Abstract
The non-rubber components present in natural rubber latex can contribute to the dark color of dried films and may cause allergic reactions. This project aimed to develop light-color rubber films with minimal protein contamination. Various additives were incorporated, and a leaching procedure was implemented to address this issue. The evaluation focused on protein content, color changes, and swelling properties of thin natural rubber films. Texapon N70 proved effective as both a latex stabilizer and leaching agent, while Uniphen P-23 served as a preservative. The combined use of these additives facilitated the removal of soluble serum through appropriate incubation, leaching, and centrifugation processes. The introduction of additional centrifugation cycles improved deproteinization and color reduction; however, it led to a loss of rubber mass and an increase in manufacturing costs. Increasing the amount of Texapon N70 and introducing alkali potassium hydroxide (KOH) further enhanced the efficiency of deproteinization and color reduction. The optimal conditions determined in this investigation were as follows: 0.5% w/w Texapon N70, 0.5% w/w KOH, 1% w/w Uniphen P-23, a 60-min incubation period, and a single leaching cycle with distilled water. These conditions resulted in a 90.57 ± 1.20% decrease in protein contamination and a color change (ΔE) of 433.69 ± 20.23. This successful condition can be replicated and scaled up for further applications.
1. Introduction
Natural rubber latex (NRL), derived from the Hevea brasiliensis tree, is a milky fluid extensively used in the manufacturing of various rubber products. NRL is a colloidal dispersion primarily composed of rubber particles surrounded by a thin layer of proteins, lipids, and long chains of fatty acids. These components impart a negative charge to the particles and maintain the colloidal latex stability. Upon centrifugation, NRL separates into three fractions. The upper fraction consists of white rubber particles, which typically represent 30 to 45% of the fresh NRL’s weight. NRL is classified as a large polymer molecule of cis-1,4-polyisoprene, comprising numerous smaller molecules of the same kind, along with two terminal groups known as chain α and ω. The ω-terminal group contains protein components, whereas the α-terminal is associated with phospholipids [1,2,3]. The C-serum phase, the second intermediate fraction, contains soluble substances found in the cytoplasm of plant cells. It encompasses proteins involved in cellular metabolism, inorganic salts, organic acids, nucleotide materials, and carbohydrates [4]. Lastly, the bottom fraction consists of non-rubber substances like lutoids and Frey-Wyssling complexes, which are biochemical organelles present in H. brasiliensis. Lutoids contain several cations, including calcium, potassium, magnesium, and copper, along with cationic proteins that cause flocculation of rubber particles. This results in a positive charge on the interior membranes of lutoids and a negative charge on the exterior. Lutoids are primarily associated with hevein, a water-soluble, anionic, high-sulfur protein known for causing allergic reactions. The Frey-Wyssling complex comprises carotenoids and lipids, which give the rubber a yellowish hue [5].
NRL is an immensely significant polymer in our society. It serves as a critical raw material in the production of over 40,000 different products. NRL is utilized in an array of applications, including medical devices, surgical gloves, aircraft and car tires, pacifiers, clothing, and toys, among many others. Despite its minimal usage in some products, the latex proteins present in NRL derived from H. brasiliensis can trigger severe allergic reactions in certain individuals. The process of purifying NRL presents a considerable challenge due to the complexity of separating the latex proteins from the NR. Considering the potential life-threatening nature of these allergies, it would be highly desirable to have a rubber alternative that does not contain these latex proteins [6,7].
Numerous studies have been conducted to elucidate methods for eliminating allergenic proteins from NRL, employing chemical, physical, and biological techniques. Some proteins are partially degraded under alkaline conditions induced by ammonia (NH3) [8]. Various approaches have been employed to remove serum proteins, including centrifugation, chemical additives, creaming, simple or ultrasound-assisted leaching, chlorination, and enzymatic methods [8,9,10,11,12,13,14]. While centrifugation solely eliminates serum proteins, alkaline treatment of NRL leads to the release of proteins bound to rubber particles, resulting in alterations to the composition of NRL proteins and affecting the physical properties of latex. This method is widely employed in the industry due to its cost-effectiveness. Moreover, the addition of different substances such as surfactants, solvents, alkalis, and polymers enhance the efficiency of protein leaching from rubber, facilitating more effective protein removal. However, the impact of these deproteinized NRL (DNRL) methods on the color change of NR films has not been previously reported.
Hence, the aim of this study is to discover and develop a method for effectively removing or reducing proteins in NRL, referred to as DNRL, while simultaneously preserving or enhancing desirable properties such as latex stability, extending shelf life, and achieving lighter-colored DNRL films. Various additives including surfactants, solvents, alkalis, and polymers were employed in the process. The evaluation encompassed the analysis of remaining proteins in DNRL, assessment of color in dried DNRL films, and examination of numerous other DNRL properties. Furthermore, the intention is to incorporate the developed protein removal or reduction process into industrial DNRL production, potentially finding applications in the pharmaceutical and medical materials sectors.
2. Materials and Methods
2.1. Materials
NRL was obtained from the rubber tree RRIM 600 clone, located in Songkhla province, Thailand. It was acquired from the supplier without any preservatives or additional additives. Two types of preservatives were employed in this study. The combination of phenoxyethanol, methylparaben, ethylparaben, propylparaben, isobutylparaben, and butylparaben, known as Uniphen P-23 and produced by Induchem AG (Dübendorf, Switzerland), was used. NH3 was purchased from Loba Chemie (Mumbai, India). Various groups of additives were utilized. Non-ionic surfactants, namely polyoxyethylene (20) sorbitan monolaurate (Tween 20), polyoxyethylene (20) sorbitan monopalmitate (Tween 40), polyoxyethylene (20) sorbitan monostearate (Tween 60), polyoxyethylene (20) sorbitan monooleate (Tween 80), sorbitan monooleate (Span 80), and sorbitan trioleate (Span 85), were supplied by Sigma-Aldrich (Darmstadt, Germany). Anionic surfactants, including 40% sodium lauryl ether sulfate (Texapon N40), 70% sodium lauryl ether sulfate (Texapon N70), sodium lauryl sulfate (SLS), and a combination of sodium laureth sulfate, glycol distearate, cocamide monoethanolamine, and laureth-10 (Euperlan PK771 Benz), were products from BASF (Ludwigshafen, Germany), sourced from P.C. Drug Center (Bangkok, Thailand). The amphoteric surfactant, cocamidopropyl betaine (Dehyton K), was a product from BASF (Ludwigshafen, Germany), also distributed by P.C. Drug Center (Bangkok, Thailand). Solvent groups, including isopropanol, propylene glycol, and butylene glycol, were supplied by P.C. Drug Center (Bangkok, Thailand). Polymer groups, consisting of poloxamer 407 (Lutral F-127) and poloxamer 188 (Lutral F-68), were gifted from BASF (Ludwigshafen, Germany). Additionally, polyvinyl alcohol (PVA) 67 K (Mowiol® 8–88) was purchased from Sigma-Aldrich (Darmstadt, Germany), while hydrolyzed collagen (Nutrilan I50) was a product from BASF (Ludwigshafen, Germany), obtained from P.C. Drug Center (Bangkok, Thailand). The alkali groups included sodium hydroxide (NaOH) from P.C. Drug Center (Bangkok, Thailand) and potassium hydroxide (KOH) distributed by RCI Labscan (Bangkok, Thailand). Distilled water was used throughout the experiment. All other chemicals were of analytical grade and used as received.
2.2. Effect of Additives on NRL Stability
To begin, fresh NRL was passed through a No.14 mesh sieve with 1.4 mm openings to eliminate any aggregated impurities. Subsequently, various types of additives were introduced to 50 mL of fresh NRL at a concentration of 2% w/w (refer to Table 1 for details). Continuous stirring was employed during the addition of additives. Following this, either 1% w/w of Uniphen P-23 or 0.2% w/w of NH3, serving as a preservative, was added to the prepared NRL. The mixture was then incubated at room temperature. Throughout the experiment, the physical characteristics of the incubated NRL, such as coagulation, color, and odor, were observed and recorded at various time intervals, including 0, 1, 2, 4, 8, 24, and 48 h.

Table 1.
Effect of additives on the stability of NRL.
2.3. Method of Protein Reduction in NRL
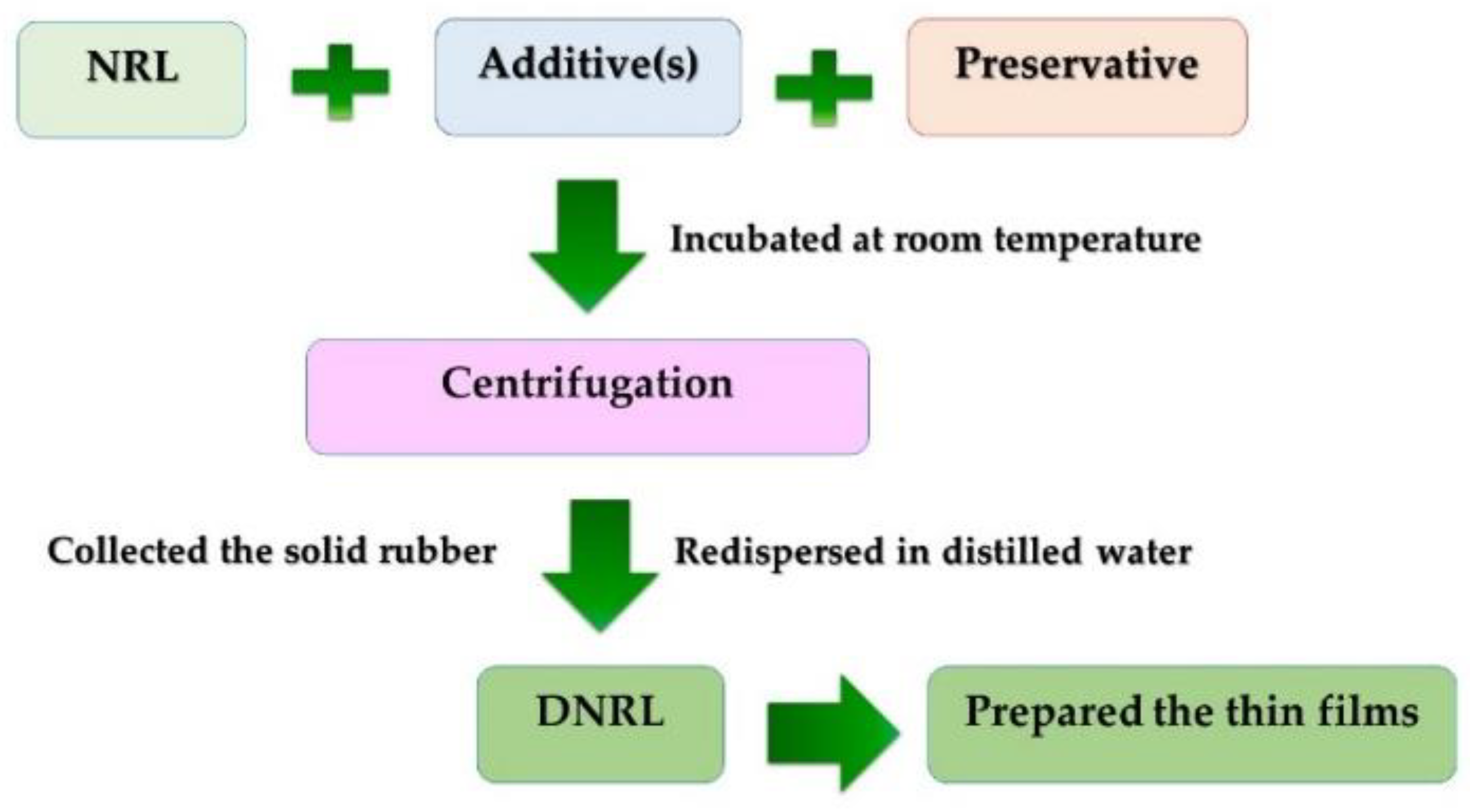
In this section, the process for protein reduction in NRL is outlined. Initially, 2% of the selected additive was added to 100 mL of freshly filtered NRL, followed by continuous stirring. Subsequently, either 1% w/w of Uniphen P-23 or 0.2% w/w of NH3 was added to the NRL mixture. The prepared NRL mixture was incubated at room temperature. To collect the rubber content, the mixture was then subjected to centrifugation using a high-speed centrifugation instrument (Hermle Z323K, Labnet International Inc., Edison, MD, USA) at 10,000 rpm for 45 min. The resulting solid rubber was collected and redispersed to the initial volume using a leaching medium. The re-dispersed mixture was centrifuged again using the same process. Finally, the obtained solid rubber was re-dispersed in distilled water to achieve the initial volume. The resulting DNRL was poured into a Petri dish and transferred to a hot air oven at 50 °C for 1–2 days to form a thin DNRL film. The overall deproteinization process is summarized in Figure 1. The effects of different additive types and amounts, washing cycles, leaching medium, and incubation times on protein reduction in NRL were investigated.
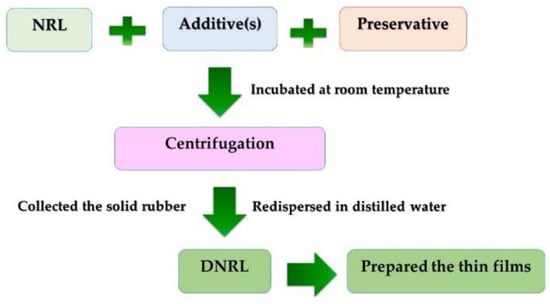
Figure 1.
A schematic diagram of the deproteinization process at laboratory scale.
2.4. Characterizations of DNRL and Its Film
2.4.1. pH Measurement
The pH value of DNRL was determined using a pH meter (SevenEasy S-20 pH meter, Mettler Toledo, Switzerland) equipped with a glass electrode. Prior to the initial use, the instrument was calibrated with pH 4.0, 7.0, and 10.0 standard buffers. Distilled water was used to rinse the electrode between sample measurements. The pH measurements were conducted in triplicate at ambient temperature.
2.4.2. Viscosity Measurement
The viscosity of DNRL was determined using a programmable rheometer (Brookfield DV-III ULTRA, Brookfield Engineering Laboratories Inc., Plymouth, MA, USA) equipped with an SC4-34 spindle. The viscosity measurements were conducted at various speeds ranging from 50 to 250 rpm. The samples were analyzed in triplicate at ambient temperature.
2.4.3. Total Solid Content (TSC)
The TSC of DNRL was determined using a modified version of the method described in ASTM D1076 [15]. Ten grams of latex were accurately weighed (W0) into a Petri dish and dried at 50 °C in a hot air oven for 1–2 days. The dried mass was subsequently cooled in a desiccator to room temperature for 24 h and then reweighed (WTSC). The percentage of TSC was calculated using Equation (1). The measurements were conducted in triplicate.
2.4.4. Nitrogen Content
The remaining protein content in the obtained DNRL was analyzed as nitrogen content using the Kjeldahl method [16], conducted by the Fooktien Group Co., Ltd., Songkhla, Thailand. In this method, the sample was digested in a strong sulfuric acid solution with Kjeldahl as a catalyst, using a mixture of K2SO4:CuSO4·5H2O (in a ratio of 9:1). This digestion process converted the amine nitrogen present in the proteins into ammonium ions. Heating and distillation techniques were employed to convert the ammonium ions into ammonia gas, which was then captured in an acid solution. The quantity of trapped ammonia, related to the nitrogen content, was determined through titration with 0.1 N NaOH. The protein content was estimated by multiplying the percentage of nitrogen by a Kjeldahl factor of 6.25. The measurements were performed in triplicate. The protein amount in the sample was compared with that of freshly raw NRL, allowing the calculation of the protein reduction efficacy using Equation (2).
where Psample represents the protein content in each sample, and PNRL represents the protein content in fresh NRL from the same production batch.
2.4.5. Physical Appearance of Dried DNRL Film
The physical homogeneity characteristics of the DNRL films were visually observed for their appearance. The color measurements were conducted at four different sites of the DNRL film samples using a colorimeter (ColorFlex spectrophotometer, HunterLab, Reston, VA, USA). Before operating the instrument, the calorimeter was calibrated using standard black and white materials. The color analysis was performed in the Commission Internationale de l’Eclairage (CIE)-L*a*b* three-dimensional color space system. The lightness (L) value represents the scale from the darkest black at 0 to the brightest white at 100. The color channels (a and b) serve as indicators of the four unique colors of human vision. The a* axis indicates the green–red opposition, with green represented by negative values and red by positive values. The b* axis represents the blue–yellow oppositions, with blue as negative values and yellow as positive values. The color of the dried DNRL films was measured in quadruplicate, and the color difference (ΔE) was calculated using Equation (3). Subsequently, the National Bureau of Standards (NBS) color difference scales were determined using Equation (4).
The NBS rating system was employed to assess the level of color change in each sample. The NBS rating scale is as follows: 0.0–0.5 = extremely slight change; 0.5–1.5 = slight change; 1.5–3.0 = perceivable change; 3.0–6.0 = marked change; 6.0–12.0 = extremely marked change; and >12.0 = change to another color [17].
2.4.6. Percentage of Swelling
For the analysis of the swelling behavior, the DNRL film specimens were prepared by cutting them into square shapes measuring 10 mm × 10 mm. The initial weight of each specimen was measured and recorded as W0. These specimens were then immersed in 50 mL of distilled water at room temperature and allowed to swell for 48 h. After removing any excess water from the hydrated films, their final weight was measured and recorded as Ws. The percentage of swelling was calculated using Equation (5), taking into account the initial and final weights. This experiment was performed with five repetitions to ensure accuracy and consistency of the results.
2.5. Statistical Data Analysis
The experimental data obtained from the study were analyzed statistically. The data are presented as mean ± standard deviation (S.D.). To determine the statistical significance, a one-way analysis of variance (ANOVA) was conducted, and a significance level of p < 0.05 was used. Subsequently, Scheffe’s post-hoc test was employed to compare the means of each group that showed significant differences. The statistical data analysis was carried out using the Microsoft® Excel® 2019 MSO program (Version 2305 Build 16.0.16501.20074).
3. Results
3.1. Effect of Additives on the Stability of NRL
The stability of NRL was investigated by incubating it at room temperature for 2 h without any additives or preservatives. It was observed that the NRL without additives or preservatives underwent coagulation with a foul smell, and its color changed from white to a dark yellowish amber color. However, when NRL was stabilized with additives and preservatives, different levels of stability were achieved, as shown in Table 1. It was found that 0.2% w/w NH3 as a preservative provided longer stability to NRL compared to 1% w/w Uniphen P-23, both with and without additives. While some additives resulted in worse stability, causing aggregation of NRL, there were certain additives that showed improved stability. Among them, Texapon N70, SLS, Dehyton K, Poloxamer 407, and KOH were identified as the best additives in their respective groups for maintaining the stability of NRL, particularly when NH3 was used as a preservative. Therefore, these additives were selected for further investigation in the subsequent study.
3.2. Properties of NRL and DNRL
Table 2 shows the various properties of NRL and DNRL. The pH value, viscosity, and TSC of NRL were determined to be 6.40 ± 0.07, 8.71 ± 0.79 cps, and 41.26 ± 0.10%, respectively. The protein content of raw NRL in the studied batch was found to be 3.50 ± 0.34%. For the scale-up batch, the pH, viscosity, TSC, and protein content of raw NRL were measured as 6.28 ± 0.27, 7.86 ± 0.47 cps, 45.00 ± 1.60%, and 3.39 ± 0.04%, respectively. These values in both batches were similar, and they were used for comparison with the protein content of each DNRL sample to calculated the protein reduction effectiveness. In this study, deproteinization was performed using various additives and processes. The pH value, viscosity, TSC, protein reduction of NRL, and color of NR films were evaluated as part of this investigation. The acceptance criteria for these properties were established as follows: the pH should not be excessively high (>12) or too low (<3) to avoid skin irritation; the viscosity should be low (<50 cps) to facilitate easy pouring during NR production processes; the TSC should be >20% to indicate a lower loss of rubber mass during the deproteinization process; the protein reduction should be >80% to signify an effective deproteinization process; and the NBS color change should be >12.0 to demonstrate a noticeable change to a different color. The statistical data analyses of protein reduction and color change are presented in the supplementary materials (Tables S1 and S2).

Table 2.
Properties of NRL and DNRL.
In the experiment, two preservatives were used in NRL: 0.2% w/w NH3 and 1% w/w Uniphen P-23. The addition of these preservatives resulted in changes in the properties of the prepared DNRL compared to raw NRL. The TSC and viscosity of DNRL decreased, while the pH value increased when compared to raw NRL. It was observed that when 0.2% w/w NH3 was used as a preservative in NRL, the increase in pH of the prepared NRL was greater than that observed in the latex using 1% w/w Uniphen P-23 as a preservative. Furthermore, the nitrogen content in NRL treated with 0.2% w/w NH3 was lower than that observed when using 1% w/w Uniphen P-23 as a preservative in NRL. The reduction in protein content in NRL was more efficient when 0.2% w/w NH3 was used as a preservative without additives, compared to 1% w/w Uniphen P-23. However, the difference between the two preservatives was not statistically significant, with a p-value of 0.1848.
Both NRL and prepared DNRL were capable of being fabricated into transparent thin films after the drying process. The thickness of each film was controlled within the range of 400 to 600 μm, and the color of the films was evaluated. The color measurements were represented by the L*, a*, and b* values of the NRL film, which were used as reference values for calculating ∆E and NBS in the DNRL films. The color results are presented in Table 3. The dried NRL film exhibited a dark yellow color, with a high b* value indicating a yellowish hue. In contrast, both NRL and DNRL films had high L* values, indicating transparency and brightness. The a* values were close to 0, indicating the absence of red or blue shades in the rubber films. The change in b* values in DNRL was an important factor indicating the color change (∆E and NBS) of the rubber films, as these values exhibited greater variations compared to both L* and a* values.

Table 3.
Color properties of dried NRL and DNRL films.
The statistical analysis revealed significant differences between the preservatives (NH3 and Uniphen P-23) in terms of the a* and ∆E values (p-value = 0.0045 and 0.0411, respectively). However, when Scheffe’s post-hoc test was conducted on the average data of these groups, it only showed significant differences in the a* values of the NRL and DNRL films with the NH3 group, as well as the DNRL films with both the NH3 and Uniphen P-23 groups. No significant differences were observed among the groups in terms of the ∆E values. Although the NBS values of both DNRL films with NH3 and Uniphen P-23 were higher than 12.0, indicating a change to other colors, the statistical tests yielded ambiguous results. This suggests that while there may be noticeable color changes in the films, the statistical analysis did not provide clear differentiation between the groups.
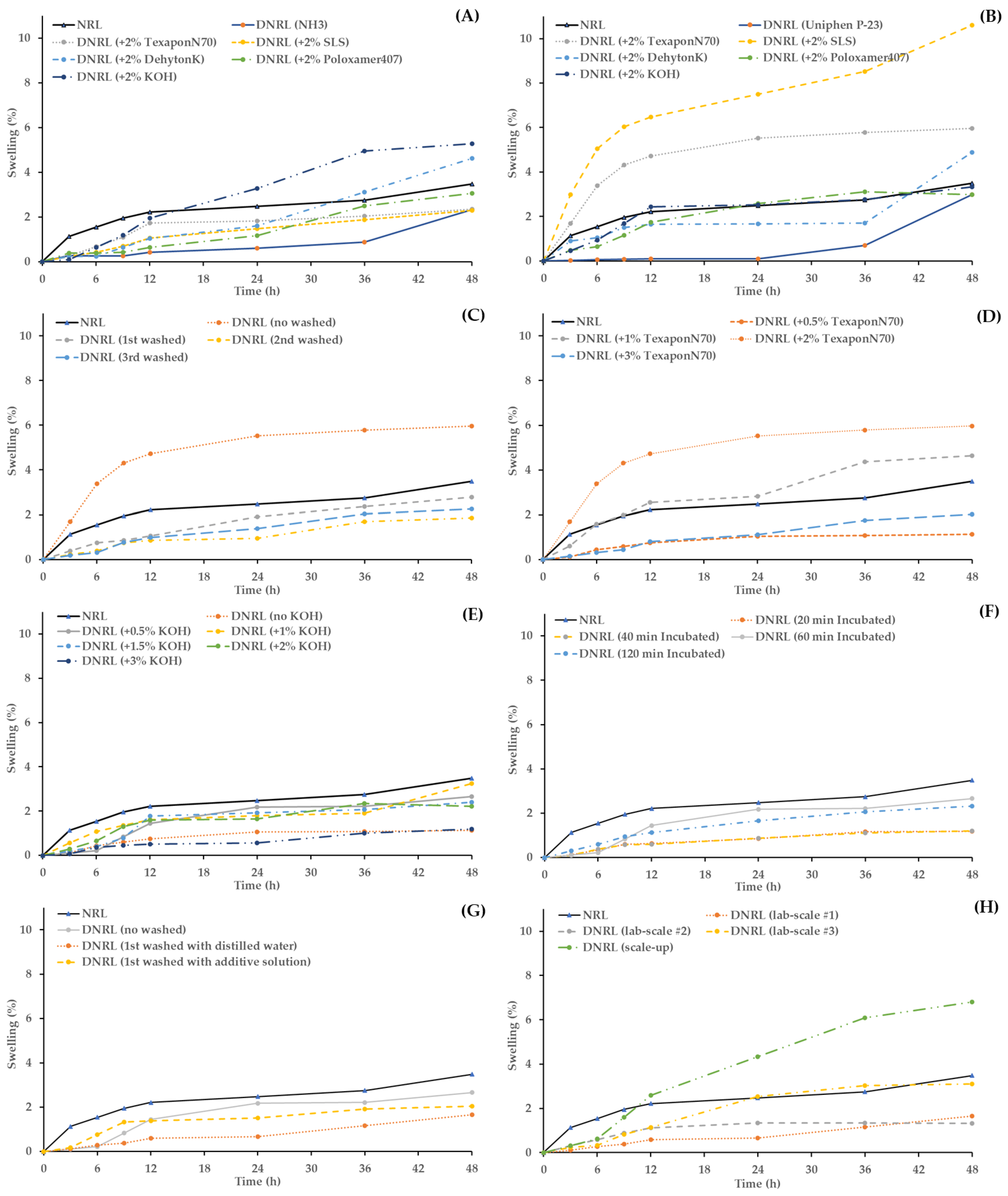
The swelling properties of both NRL and prepared DNRL films in distilled water over a 48-h period were evaluated, and the results are shown in Figure 2. The error bars for each time point were removed from the figures due to overlapping lines. NRL, being a hydrophobic rubber polymer, exhibited low swelling (3.49 ± 0.45% at 48 h). However, DNRL demonstrated even lower swelling properties compared to NRL in both films prepared using NH3 and Uniphen P-23 as preservatives.
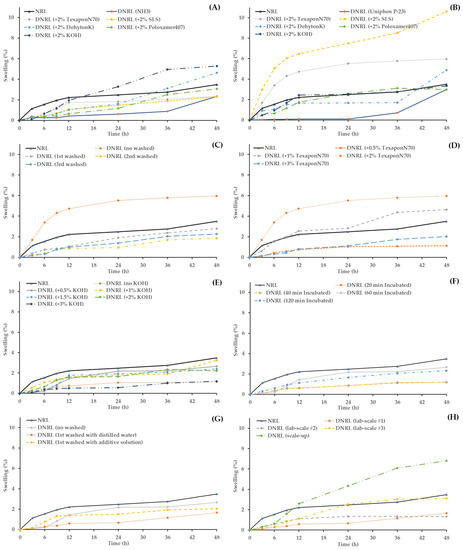
Figure 2.
Swelling properties of dried NRL and DNRL films: Effect of (A) additive with 0.2% w/w NH3 as a preservative, (B) additive with 1% w/w Uniphen P-23 as a preservative, (C) washing cycle with 1% w/w Uniphen P-23 and 2% w/w Texapon N70, (D) concentration of Texapon N70 with 1% w/w Uniphen P-23, (E) concentration of KOH with 1% w/w Uniphen P-23 and 0.5% w/w Texapon N70, (F) incubation time with 1% w/w Uniphen P-23, 0.5% w/w Texapon N70, and 0.5% w/w KOH, (G) washing solution with 1% w/w Uniphen P-23, 0.5% w/w Texapon N70, and 0.5% w/w KOH, and (H) reproducibility and scale-up of optimum DNRL formulation.
3.2.1. Effect of Additive
The effect of various additives on the properties of DNRL was investigated. The results showed an interesting trend in the properties of prepared DNRL with different additives. The one-way ANOVA analysis revealed significant differences in the protein reduction among the films treated with additives using NH3 and Uniphen P-23 as preservatives (p-value = 0.0064 and <0.0001, respectively). For films treated with NH3, the Scheffe’s post-hoc analysis indicated significant differences in protein reduction means between without additive/Texapon N70 group, Texapon N70/Poloxamer 407 group, and SLS/Poloxamer 407 group. In the case of films treated with Uniphen P-23, the Scheffe’s post-hoc analysis showed significant differences in protein reduction means between the groups without additive and all studied additives groups, as well as between the Texapon N70/Dehyton K group and the SLS/Dehyton K, Poloxamer 407, and KOH groups. Overall, the combination of Uniphen P-23 and additives resulted in higher protein reduction compared to the combination of NH3 and additives.
The addition of different additives and preservatives during the deproteinization process had an impact on the color properties of the prepared DNRL films. The films became brighter, as indicated by increased L* values, but also started to exhibit a yellow shade, leading to decreased b* values. This change in color resulted in higher ∆E and NBS values. When comparing the effects of different preservatives, it was found that 1% w/w Uniphen P-23, when used in conjunction with Texapon N70, Dehyton K, and Poloxamer 407, was more effective in reducing the color of DNRL films compared to 0.2% w/w NH3. However, when 0.2% w/w NH3 was used as a preservative along with SLS and KOH, as well as in the absence of additives, it proved to be effective in diminishing the color of DNRL films.
In the groups using NH3 as a preservative, significant differences were observed in the L*, a*, b*, and ∆E values when different additives were added (p-value < 0.0001 for all parameters). The Scheffe’s post-hoc test for ∆E showed significant differences between the no additive/SLS group, Texapon N70/SLS and Poloxamer 407 groups, SLS/Dehyton K, Poloxamer 407, and KOH groups, as well as the Poloxamer 407/KOH group. This indicates that SLS contributed the most to the discoloration of DNRL films in this preservative group. On the other hand, in the Uniphen P-23 groups with different additives, significant differences were found in the a*, b*, and ∆E values (p-value = 0.0049, <0.0001, and <0.0001, respectively). However, after the Scheffe’s post-hoc test, only the b* and ∆E values showed significant differences among the groups. The summarized ∆E data indicated significant differences between the no additive/Texapon N70, SLS, and Dehyton K groups, Texapon N70/Poloxamer 407 and KOH groups, and SLS/Dehyton K, Poloxamer 407, and KOH groups. This suggests that the surfactant performed better than other additives in terms of color change in DNRL films. Based on these results, 2% w/w Texapon N70 was selected as the additive for the next step.
When various additives and preservatives were added, the swelling percentages of the dried films generally decreased. However, it was observed that 2% w/w KOH increased the swelling percentages of the dried films when 0.2% w/w NH3 was used as a preservative (Figure 2A). Additionally, 2% w/w SLS and 2% w/w Texapon N70 increased the swelling percentages of the dried films when 1% w/w Uniphen P-23 was used as a preservative (Figure 2B). These results indicate an increase in the polarity of alkali (KOH) and surfactants (SLS and Texapon N70) in the rubber films, which led to increased swelling.
3.2.2. Effect of Washing Cycle
The effect of the washing cycle on DNRL properties was investigated, and it was observed that increasing the washing cycle using distilled water as a medium resulted in a significant decrease in various DNRL properties (p-value = 0.0056). Specifically, in the 2nd and 3rd washing cycles, there was a significant difference in protein reduction compared to the group without washing. The washing cycle also had a significant impact on the L*, b*, and ∆E values of DNRL films (p-value < 0.0001 for all three parameters). The Scheffe’s post-hoc test indicated a significant color change between the no washing group and the groups subjected to washing with distilled water. The 3rd washing cycle showed a significant color difference compared to the lower degrees of washing, while the 1st and 2nd washes did not exhibit a significant color difference. Although increasing the washing cycle resulted in reduced nitrogen content and improved decoloration of the thin film, it could also reduce the TSC and increase production costs. Therefore, for DNRL preparation in this study, it was determined that the 1st washing cycle was appropriate.
The DNRL prepared from 2% w/w Texapon N70 and 1% w/w Uniphen P-23 showed higher swelling properties compared to NRL. However, the washing process with distilled water had a decreasing effect on the swelling properties of DNRL films, and their swelling percentages were also lower than that of NRL. Interestingly, the dried films’ swelling percentages of DNRL with 1–3 washing cycles did not show significant differences (Figure 2C). This suggests that the number of washing cycles did not have a significant impact on the swelling properties of DNRL films prepared with Texapon N70 and Uniphen P-23.
3.2.3. Effect of Concentration of Additive
The concentration of Texapon N70 used as an additive in DNRL had no significant effect on various properties except for protein reduction efficacy (p-value < 0.0001). Increasing the concentration of Texapon N70 resulted in a significant reduction in nitrogen content, indicating improved protein reduction. The concentration of Texapon N70 also had a significant impact on the L*, a*, b*, and ΔE values (p-value < 0.0001 in all parameters) of DNRL films, indicating changes in color. Scheffe’s post-hoc test revealed significant differences in color between higher concentrations (2 or 3% w/w) and lower concentrations (0, 0.5, or 1% w/w) of Texapon N70. This highlights the role of surfactants in both deproteinization and decolorization of the rubber films. However, it is important to note that high concentrations of surfactants may cause skin irritation and other adverse effects. Therefore, the lowest concentration of Texapon N70 (0.5% w/w) was chosen for further study, and additional methods were considered to achieve both color and protein reduction in DNRL.
Regarding the swelling properties of DNRL films with 1% w/w Uniphen P-23 preservative, the dried films’ swelling percentages tended to increase with the increasing concentration of Texapon N70 up to 2% w/w. However, when 3% w/w Texapon N70 was used, the swelling percentage decreased (Figure 2D).
3.2.4. Effect of Alkali Solution
The addition of KOH as an alkali solution, together with 0.5% w/w Texapon N70, had several effects on DNRL. It increased the TSC and pH value of DNRL, as shown in Table 2. Moreover, the inclusion of KOH resulted in a significant decrease in nitrogen content, indicating effective protein reduction (p-value < 0.0001). All concentrations of KOH showed significant protein reduction compared to the condition without KOH. Although increasing the concentration of KOH led to a slight decrease in protein content in DNRL, the difference was not statistically significant. Therefore, for the purpose of minimizing the use of alkali substance in the protein reduction process, 0.5% w/w KOH was selected for further study in combination with 0.5% w/w Texapon N70.
Regarding the decoloration factor, the concentration of KOH significantly influenced the L*, a*, b*, and ∆E values (p-value < 0.0001 in all parameters). All DNRL films with KOH exhibited higher ∆E values compared to NRL films. However, increasing the concentration of KOH tended to decrease the ∆E values, indicating improved decoloration. Scheffe’s post-hoc test showed a significant color change between all groups, except between the 2% and 3% w/w KOH concentrations. This suggests that lower concentrations of KOH were more effective in achieving decoloration.
In terms of swelling properties, the dried DNRL films prepared with 1% w/w Uniphen P-23 + 0.5% w/w Texapon N70 exhibited lower percentage swelling compared to dried NRL films. Additionally, the inclusion of KOH as an additive also resulted in lower percentage swelling compared to dried NRL films. The dried DNRL films’ swelling percentages did not differ significantly when the concentration of KOH was increased up to 2% w/w. However, the swelling percentage decreased when 3% w/w KOH was incorporated (Figure 2E).
3.2.5. Effect of Incubation Time
The properties of DNRL films fabricated with different incubation times in the protein reduction process were examined. The pH, viscosity, and TSC properties of DNRL did not show any significant differences based on the incubation time. However, the protein reduction efficiency was significantly affected by the incubation time (p-value = 0.0054), although in an inconsistent manner. Initially, increasing the incubation time led to higher protein reduction efficiency. However, after a prolonged incubation period, the efficiency of protein reduction decreased. The protein reduction achieved with 120 min of incubation time was significantly different compared to both 40 and 60 min of incubation. Therefore, for the next step, an incubation time of 60 min was chosen due to its efficiency in reducing nitrogen content.
The different incubation times had a significant impact on the color change of DNRL films in all parameters (p-value = 0.0001, <0.0001, <0.0001, and 0.0008 for L*, a*, b*, and ∆E, respectively). Scheffe’s post-hoc test revealed a significant difference between 20 and 40 min, as well as between 40 and 60 min of incubation time. Although the 60-min incubation time resulted in the lowest color change (∆E and NBS), some discoloration was still noticeable.
The incubation time, when combined with all additives, had a slight effect on the swelling properties of DNRL films. The incubation times of 20 and 40 min exhibited the lowest swelling percentages, while an increase in the incubation time to 1 and 2 h tended to increase the dried films’ swelling percentages (Figure 2F). However, all the swelling profiles were lower than that of NRL films.
3.2.6. Effect of Washing Solution
The washing solution used in the deproteinization process had a significant impact on the properties of DNRL. When distilled water was used as the washing solution, it resulted in a decrease in TSC and nitrogen content. On the other hand, when an additive solution was used as the washing solution, it led to an increase in pH value and viscosity, but a decrease in TSC and effective reduction in nitrogen content compared to distilled water. The washing process, regardless of the solution used, significantly reduced the protein content compared to the unwashed batch (p-value < 0.0001). Additionally, after Scheffe’s post-hoc test, the protein reductions achieved with distilled water and additive solution washing were significantly different. However, distilled water was considered more suitable as a washing solution as it resulted in a lower concentration of additives in the final DNRL products.
The washing solution, whether distilled water or an additive solution, significantly altered the color of DNRL films in all parameters (p-value = 0.0001, <0.0001, <0.0001, and <0.0001 for L*, a*, b*, and ∆E, respectively). Scheffe’s post-hoc test indicated a significant difference between the average data of all groups, highlighting the impact of the washing solution on the color change.
The washing solution tended to decrease the swelling percentages of dried films compared to NRL and unwashed DNRL films (Figure 2G). Specifically, the dried films’ swelling profile was lower when washed with distilled water compared to films washed with an additive solution.
3.2.7. Reproducibility of Optimum DNRL Formulation
The reproducibility of the optimum DNRL formulation, which consisted of NRL, 1% w/w Uniphen P-23, 0.5% w/w Texapon N70, 0.5% w/w KOH, incubated for 60 min and washed with distilled water once, was evaluated. It was found that there was no significant difference in each batch in terms of pH, viscosity, TSC, protein reduction, and discoloration of DNRL films. This indicates that the deproteinization process was reproducible and consistently reduced the protein content by more than 90.57 ± 1.20% (data averaged from 3 reproducibility batches). Moreover, the process was successful in reducing the color of the thin films. However, there was only a small difference observed in the swelling percentages of the optimum DNRL films in each batch (Figure 2H).
3.2.8. Scale-Up of Optimum DNRL Formulation for Industrial Use
During the scale-up of the optimum DNRL formulation for industrial use, a larger batch of 20 L fresh NRL was used as the raw material, and a pilot-scale centrifugation instrument was operated for concentrated rubber production (Figure 3). The concentrated DNRL with 60% TSC was obtained from this instrument and then diluted with distilled water to reach the initial volume, which was similar to the laboratory scale. However, some differences were observed in the scaled-up process compared to the laboratory scale. The pH value and viscosity of the DNRL slightly increased, while the TSC significantly decreased. This reduction in TSC was attributed to the limitations of the industrial centrifugation process, which could not remove the aqueous serum from the DNRL completely. The deproteinization effectiveness of the scaled-up process was also lower than that of the laboratory scale, but it was still high enough for DNRL production (78.54 ± 0.90% effectiveness, 0.73 ± 0.03% protein content). This lower effectiveness was primarily due to the capacity limitations of the industrial centrifugation, which could not achieve the same level of protein reduction as the laboratory-scale process.

Figure 3.
Scale-up industrial centrifugation instrument for DNRL preparation.
In terms of the dried films’ swelling percentages, the scale-up process resulted in higher swelling compared to the DNRL films fabricated in the laboratory scale (Figure 2H). The swelling profile of the scaled-up process was also higher than that of an NRL film. Additionally, Scheffe’s post-hoc test revealed a significant difference in the discoloration effectiveness between the scale-up and laboratory levels. The lower color reduction observed in the scaled-up process could be attributed to the lower contaminant removal capacity of the industrial-scale equipment. Despite these differences, the scaled-up DNRL process still demonstrated acceptable protein reduction and color change, making it suitable for industrial production. Further optimization and adjustments may be necessary to improve the scale-up process and achieve even better results.
4. Discussion
NRL contains over 200 different types of proteins, among which 15 proteins (Hev b1 to Hev b15) have been officially listed as allergenic proteins by the World Health Organization (WHO)/International Union of Immunological Societies (IUIS) Allergen Nomenclature Sub-Committee [18]. These natural proteins in rubber have been associated with asymptomatic sensitization as well as IgE-mediated hypersensitivity. Various methods have been attempted to reduce protein contamination in NRL to produce low hypersensitive NRL materials [8,9,10,12,13,14,19,20,21,22,23]. The centrifugation method is commonly employed at an industrial scale for the commercial production of concentrated NRL. This process can reduce some proteins to obtain DNRL [24,25,26]. However, the efficiency of protein reduction is not yet perfect, and thus, a combination of chemical and physical methods should be developed to minimize the allergenicity risk of NRL proteins. Additionally, the color of the NR matrix is an important quality aspect that needs consideration. Pale-colored NR blocks or thin films are desirable features for high-quality products and can yield higher economic value [27]. Therefore, in this study, the use of additives and preservatives was investigated to reduce these proteins in NRL while maintaining its properties such as stability, extended shelf life, film formation, and film properties. Furthermore, the removal of other constituents apart from the rubber polymer in the latex is crucial as they may affect certain rubber properties, particularly the discoloration of NRL films. Ultimately, this developed process may also yield pale-colored rubber products.
In this study, various groups of additives were tested to assess their impact on protein removal and color properties of NRL. Firstly, two additives were examined: 0.2% w/w NH3 and 1% w/w Uniphen P-23, which were used as preservatives. These substances directly influenced the stability of NRL during the deproteinization process by inhibiting microbial growth during the latex incubation. The use of NH3 as a preservative in NRL technology has been widely reported in other studies [28,29]. It induces protein denaturation and precipitation, thus preserving the antimicrobial effect in NRL. Furthermore, NH3 helps to prevent rubber particle coagulation or clumping, extending the latex’s storage period [30]. Uniphen P-23, on the other hand, is composed of several parabens and phenoxyethanol compounds. It serves as a potent bacteriostatic agent against various microorganisms. The paraben compounds in Uniphen P-23 interfere with cellular membrane transfer processes and inhibit the synthesis of DNA, RNA, and enzymes in bacterial cells [31]. Additionally, phenoxyethanol enhances the permeability of the cell membrane to potassium ions and inhibits DNA and RNA synthesis [32]. The results of the study demonstrated that both preservatives effectively preserved the latex by preventing microbial growth and rubber particle coagulation.
However, it should be noted that certain additives, particularly non-ionic surfactants, solvents, and certain polymers, can cause rubber agglomeration even when preservatives are used. While non-ionic surfactants have been reported to enhance the stability of rubber latex due to their compatibility, the mechanism by which they stabilize rubber droplets is through steric stabilization on the droplet surface. Therefore, it is crucial to use appropriate concentrations of non-ionic surfactants to achieve optimal stability [33]. Using an excessive number of additives or rapidly introducing them into the latex can disrupt the natural latex stabilization mechanism, initiate rubber nucleation, and accelerate the formation of clumps in NRL. Similar issues can arise with solvent and polymer additives [34,35,36]. However, it is worth noting that a previous study by Wathen and Gent reported the stabilization of NRL using ethylene glycol [37]. In this study, the appropriate additives that prolonged the shelf life of NRL were found to be anionic and amphoteric surfactants, certain types of polymers, and alkalis. Both KOH and NaOH, as alkalis, were identified as the most effective additives in extending the shelf life due to their high pH, which enhances the negative charge on the surface of rubber particles. Similarly, anionic and amphoteric surfactants also increased the surface charges of the particles. Poloxamer 407, on the other hand, provided stability to NRL through a steric effect. These findings indicate that the mechanical stability resulting from the repulsive effect of surface charge is more crucial than steric stabilization.
The removal of proteins from NRL was successfully achieved by using additives in combination with high-speed centrifugation. The additives used in this study were found to increase the solubility of proteins present in natural latex [38]. Additionally, alkali substances were effective in breaking down the protein matrix and increasing protein solubility [39]. Centrifugation played a crucial role in separating the soluble proteins from rubber latex, leading to increased protein reduction in NRL [24,25,26,40]. The effectiveness of the additives in protein removal was ranked as follows: SLS, Texapon N70, KOH, Dehyton K, and Poloxamer 407. Among them, the anionic surfactant SLS demonstrated superior protein removal ability compared to the other additives. Both 2% w/w Texapon N70 and 2% w/w SLS exhibited significant reduction in nitrogen content in NRL when used in combination with either 0.2% w/w NH3 or 1% w/w Uniphen P-23 as a preservative. Moreover, both surfactants effectively reduced the coloration of DNRL films. It is worth noting that SLS, while highly efficient in protein removal, can be irritating to the skin and may cause darkening of the skin with prolonged use [41,42]. SLS has also been reported as an additive for protein reduction in NRL in previous studies [9,19,43,44]. On the other hand, Texapon N70 was considered safer for use and contact with the skin compared to SLS. Therefore, Texapon N70 was selected as the additive for further investigation in this work, as it had not been previously reported for protein removal in NRL. Furthermore, Uniphen P-23, a pharmaceutical additive, showed potential as a preservative when combined with other additives. It is widely used as a preservative in the cosmetic and pharmaceutical industries due to its high safety profile. In contrast, NH3 can cause skin irritation. Thus, 1% w/w Uniphen P-23 and 2% w/w Texapon N70 were chosen as the preservatives and additives for the subsequent study to explore their potential in DNRL technology.
The concentration of Texapon N70, an additive used for protein removal in NRL, had a direct impact on the efficiency of protein reduction. Increasing the concentration of Texapon N70 resulted in higher protein removal and the production of pale-colored NR films. However, it is important to note that higher concentrations of Texapon N70 may cause skin irritation. Therefore, it is recommended to use a lower concentration of Texapon N70 to maintain safety. KOH, another additive, can be used in combination with Texapon N70. KOH increases the pH above the isoelectric point (pI) value of proteins in NRL, which is approximately 4.0–11.2 [45]. This leads to a higher negative charge on the proteins, enhancing their solubility and facilitating their separation from NRL, thereby increasing the efficiency of protein removal [46]. Additionally, the color of dried DNRL films is also reduced with the use of KOH. However, there was no significant difference observed in protein removal and color change of DNRL films with increasing KOH concentrations. It is important to consider that higher concentrations of KOH can cause skin irritation. Therefore, the concentration of KOH should be used at the lowest effective concentration, which is 0.5% w/w. In summary, the chosen additives for this study were 0.5% w/w Texapon N70 and 0.5% w/w KOH. This combination provided effective protein removal from NRL and resulted in reduced coloration of DNRL films while considering both efficiency and safety considerations.
Several process parameters were found to have an impact on protein reduction and color change in rubber films. Increasing the number of washing cycles led to a decrease in the remaining protein content in NRL, resulting in reduced coloration of the dried DNRL film. However, it should be noted that this process also resulted in some loss of rubber mass. Furthermore, increasing the duration of the centrifugation process may lead to higher production costs and longer processing times. The optimum incubation time for the deproteinization process was determined to be 60 min. This specific time frame resulted in significantly higher protein reduction efficiency compared to shorter or longer incubation times. The choice of washing solution used in the leaching process also played a role in the properties of DNRL, particularly in terms of protein reduction and color change. Distilled water was found to be an effective washing solution, capable of minimizing the presence of contaminants in DNRL. Therefore, distilled water was selected as the preferred washing solution for the process. By optimizing these process parameters, it is possible to achieve efficient protein reduction and minimize coloration in DNRL while considering factors such as rubber mass loss, production costs, and processing time.
Furthermore, literature studies report that the development of light-colored NR has been achieved through the use of enzymatic browning inhibitors, such as sodium metabisulphite. Additionally, various chemicals, including NaOH and proteolytic enzymes, have been utilized to discolor the rubber. It has been observed that deproteinized rubber or rubber treated with urea exhibits a color similar to centrifuged rubber, while rubber treated with NaOH or subjected to saponification demonstrates the lightest color index. The decolorization of NR is minimally affected by treatment with sodium metabisulphite. Given their low nitrogen concentration, deproteinized rubber and saponified rubber are considered suitable methods for producing light-colored NR [47,48]. Therefore, this study aimed to focus on the development of DNRL preparations that result in light-colored rubber films. The selection of safe additives and the optimization of the centrifugation process were conducted to make it suitable. The results showed that the process of reducing protein content in NRL not only effectively reduces the amount of protein but can also have an impact on other properties, particularly in terms of decolorization. As the protein is eliminated, it may also result in the removal of other components present in the latex, leading to a noticeable reduction in the color change of the rubber [27]. It is important to note that while the deproteinization process in NRL promotes discoloration of the rubber during drying, it does not significantly alter other important properties of rubber, such as hydrophobicity or swelling of the rubber film. This indicates that the process can selectively target proteins while preserving other desired characteristics of the rubber material. This aspect is beneficial as it enhances the value of rubber raw materials, making them suitable for use in high-value industries such as medical products.
In summary, the optimum formulation for the production of DNRL involves the use of 1% w/w Uniphen P-23 as a preservative, 0.5% w/w Texapon N70 as an additive, and 0.5% w/w KOH as a co-additive. The deproteinization process includes incubating the latex at room temperature for 60 min, followed by centrifugation. The resulting rubber solid is washed with distilled water and centrifuged again. This optimized process can be reliably replicated on a laboratory scale, and the scale-up process using a pilot-scale centrifugation instrument for concentrated rubber production was successful. While the efficiency of protein removal and decolorization achieved on the pilot scale may be slightly lower than that achieved in the laboratory, it is still sufficiently effective for industrial application. Additionally, this method offers the advantages of simplicity, shorter processing time, and the ability to store the DNRL at room temperature for an extended shelf life. Overall, the developed process and formulation provide a practical and effective means to produce DNRL with reduced protein content and improved color properties, making it suitable for various industrial applications.
5. Conclusions
This study focused on developing a deproteinization process for natural rubber latex (NRL) using various additives and preservatives. The aim was to achieve deproteinized NRL (DNRL) with improved properties such as enhanced latex stability, extended shelf life, and lighter color in the resulting films. Additives including surfactants, preservatives, solvents, polymers, and alkali solutions were selected to influence the shelf life of NRL. Texapon N70 and Uniphen P-23 were identified as suitable surfactants and preservatives, respectively, for the deproteinization process. The process involved incubating the NRL with the selected additives for an appropriate duration, followed by leaching and centrifugation to remove the soluble serum. Increasing the amount of Texapon N70 and the number of re-centrifugation cycles led to improved deproteinization and color reduction, as well as enhanced properties of the dried DNRL films. KOH was found to be a suitable co-additive that enhanced the deproteinization process of NRL and reduced the required amount of Texapon N70. The optimized conditions determined in this study were as follows: 1% w/w Uniphen P-23, 0.5% w/w Texapon N70, 0.5% w/w KOH, a 60-min incubation time, and one cycle of leaching with distilled water. These conditions resulted in a significant reduction in protein contamination by 90.57 ± 1.20% and a color change (∆E) of 433.69 ± 20.23. Importantly, the developed fabrication conditions were reproducible and could be scaled up to pilot-scale proportions. In conclusion, this study successfully developed a deproteinization process for NRL using additives and preservatives, resulting in DNRL with improved properties. The optimized conditions provide a practical approach for producing DNRL on a larger scale while maintaining or enhancing its desirable characteristics. Further investigations should be conducted to evaluate the influence of these effective additives on the molecular mass of the NR polymer and its viscoelastic properties. Understanding the specific mechanisms by which these additives influence NR protein reduction and related properties will provide valuable insights for optimizing the deproteinization process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su151310015/s1.
Author Contributions
Conceptualization, J.S. and W.P.; methodology, J.S. and W.P.; software, J.S.; validation, J.S., W.T. and W.P.; formal analysis, J.S., W.T., T.P. and W.P.; investigation, C.B. and N.P.; resources, W.P.; data curation, J.S. and W.P.; writing—original draft preparation, J.S. and W.P.; writing—review and editing, T.P. and W.P.; visualization, W.P.; supervision, W.P.; project administration, W.P.; funding acquisition, W.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Thai Government Budget Grant (Government Strategy) Year 2015, under grant number PHA580687a. The financial assistance provided by this funding source was crucial in conducting the study and achieving its objectives.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to the Songkhla Rubber Research Center, Rubber Authority of Thailand, for providing the pilot-scale concentrated rubber production instrument, which was instrumental in conducting this study. Special thanks are extended to Saffanah Mohd Ab Azid for her diligent proofreading of this paper, ensuring its accuracy and readability. The support and contributions from these individuals and institutions have greatly facilitated the successful execution of this research project.
Conflicts of Interest
The authors declare that they have no conflict of interest regarding the publication of this research.
References
- Yunyongwattanakorn, J.; Tanaka, Y.; Kawahara, S.; Klinklai, W.; Sakdapipanich, J. Effect of non-rubber components on storage hardening and gel formation of natural rubber during accelerated storage under various conditions. Rubber Chem. Technol. 2003, 76, 1228–1240. [Google Scholar] [CrossRef]
- Tarachiwin, L.; Sakdapipanich, J.; Ute, K.; Kitayama, T.; Tanaka, Y. Structural characterization of α-terminal group of natural rubber. 2. decomposition of branch-points by phospholipase and chemical treatments. Biomacromolecules 2005, 6, 1858–1863. [Google Scholar] [CrossRef]
- Oouchi, M.; Ukawa, J.; Ishii, Y.; Maeda, H. Structural analysis of the terminal groups in commercial hevea natural rubber by 2D-NMR with DOSY filters and multiple-WET methods using ultrahigh-field NMR. Biomacromolecules 2019, 20, 1394–1400. [Google Scholar] [CrossRef]
- Ernst Kerche-Silva, L.; Gomes Silva Morais Cavalcante, D.; Silva Danna, C.; Silva Gomes, A.; Moratto Carrara, I.; Lourenço Cecchini, A.; Yoshihara, E.; Eloizo Job, A. Free-radical Scavenging properties and Cytotoxic Activity Evaluation of Latex C-serum from Hevea brasiliensis RRIM 600. Free Rad. Antioxid. 2016, 7, 107–114. [Google Scholar] [CrossRef]
- Guerra, N.B.; Sant’Ana Pegorin, G.; Boratto, M.H.; de Barros, N.R.; de Oliveira Graeff, C.F.; Herculano, R.D. Biomedical applications of natural rubber latex from the rubber tree Hevea brasiliensis. Mater. Sci. Eng. C. 2021, 126, 112126. [Google Scholar] [CrossRef] [PubMed]
- Deval, R.; Ramesh, V.; Prasad, G.B.; Jain, A.K. Natural rubber latex allergy. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 304–310. [Google Scholar] [CrossRef]
- Jankangram, W. Natural rubber latex protein. Asia Pac. J. Sci. Technol. 2017, 18, 996–1002. [Google Scholar]
- Perrella, F.W.; Gaspari, A.A. Natural rubber latex protein reduction with an emphasis on enzyme treatment. Methods 2002, 27, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Pichayakorn, W.; Suksaeree, J.; Boonme, P.; Taweepreda, W.; Ritthidej, G.C. Preparation of deproteinized natural rubber latex and properties of films formed by itself and several adhesive polymer blends. Ind. Eng. Chem. Res. 2012, 51, 13393–13404. [Google Scholar] [CrossRef]
- Pichayakorn, W.; Suksaeree, J.; Taweepreda, W. Improved deproteinization process for protein-free natural rubber latex. Adv. Mater. Res. 2014, 844, 474–477. [Google Scholar] [CrossRef]
- Jayadevan, J.; Alex, R.; Gopalakrishnapanicker, U. Deproteinised natural rubber latex grafted poly(dimethylaminoethyl methacrylate)-poly(vinyl alcohol) blend membranes: Synthesis, properties and application. Int. J. Biol. Macromol. 2018, 107, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Suksaeree, J.; Waiprib, R.; Pichayakorn, W. Improving the hydrophilic properties of deproteinized natural rubber latex films for lidocaine transdermal patches by starch blending. J. Polym. Environ. 2022, 30, 1574–1586. [Google Scholar] [CrossRef]
- Nanti, S.; Wongputtisin, P.; Sakulsingharoj, C.; Klongklaew, A.; Chomsri, N. Removal of allergenic protein in natural rubber latex using protease from Bacillus sp. Food Appl. Biosci. J. 2017, 2, 216–223. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nghia, P.T.; Klinklai, W.; Saito, T.; Kawahara, S. Removal of proteins from natural rubber with urea and its application to continuous processes. J. Appl. Polym. Sci. 2008, 107, 2329–2332. [Google Scholar] [CrossRef]
- ASTM D1076; Standard Practice for Standard Specification for Rubber-Concentrated, Ammonia Preserved, Creamed, and Centrifuged Natural Latex D1076. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM D3533; Standard Test Method for Rubber-Nitrogen Content D3533. ASTM International: West Conshohocken, PA, USA, 2001.
- Al Qahtani, M.Q.; Binsufayyan, S.S. Color change of direct resin-based composites after bleaching: An in vitro study. King Saud Univ. J. Dent. Sci. 2011, 2, 23–27. [Google Scholar] [CrossRef]
- Parisi, C.A.S.; Kelly, K.J.; Ansotegui, I.J.; Gonzalez-Díaz, S.N.; Bilò, M.B.; Cardona, V.; Park, H.-S.; Braschi, M.C.; Macias-Weinmann, A.; Piga, M.A.; et al. Update on latex allergy: New insights into an old problem. World Allergy Organ. J. 2021, 14, 100569. [Google Scholar] [CrossRef] [PubMed]
- Chaikumpollert, O.; Yamamoto, Y.; Suchiva, K.; Kawahara, S. Protein-free natural rubber. Colloid Polym Sci. 2012, 290, 331–338. [Google Scholar] [CrossRef]
- Nawamawat, K.; Sakdapipanich, J.T.; Ho, C.C. Effect of Deproteinized Methods on the Proteins and Properties of Natural Rubber Latex during Storage. Macromol. Symp. 2010, 288, 95–103. [Google Scholar] [CrossRef]
- Kongkaew, C.; Loykulnant, S.; Chaikumpollert, O.; Suchiva, K. Creaming of Skim Natural Rubber Latex with Chitosan Derivatives. J. Appl. Polym. Sci. 2010, 115, 1022–1031. [Google Scholar] [CrossRef]
- Kawahara, S.; Klinklai, W.; Kuroda, H.; Isono, Y. Removal of proteins from natural rubber with urea. Polym. Adv. Technol. 2004, 15, 181–184. [Google Scholar] [CrossRef]
- Nurulhuda, A.; Aziana, A.H.; Norazreen, A.R.; Aziah, A.N.; Qamarina, M.S.N. Urea as a single denaturing agent in deproteinisation of natural rubber latex. J. Rubber Res. 2019, 22, 99–107. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Saiwari, S.; Soontaranon, S.; Masa, A. Influence of Centrifugation Cycles of Natural Rubber Latex on Final Properties of Uncrosslinked Deproteinized Natural Rubber. Polymers 2022, 14, 2713. [Google Scholar] [CrossRef]
- Sekhar, B.C. Improvements to Centrifugation of Rubber Latex. WO2005007704A1, 21 July 2003. [Google Scholar]
- Shabinah Filza, M.S.; Siti Nor Qamarina, M.; Nurulhuda, A.; Norhanifah, M.Y.; Mohamad Akmal, A.R. Purified natural rubber latex: Investigating effect of single anionic surfactant and centrifugation process to latex properties and addition of bio-filler towards latex film performance. NSPM 2017 AIP Conf. Proc. 1985 2018, 1985, 040008. [Google Scholar] [CrossRef]
- Lv, M.; Fang, L.; Yu, H.; Rojruthai, P.; Sakdapipanich, J. Discoloration Mechanisms of Natural Rubber and Its Control. Polymers 2022, 14, 764. [Google Scholar] [CrossRef]
- Ho, C.C.; Ng, W.L. Surface study on the rubber particles in pretreated Hevea latex system. Colloid Polym. Sci. 1979, 257, 406–412. [Google Scholar] [CrossRef]
- Singh, M.; Esquena, J.; Solans, C.; Booten, K.; Tadros, T.F. Influence of hydrophobically modified inulin on the stability of vulcanized natural rubber latex. Colloids Surf. A Physicochem. Eng. Aspects. 2014, 451, 90–100. [Google Scholar] [CrossRef]
- Lu, L.J.; Kurup, V.P.; Fink, J.N.; Kelly, K.J. Comparison of latex antigens from surgical gloves, ammoniated and nonammoniated latex: Effect of ammonia treatment on natural rubber latex proteins. J. Lab. Clin. Med. 1995, 126, 161–168. [Google Scholar] [PubMed]
- Valkova, N.; Lépine, F.; Valeanu, L.; Dupont, M.; Labrie, L.; Bisaillon, J.-G.; Beaudet, R.; Shareck, F.; Villemur, R. Hydrolysis of 4-hydroxybenzoic acid esters (parabens) and their aerobic transformation into phenol by the resistant Enterobacter cloacae strain EM. Appl. Environ. Microbiol. 2001, 67, 2404–2409. [Google Scholar] [CrossRef]
- Gilbert, P.; Beveridge, E.C.; Crone, P.B. Effect of phenoxyethanol on the permeability of Escherichra coli NCTC 5933 to inorganic ions. Microbios. 1977, 19, 17–26. [Google Scholar]
- Singh, M.; Mei, E.L.H. Surfactants and their Use in Latex Technology. MRB Rubber Technol. Dep. 2013, 13, 33–36. [Google Scholar]
- Ng, J.W.; Othman, N.; Yusof, N.H. Various coagulation techniques and their impacts towards the properties of natural rubber latex from Hevea brasiliensis—A comprehensive review related to tyre application. Ind. Crops Prod. 2022, 181, 114835. [Google Scholar] [CrossRef]
- Zong-Qiang, Z.; He-Ping, Y.; Qi-Fang, W.; Guang, L. Effects of coagulation processes on properties of epoxidized natural rubber. J. Appl. Polym. Sci. 2008, 109, 1944–1949. [Google Scholar] [CrossRef]
- Chaiprapat, S.; Wongchana, S.; Loykulnant, S.; Kongkaew, C.; Charnnok, B. Evaluating sulfuric acid reduction, substitution, and recovery to improve environmental performance and biogas productivity in rubber latex industry. Process Saf. Environ. Prot. 2015, 94, 420–429. [Google Scholar] [CrossRef]
- Wathen, G.D.; Gent, A.N. Slow Crystallisation of Natural Rubber Latex. J. Nat. Rubb. Res. 1990, 5, 178–181. [Google Scholar]
- Parra, D.F.; Pinto Martins, C.F.; Collantes, C.H.D.; Lugao, A.B. Extractable proteins from field radiation vulcanized natural rubber latex. Nucl. Instrum. Methods Phys. Res. B 2005, 236, 508–512. [Google Scholar] [CrossRef]
- Deleu, L.J.; Lambrecht, M.A.; de Vondel, J.V.; Delcour, J.A. The impact of alkaline conditions on storage proteins of cereals and pseudo-cereals. Curr. Opin. Food Sci. 2019, 25, 98–103. [Google Scholar] [CrossRef]
- Sriring, M.; Nimpaiboon, A.; Kumarn, S.; Higaki, K.; Higaki, Y.; Kojio, K.; Takahara, A.; Ho, C.C.; Sakdapipanich, J. Film formation process of natural rubber latex particles: Roles of the particle size and distribution of non-rubber species on film microstructure. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124571. [Google Scholar] [CrossRef]
- Bondi, C.A.M.; Marks, J.L.; Wroblewski, L.B.; Raatikainen, H.S.; Lenox, S.R.; Gebhardt, K.E. Human and environmental toxicity of sodium lauryl sulfate (SLS): Evidence for safe use in household cleaning products. Environ. Health Insights 2015, 9, EHI.S31765. [Google Scholar] [CrossRef]
- Heetfeld, A.B.; Schill, T.; Schröder, S.S.; Forkel, S.; Mahler, V.; Pfützner, W.; Schön, M.P.; Geier, J.; Buhl, T. Challenging a paradigm: Skin sensitivity to sodium lauryl sulfate is independent of atopic diathesis. Br. J. Dermatol. 2020, 183, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Boonme, P.; Taweepreda, W.; Pichayakorn, W. Novel process in preparation of deproteinized natural rubber latex. Adv. Mater. Res. 2014, 844, 462–465. [Google Scholar] [CrossRef]
- Suksaeree, J.; Taweepreda, W.; Pichayakorn, W. Surfactant Treatment and Leaching Combination Process for Preparation of Deproteinized Natural Rubber Latex. Key Eng. Mater. 2015, 659, 500–504. [Google Scholar] [CrossRef]
- Dai, L.; Kang, G.; Li, Y.; Nie, Z.; Duan, C.; Zeng, R. In-depth proteome analysis of the rubber particle of Hevea brasiliensis (para rubber tree). Plant Mol. Biol. 2013, 82, 155–168. [Google Scholar] [CrossRef]
- Siti Maznah, K.; Baharin, A.; Hanafi, I.; Azhar, M.E.; Mas Rosemal Hakim, M.H. Effect of soaking in potassium hydroxide solution on the curing, tensile properties and extractable protein content of natural rubber latex films. Polym. Test. 2008, 27, 1013–1016. [Google Scholar] [CrossRef]
- Boonyang, H.; Sakdapipanich, J. The method to produce light-color natural rubber. Adv. Mater. Res. 2014, 844, 85–88. [Google Scholar] [CrossRef]
- Rojruthai, P.; Pareseecharoen, C.; Sakdapipanich, J. Physical decoloration in the concentration process of natural rubber. SPE Polym. 2021, 2, 210–216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).