58 | 16.37 | 8.54 | 7.83 | 27.85 |
| Mw7a | 150 | 56.9 | 1.84 | 22.73 | 12.00 | 10.73 | 32.33 |
| Mw8a | 200 | 54.2 | 2.56 | 26.17 | 14.97 | 11.20 | 25.46 |
| Rp1a | 900 | 39.7 | 3.45 | 11.86 | 5.37 | 6.49 | 24.39 |
| Rp2a | 950 | 26.0 | 1.76 | 10.26 | 4.77 | 5.50 | 13.98 |
| Rp3a | 1050 | 39.9 | 4.28 | 17.87 | 7.51 | 10.36 | 17.75 |
| LSD | 3.099 | 0.485 | 3.142 | 2.742 | 1.240 | 1.314 | |
| Grassland | 52.3 | 1.993 | 21.757 | 11.837 | 9.92 | 28.547 | |
| SD | 6.99 | 0.47 | 4.67 | 3.17 | 1.72 | 3.15 | |
| Land use | Riparian forest | 35.4 | 3.163 | 13.330 | 5.883 | 7.45 | 18.707 |
| SD | 5.26 | 1.17 | 3.66 | 1.35 | 2.32 | 4.74 | |
| LSD | 2.017 | 0.316 | 2.045 | 1.785 | 0.807 | 0.855 | |
| Factor | Distance from River (m) | HD | HR | HI | CHA/CFA | E4/E6 |
|---|---|---|---|---|---|---|
| Mw6a | 120 | 16.37 | 0.41 | 21.51 | 1.09 | 3.25 |
| Mw7a | 150 | 22.73 | 0.86 | 44.97 | 1.11 | 3.16 |
| Mw8a | 200 | 26.17 | 0.66 | 37.51 | 1.34 | 3.65 |
| Rp1a | 900 | 11.86 | 0.26 | 11.72 | 0.83 | 3.90 |
| Rp2a | 950 | 10.27 | 0.18 | 8.38 | 0.87 | 3.82 |
| Rp3a | 1050 | 17.87 | 0.33 | 13.84 | 0.72 | 3.66 |
| LSD | 3.91 | 0.06 | 5.60 | n.s. | n.s. | |
| Grassland | 21.76 | 0.64 | 34.66 | 1.18 | 3.35 | |
| Land use | SD | 4.81 | 0.20 | 11.08 | 0.14 | 0.33 |
| Riparian forest | 13.33 | 0.26 | 11.32 | 0.81 | 3.79 | |
| SD | 3.66 | 0.22 | 2.52 | 0.07 | 0.26 | |
| LSD | 2.55 | 0.04 | 3.64 | 0.09 | 0.43 |
| Object | C (SD) | H (SD) | N (SD) | S (SD) | O | H/C | O/C | ω |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| Mw6a | 51.88 a (0.16) | 5.57 a (0.37) | 4.99 a (0.06) | 0.85 a (0.01) | 36.71 | 1.29 | 0.53 | 0.0208 |
| Mw7a | 51.48 ac (0.07) | 5.62 a (0.36) | 4.97 a (0.04) | 0.86 a (0.01) | 37.06 | 1.31 | 0.54 | 0.0177 |
| Mw8a | 50.00 b (0.26) | 5.58 a (0.36) | 5.37 b (0.05) | 0.92 a (0.03) | 38.13 | 1.34 | 0.57 | 0.0817 |
| Rp1a | 50.95 c (0.01) | 5.80 a (0.25) | 5.06 a (0.05) | 0.66 b (0.03) | 37.54 | 1.37 | 0.55 | −0.0046 |
| Rp2a | 51.90 a (0.13) | 5.75 a (0.36) | 4.90 a (0.06) | 0.67 b (0.02) | 36.77 | 1.33 | 0.53 | −0.0247 |
| Rp3a | 52.86 d (0.08) | 6.00 a (0.40) | 5.39 b (0.04) | 0.65 b (0.02) | 38.04 | 1.36 | 0.54 | −0.0205 |
| Spin Concentration per Gram × 1017 | g Parameter | |
|---|---|---|
| Rp1a | 2.35 | 2.0034 |
| Rp2a | 1.73 | 2.0034 |
| Rp3a | 1.34 | 2.0034 |
| Average Rp | 1.81 (SD 0.5) | -- |
| Mw6a | 1.37 | 2.0033 |
| Mw7a | 1.22 | 2.0033 |
| Mw8a | 1.47 | 2.0032 |
| Average Mw | 1.35 (SD 0.13) |
| TEC | CHA | CFA | CR | HD | CHA/ CFA | Spin Concentration | Alcoholmethoxy | Aromatic | Attached to Benzene | |
|---|---|---|---|---|---|---|---|---|---|---|
| Distance from the river | −0.407 | −0.622 | 0.008 | −0.342 | −0.407 | −0.987 * | 0.521 | −0.972 * | −0.983 * | −0.447 |
| TOC | 0.827 * | 0.774 | 0.752 | 0.8322 | 0.827 * | 0.417 | −0.351 | 0.322 | 0.410 | −0.253 |
| CAC | −0.358 | −0.475 | −0.105 | −0.801 | −0.358 | −0.835 * | 0.336 | −0.691 | −0.853 * | −0.406 |
| TEC | - | 0.954 * | 0.881 * | 0.384 | 0.999 * | 0.394 | −0.592 | 0.377 | 0.385 | −0.514 |
| CHA | - | 0.698 | 0.367 | 0.954 * | 0.615 | −0.555 | 0.6243 | 0.578 | −0.348 | |
| CFA | - | 0.336 | 0.881 * | −0.028 | −0.537 | −0.085 | 0.008 | −0.675 | ||
| HI | −0.005 | 0.501 | 0.674 | −0.822 * | 0.579 | 0.745 | −0.031 | |||
| CHA/CFA | 0.394 | - | −0.436 | 0.963 * | 0.982 * | 0.404 | ||||
| Methylen | 0.131 | −0.089 | 0.181 | 0.197 | 0.889 * | |||||
| Alcoholmethoxy | −0.373 | - | 0.919 * | 0.411 |
| Proton Type | Methyl | Methylene | Attached to Benzene | Alcohol Methoxy | Aromatic |
|---|---|---|---|---|---|
| ppm | |||||
| 0–1.77 | 1.77–2.33 | 2.33–2.8 | 2.8–4.38 | 6–8.27 | |
| Rp1a | 1 | 0.19 | 0.06 | 0.47 | 0.03 |
| Rp2a | 1 | 0.12 | 0.02 | 0.37 | 0.06 |
| Rp3a | 1 | 0.16 | 0.03 | 0.35 | 0.01 |
| Mw6a | 1 | 0.18 | 0.07 | 0.66 | 0.28 |
| Mw7a | 1 | 0.24 | 0.07 | 0.67 | 0.23 |
| Mw8a | 1 | 0.10 | 0.02 | 0.71 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawałko, D.; Jamroz, E.; Jerzykiewicz, M.; Ćwieląg-Piasecka, I. Characteristics of Humic Acids in Drained Floodplain Soils in Temperate Climates: A Spectroscopic Study. Sustainability 2023, 15, 11417. https://doi.org/10.3390/su151411417
Kawałko D, Jamroz E, Jerzykiewicz M, Ćwieląg-Piasecka I. Characteristics of Humic Acids in Drained Floodplain Soils in Temperate Climates: A Spectroscopic Study. Sustainability. 2023; 15(14):11417. https://doi.org/10.3390/su151411417
Chicago/Turabian StyleKawałko, Dorota, Elżbieta Jamroz, Maria Jerzykiewicz, and Irmina Ćwieląg-Piasecka. 2023. "Characteristics of Humic Acids in Drained Floodplain Soils in Temperate Climates: A Spectroscopic Study" Sustainability 15, no. 14: 11417. https://doi.org/10.3390/su151411417
APA StyleKawałko, D., Jamroz, E., Jerzykiewicz, M., & Ćwieląg-Piasecka, I. (2023). Characteristics of Humic Acids in Drained Floodplain Soils in Temperate Climates: A Spectroscopic Study. Sustainability, 15(14), 11417. https://doi.org/10.3390/su151411417









