Flash-Calcined Sediments for Zinc Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediments and Calcination Method
2.2. Zinc Removal Experiments
2.2.1. Aqueous Zn/Calcined Sediments: Batch Experiments
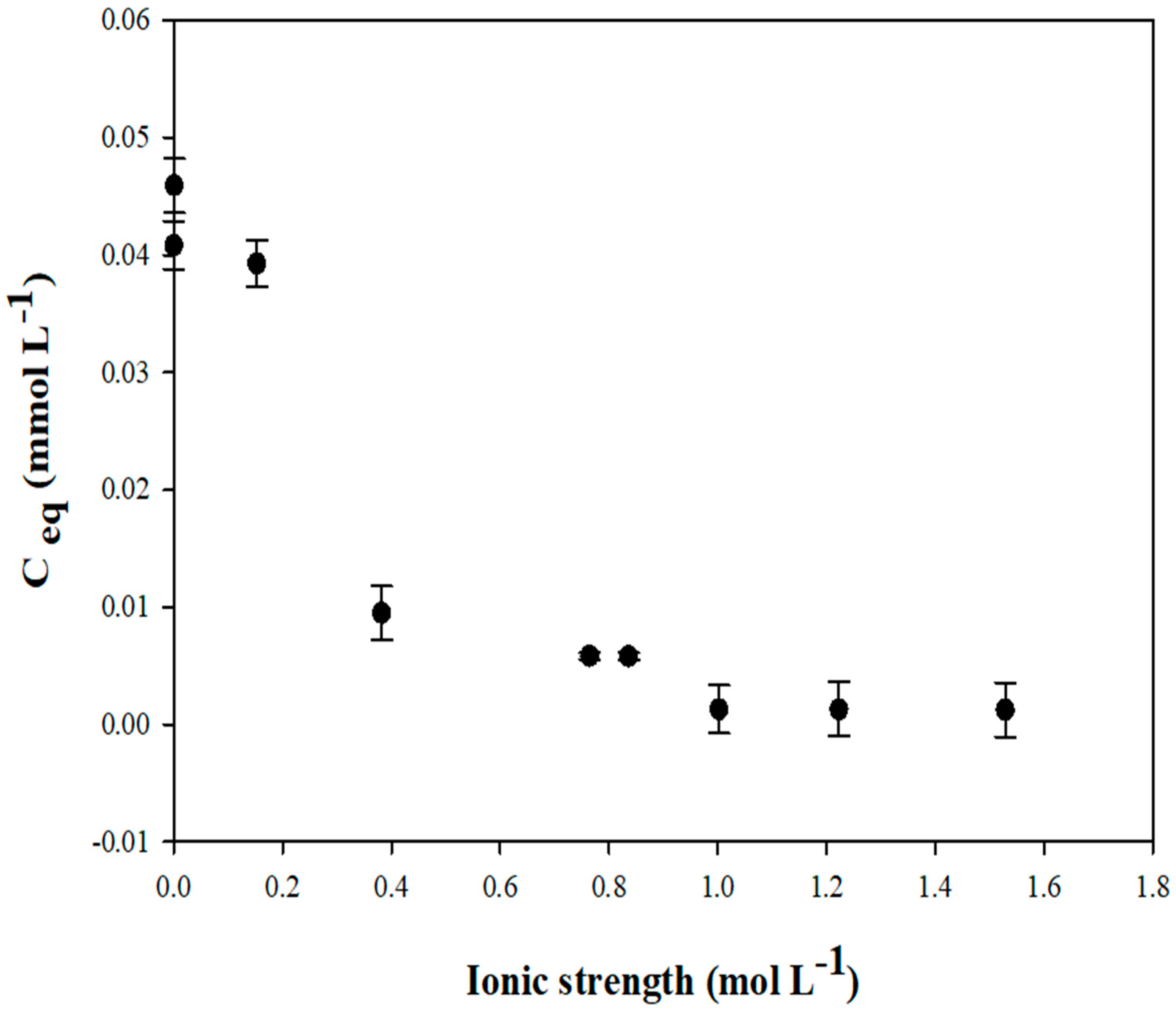
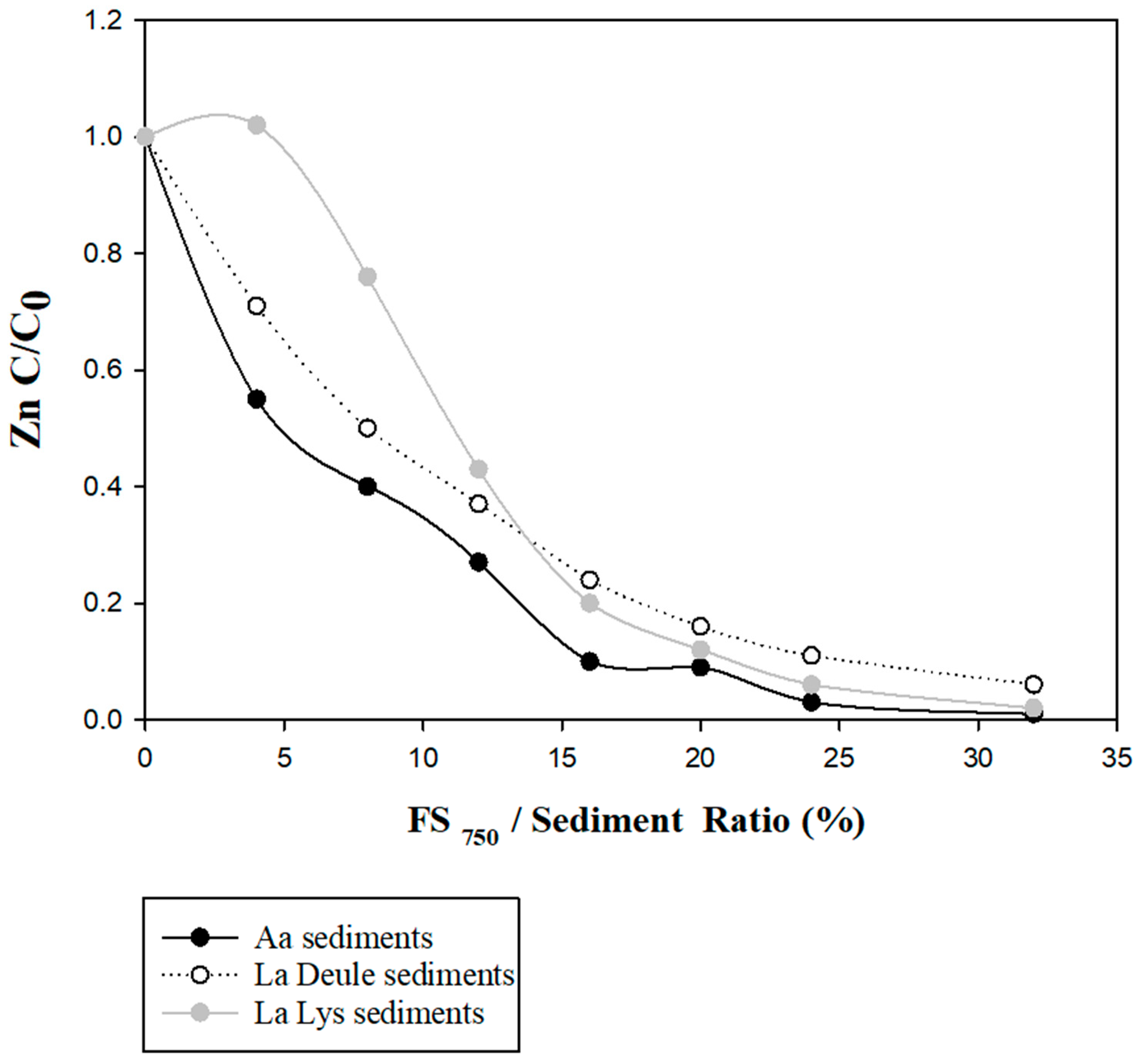
2.2.2. Stabilization of Zn-Spiked Sediments Using the Calcined Sediment
Spiking Experiments
Spiked Sediments’ Stabilization Tests
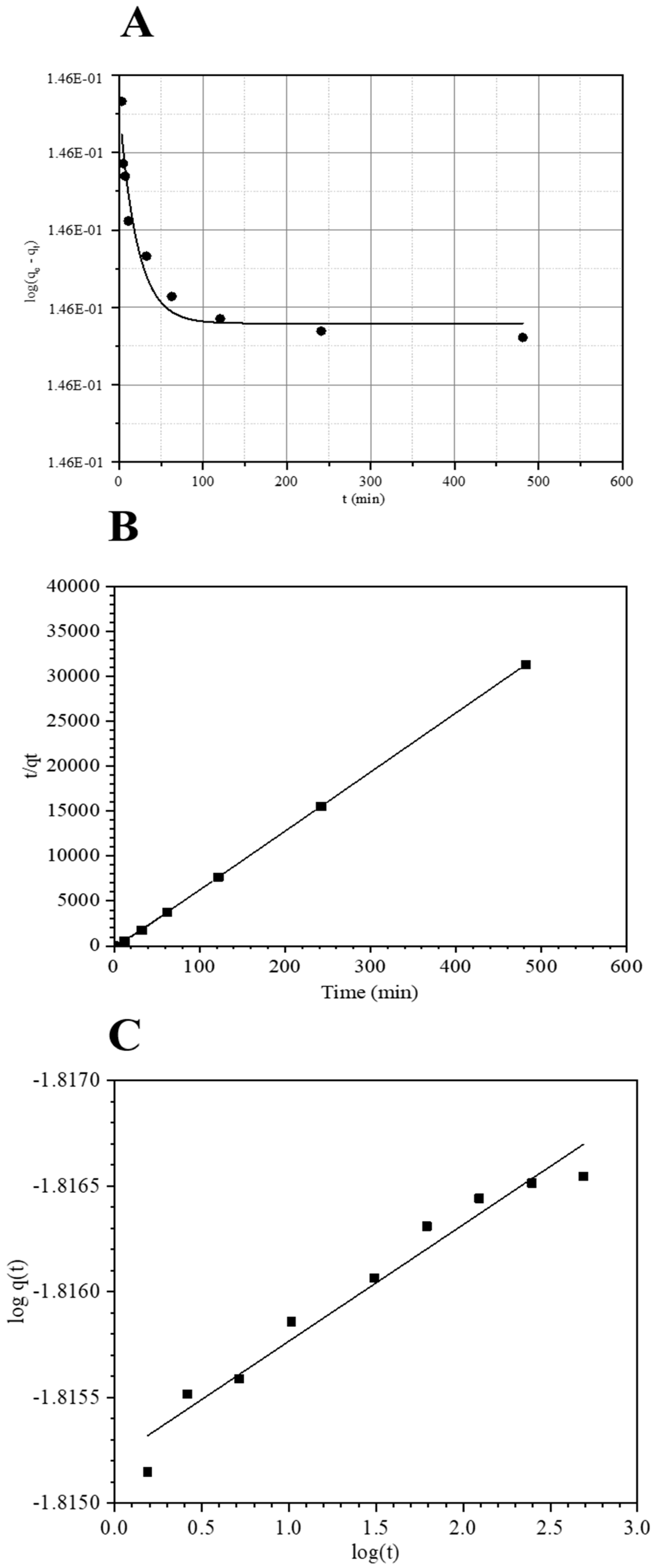
2.3. Kinetics and Sorption Modelling
2.3.1. Kinetic and Reliability
Consistency of the Kinetic Models
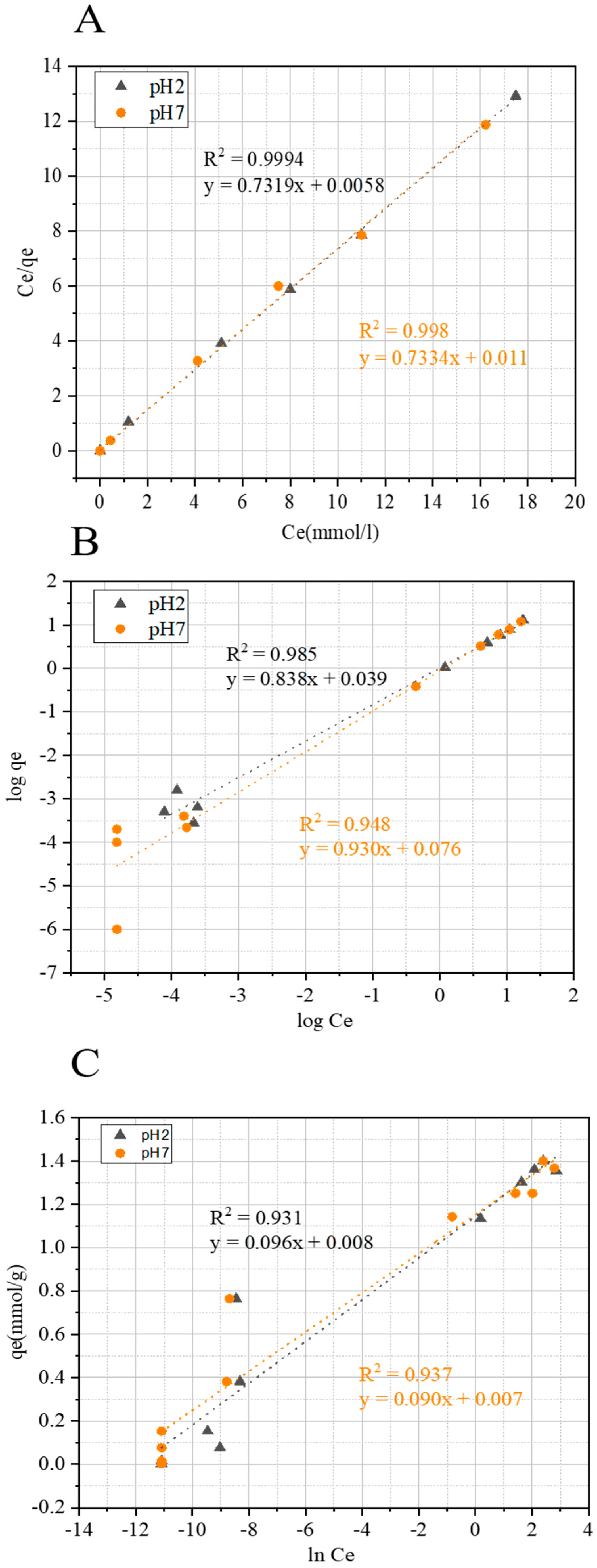
2.3.2. Adsorption Isotherm Models
2.4. Characterization of Adsorbent
3. Results and Discussion
3.1. Physico-Chemical Properties of Sediments before and after Flash Calcination
3.2. Aqueous Interactions of FCS750 with Zn
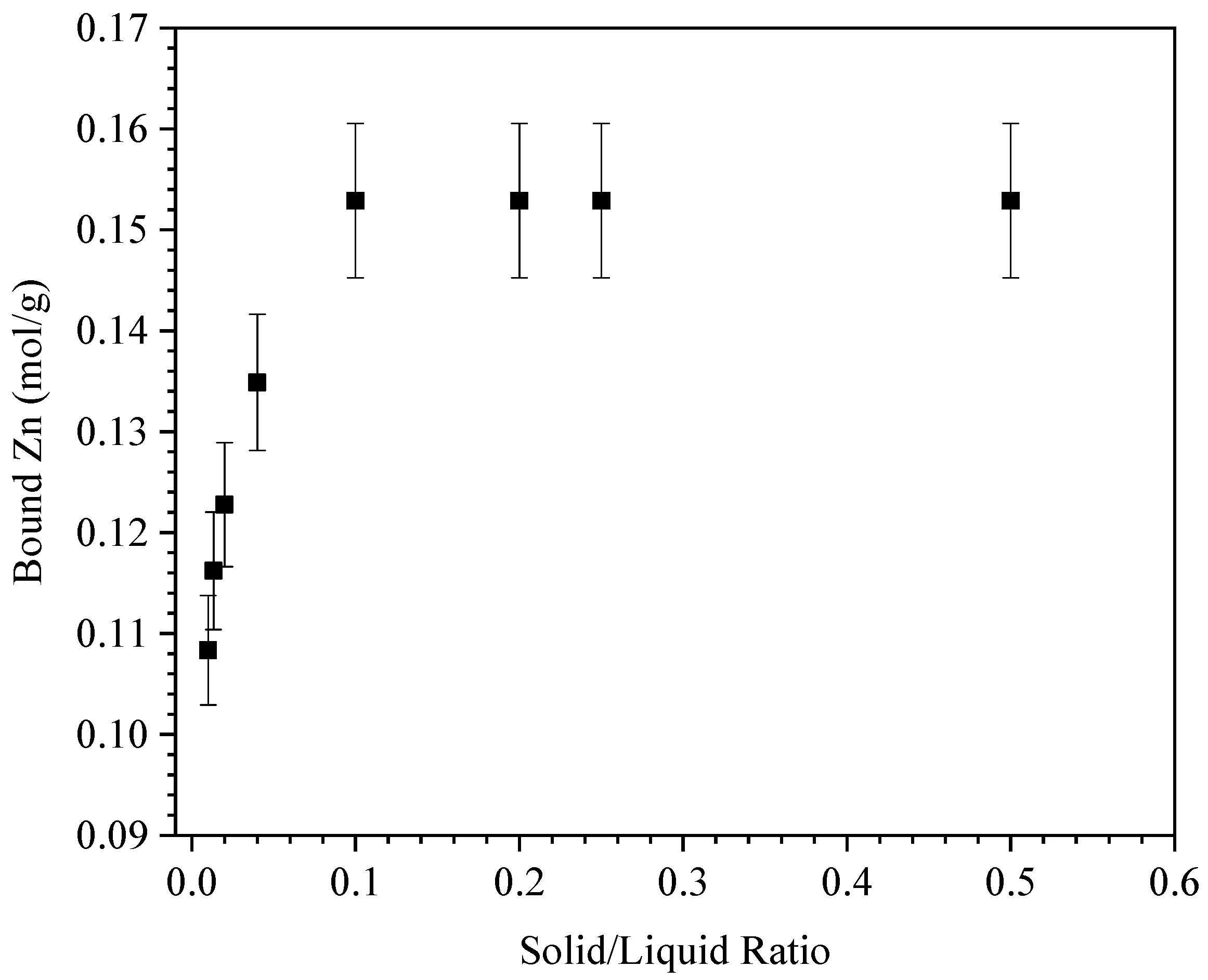
3.2.1. Adsorbent/Adsorbate Ratios and Interaction Kinetics
3.2.2. Adsorption Capacity
3.2.3. Removing Capacity of FCS750 Compared to Other Sorbents
3.3. FCS750 Application on Spiked Solid Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yong, Y.S.; Lim, Y.A.; Ilankoon, I. An analysis of electronic waste management strategies and recycling operations in Malaysia: Challenges and future prospects. J. Clean. Prod. 2019, 224, 151–166. [Google Scholar] [CrossRef]
- Rene, E.R.; Sethurajan, M.; Ponnusamy, V.K.; Kumar, G.; Dung, T.N.B.; Brindhadevi, K.; Pugazhendhi, A. Electronic waste generation, recycling and resource recovery: Technological perspectives and trends. J. Hazard. Mater. 2021, 416, 125664. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, M.S.; Kumar, R.; Prasad, L. Zinc Impurity in Drinking Water and Its Toxic Effect on Human Health. Indian Internet J. Forensic Med. Toxicol. 2019, 17, 84. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Ahmed, J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process. Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Vasquez, Y.; Escobar, M.C.; Saenz, J.S.; Quiceno-Vallejo, M.F.; Neculita, C.M.; Arbeli, Z.; Roldan, F. Canada Research Chair in Treatment of Contaminated Mine Water, Research Institute on. Bioresour. Technol. 2018, 247, 624–632. [Google Scholar] [CrossRef]
- Cunha, M.P.; Ferraz, R.M.; Sancinetti, G.P.; Rodriguez, R.P. Long-term performance of a UASB reactor treating acid mine drainage: Effects of sulfate loading rate, hydraulic retention time, and COD/SO42− ratio. Biodegradation 2018, 30, 47–58. [Google Scholar] [CrossRef]
- Ahmat, A.M.; Mamindy-Pajany, Y. Over-sulfated soils and sediments treatment: A brief discussion on performance disparities of biological and non-biological methods throughout the literature. Waste Manag. Res. J. A Sustain. Circ. Econ. 2021, 39, 528–545. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, A.; Kirrolia, A.; Kumar, R.; Yadav, N.; Bishnoi, N.R.; Lohchab, R.K. Removal of sulphate, COD and Cr(VI) in simulated and real wastewater by sulphate reducing bacteria enrichment in small bioreactor and FTIR study. Bioresour. Technol. 2011, 102, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, H.; Han, X. Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment. Chemosphere 2016, 154, 215–223. [Google Scholar] [CrossRef]
- Hu, Y.; Jing, Z.; Sudo, Y.; Niu, Q.; Du, J.; Wu, J.; Li, Y.-Y. Effect of influent COD/SO42− ratios on UASB treatment of a synthetic sulfate-containing wastewater. Chemosphere 2015, 130, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, M.; Arivizhivendhan, K.V.; Nivetha, K.; Swarnalatha, S.; Sekaran, G. Anaerobic digestion of sulphate-rich post-tanning wastewater at different COD/sulphate and F/M ratios. 3 Biotech 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Brahmacharimayum, B.; Ghosh, P.K. Sulfate bioreduction and elemental sulfur formation in a packed bed reactor. J. Environ. Chem. Eng. 2014, 2, 1287–1293. [Google Scholar] [CrossRef]
- Najib, T.; Solgi, M.; Farazmand, A.; Heydarian, S.M.; Nasernejad, B. Optimization of sulfate removal by sulfate reducing bacteria using response surface methodology and heavy metal removal in a sulfidogenic UASB reactor. J. Environ. Chem. Eng. 2017, 5, 3256–3265. [Google Scholar] [CrossRef]
- Choi, H.-J.; Yu, S.-W.; Kim, K.H. Efficient use of Mg-modified zeolite in the treatment of aqueous solution contaminated with heavy metal toxic ions. J. Taiwan Inst. Chem. Eng. 2016, 63, 482–489. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Ma, J.; Khan, M.A.; Xia, M.; Fu, C.; Zhu, S.; Chu, Y.; Lei, W.; Wang, F. Effective adsorption of heavy metal ions by sodium lignosulfonate reformed montmorillonite. Int. J. Biol. Macromol. 2019, 138, 188–197. [Google Scholar] [CrossRef]
- Spark, K.; Wells, J.; Johnson, B. Characterizing trace metal adsorption on kaolinite. Eur. J. Soil Sci. 1995, 46, 633–640. [Google Scholar] [CrossRef]
- Li, T.; Huang, X.; Wang, Q.; Yang, G. Adsorption of metal ions at kaolinite surfaces: Ion-specific effects, and impacts of charge source and hydroxide formation. Appl. Clay Sci. 2020, 194, 105706. [Google Scholar] [CrossRef]
- Oren, A.H.; Kaya, A. Factors affecting adsorption characteristics of Zn2+ on two natural zeolites. J. Hazard. Mater. 2006, 131, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Abriak, N.E.; Zentar, R.; Ballivy, G. The use of marine sediments as a pavement base material. Waste Manag. 2009, 29, 774–782. [Google Scholar] [CrossRef]
- Dang, T.A.; Kamali-Bernard, S.; Prince, W.A. Design of new blended cement based on marine dredged sediment. Constr. Build. Mater. 2013, 41, 602–611. [Google Scholar] [CrossRef]
- Lirer, S.; Liguori, B.; Capasso, I.; Flora, A.; Caputo, D. Mechanical and chemical properties of composite materials made of dredged sediments in a fly-ash based geopolymer. J. Environ. Manag. 2017, 191, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Snellings, R.; Cizer, Ö.; Horckmans, L.; Durdziński, P.T.; Dierckx, P.; Nielsen, P.; Van Balen, K.; Vandewalle, L. Properties and pozzolanic reactivity of flash calcined dredging sediments. Appl. Clay Sci. 2016, 129, 35–39. [Google Scholar] [CrossRef]
- Snellings, R.; Horckmans, L.; Van Bunderen, C.; Vandewalle, L.; Cizer, Ö. Flash-calcined dredging sediment blended cements: Effect on cement hydration and properties. Mater. Struct. 2017, 50, 241. [Google Scholar] [CrossRef]
- Van Bunderen, C.; Snellings, R.; Vandewalle, L.; Cizer, Ö. Early-age hydration and autogenous deformation of cement paste containing flash calcined dredging sediments. Constr. Build. Mater. 2018, 200, 104–115. [Google Scholar] [CrossRef]
- Rachid, H.S.; Nadia, B.; Mahfoud, T.; Omar, B.; Fatima, T.; Khalil, B.; Omar, S. Proposal of industrialization process of dredged sediments. MATEC Web Conf. 2018, 149, 02050. [Google Scholar] [CrossRef]
- Van Bunderen, C.; Benboudjema, F.; Snellings, R.; Vandewalle, L.; Cizer, Ö. Experimental analysis and modelling of mechanical properties and shrinkage of concrete recycling flash calcined dredging sediments. Cem. Concr. Compos. 2020, 115, 103787. [Google Scholar] [CrossRef]
- Chu, D.C.; Amar, M.; Kleib, J.; Benzerzour, M.; Betrancourt, D.; Abriak, N.-E.; Nadah, J. The Pozzolanic Activity of Sediments Treated by the Flash Calcination Method. Waste Biomass-Valorization 2022, 13, 4963–4982. [Google Scholar] [CrossRef]
- Ramos, R.L.; Jacome, L.A.B.; Barron, J.M.; Rubio, L.F.; Coronado, R.M.G. Adsorption of zinc (II) from an aqueous solution onto activated carbon. J. Hazard. Mater. 2002, 90, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2020, 264, 128455. [Google Scholar] [CrossRef] [PubMed]
- San Nicolas, R. Approche Performantielle des Bétons Avec Métakaolins Obtenus par Calcination Flash. Ph.D. Thesis, Université Paul Sabatier-Toulouse III, Toulouse, France, 2011. [Google Scholar]
- Nicolas, R.S.; Cyr, M.; Escadeillas, G. Characteristics and applications of flash metakaolins. Appl. Clay Sci. 2013, 83–84, 253–262. [Google Scholar] [CrossRef]
- Berenger, A.; Olivier, G.; Lanos, C.; Daiguebonne, C.; Freslon, S.; Tessier, C.; Laurans, M.; Baux, C.; Greffet, H. A new calcium sulfate-based plaster composed of composite particles. Mater. Struct. 2014, 48, 2685–2696. [Google Scholar] [CrossRef]
- Teklay, A.; Yin, C.; Rosendahl, L.; Køhler, L.L. Experimental and modeling study of flash calcination of kaolinite rich clay particles in a gas suspension calciner. Appl. Clay Sci. 2015, 103, 10–19. [Google Scholar] [CrossRef]
- Tribout, C. Valorisation de Sédiments Traités en Techniques Routières: Contribution à la Mise en Place d’un Protocole D’acceptabilité. Ph.D. Thesis, Thèse de Doctorant de l’Université Toulouse III, Toulouse, France, 2010. [Google Scholar]
- Ho, K.S.; Mckaf, G. The Kinetics of Sorption of Basic Dyes fi-om Aqueous Solution by Sphagnum Moss Peat. Can. J. Chem. Eng. 2000, 76, 822–827. [Google Scholar] [CrossRef]
- Thiebault, T.; Boussafir, M.; Guégan, R.; Le Milbeau, C.; Le Forestier, L. Clayey–sand filter for the removal of pharmaceuticals from wastewater effluent: Percolation experiments. Environ. Sci. Water Res. Technol. 2016, 2, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The adsorption of gases on plan surface of glass, mica, and platinum. J. Am. Chem. Soc. 1918, 345, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhang, B.; Mei, D.; Zhang, H.; Liu, J. Adsorption of methyl violet from aqueous solution by halloysite nanotubes. Desalination 2011, 268, 111–116. [Google Scholar] [CrossRef]
- Ghasemi, N.; Ghasemi, M.; Moazeni, S.; Ghasemi, P.; Alharbi, N.S.; Gupta, V.K.; Agarwal, S.; Burakova, I.V.; Tkachev, A.G. Zn (II) removal by amino-functionalized magnetic nanoparticles: Kinetics, isotherm, and thermodynamic aspects of adsorption. J. Ind. Eng. Chem. 2018, 62, 302–310. [Google Scholar] [CrossRef]
- Gasparini, E.; Tarantino, S.C.; Ghigna, P.; Riccardi, M.P.; Cedillo-González, E.I.; Siligardi, C.; Zema, M. Thermal dehydroxylation of kaolinite under isothermal conditions. Appl. Clay Sci. 2013, 80–81, 417–425. [Google Scholar] [CrossRef]
- Ferreiro, S.; Canut, M.; Lund, J.; Herfort, D. Influence of fineness of raw clay and calcination temperature on the performance of calcined clay-limestone blended cements. Appl. Clay Sci. 2018, 169, 81–90. [Google Scholar] [CrossRef]
- Badogiannis, E.; Kakali, G.; Tsivilis, S. Metakaolin as supplementary cementitious material optimization of kaolin to metakaolin conversion. J. Therm. Anal. Calorim. 2005, 81, 457–462. [Google Scholar] [CrossRef]
- Kwon, J.-S.; Yun, S.-T.; Lee, J.-H.; Kim, S.-O.; Jo, H.Y. Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic(III) from aqueous solutions using scoria: Kinetics and equilibria of sorption. J. Hazard. Mater. 2010, 174, 307–313. [Google Scholar] [CrossRef]
- Yang, K.; Yan, L.-G.; Yang, Y.-M.; Yu, S.-J.; Shan, R.-R.; Yu, H.-Q.; Zhu, B.-C.; Du, B. Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: Kinetics, isotherms and mechanisms. Sep. Purif. Technol. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Sreejalekshmi, K.; Vimexen, V.; Dev, V.V. Evaluation of adsorption properties of sulphurised activated carbon for the effective and economically viable removal of Zn(II) from aqueous solutions. Ecotoxicol. Environ. Saf. 2016, 124, 418–425. [Google Scholar] [CrossRef]
- Mahamat Ahmat, A.; Maherzi, W.; Le Milbeau, C.; Benzerzour, M.; Abriak, N.E. Modified Red Muds and Slag Based Hydraulic Binders for Zn Removal: A Matrix-Spiking Approach Applied on Clayey Sediments. Minerals 2021, 11, 1189. [Google Scholar] [CrossRef]
- Ahmat, A.M.; Thiebault, T.; Guégan, R. Phenolic acids interactions with clay minerals: A spotlight on the adsorption mechanisms of Gallic Acid onto montmorillonite. Appl. Clay Sci. 2019, 180, 105188. [Google Scholar] [CrossRef] [Green Version]
- Manyangadze, M.; Chikuruwo, N.; Narsaiah, T.; Chakra, C.; Radhakumari, M.; Danha, G. Enhancing adsorption capacity of nano-adsorbents via surface modification: A review. S. Afr. J. Chem. Eng. 2020, 31, 25–32. [Google Scholar] [CrossRef]
- Abu Al-Rub, F.A. Biosorption of Zinc on Palm Tree Leaves: Equilibrium, Kinetics, and Thermodynamics Studies. Sep. Sci. Technol. 2006, 41, 3499–3515. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; Issa, A.A.; Khraisheh, M.A.; Walker, G.M. Sorption of Zn (II), Pb (II), and Co (II) using natural sorbents: Equilibrium and kinetic studies. Water Res. 2006, 40, 2645–2658. [Google Scholar] [CrossRef] [PubMed]
- Anitha, T.; Kumar, P.S.; Kumar, K.S. Binding of Zn (II) Ions to Chitosan-PVA Blend in aqueous Envionment: Adsorp-tion kinetics and Equilibrium studies. Environ. Prog. Sustain. Energy 2014, 34, 15–22. [Google Scholar] [CrossRef]
- Arias, F.; Sen, T.K. Removal of zinc metal ion (Zn2+) from its aqueous solution by kaolin clay mineral: A kinetic and equilibrium study. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 100–108. [Google Scholar] [CrossRef]
- Bao, S.; Tang, L.; Ning, P.; Peng, J.; Guo, H.; Zhu, T.; Liu, Y. Highly selective removal of Zn (II) ion from hot-dip gal-vanizing pickling waste with amino-functionalized Fe3O4@SiO2 magnetic nano-adsorbent. J. Colloid Interface Sci. 2015, 462, 235–242. [Google Scholar] [CrossRef]
- Bazargan-Lari, R.; Bahrololoom, M.E.; Nemati, A. Sorption behavior of Zn (II) ions by low cost and biological natural hydroxyapatite/chitosan composite from industrial wastewater. J. Food Agric. Environ. 2011, 9, 892–897. [Google Scholar]
- Castaldi, P.; Santona, L.; Enzo, S.; Melis, P. Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. J. Hazard. Mater. 2008, 156, 428–434. [Google Scholar] [CrossRef]
- Chen, A.-H.; Liu, S.-C.; Chen, C.-Y. Comparative adsorption of Cu(II), Zn(II), and Pb(II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J. Hazard. Mater. 2008, 154, 184–191. [Google Scholar] [CrossRef]
- Chubar, N.; Carvalho, J.R.; Neiva Correia, M.J. Cork biomass as biosorbent for Cu (II), Zn (II) and Ni (II). Colloids Surf. A Physicochem. Eng. Asp. 2004, 230, 57–65. [Google Scholar] [CrossRef]
- Ding, P.; Huang, K.-L.; Li, G.-Y.; Liu, Y.-F.; Zeng, W.-W. Kinetics of adsorption of Zn(II) ion on chitosan derivatives. Int. J. Biol. Macromol. 2006, 39, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Luo, C.; Li, X.; Lu Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to en-hance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kubilay, S.; Gürkan, R.; Sarvan, A.; Sahan, T. Removal of Cu (II) Zn (II) and Co (II) ions form aqueous solutions by adsorption onto natural bentonite. Adsorption 2007, 13, 41–51. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and adsorption application of suc-cinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem. Eng. J. 2015, 229, 438–448. [Google Scholar] [CrossRef]
- Li, L.; Bi, S.; Liu, L.; Dong, W.; Wang, X. Separation and accumulation of Cu (II), Zn (II) and Cr (VI) from aqueous solu-tion by magnetic chitosan modified with diethylenetriamine. Desalination 2011, 278, 397–404. [Google Scholar] [CrossRef]
- Maheshwari, U.; Bhuvanesh, M.; Gupta, S. Efficient Adsorbent for simultaneous removal of Cu (II), Zn (II), Cr (VI): Kinetic, thermodynamic, and mass transfer mechanism. Process Saf. Environ. Prot. 2015, 98, 198–210. [Google Scholar] [CrossRef]
- Mellah, A.; Chegrouche, S. The removal of zinc from aqueous solutions by natural bentonite. Water Res. 1997, 31, 621–629. [Google Scholar] [CrossRef]
- Meshko, V.; Markovska, L.; Marinkovski, M. Experimental study and modelling of zinc adsorption by granular acti-vated carbon and natural zeolite. Int. J. Environ. Pollut. 2006, 27, 4. [Google Scholar] [CrossRef]
- Monier, M. Adsorption of Hg2+, Cu2+ and Zn2+ ions from aqueous solution using formaldehyde cross-linked modi-fied chitosan-thioglyceraldehyde Schiff’s base. Int. J. Biol. Macromol. 2012, 50, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Monier, M.; Abdel-Latif, D.A. Preparation of cross-linked magnetic chitosanphenylthiourea resin for adsorption of Hg(II), Cd(II) and Zn(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 209–210, 40–249. [Google Scholar]
- Muthukrishnaraj, A.; Manokaran, J.; Vanitha, M.; Thiruvengadaravi, K.V.; Baskaralingam, P.; Balasubramanian, N. Equilibrium, kinetic and thermodynamic studies for the removal of Zn(II) and Ni(II) ions using magnetically recoverable graphene/Fe3O4 composite. Desalination Water Treat. 2014, 56, 1–17. [Google Scholar]
- Nibou, D.; Mekatel, H.; Amokrane, S.; Barkkat, M.; Trari, M. Adsorption of Zn2+ ions onto NaA and NaX zeolites: Kinetic, equilibrium and thermodynamic studies. J. Hazard. Mater. 2010, 173, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Chang, X.; He, Q.; Hu, Z.; Li, Z. Preparation of p-tert[(dimethylamino)methyl]-calix[4]arene functionalized aminopropylpolysiloxane resin for selective solid-phase extraction and preconcentration of metal ions. J. Hazard. Mater. 2009, 169, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Pentari, D.; Perdikatsis, V.; Katsimicha, D.; Kanaki, A. Sorption properties of low calorific value greek lignites: Re-moval of lead, cadmium, zinc and copper ions from aqueous solutions. J. Hazard. Mater. 2009, 168, 1017–1021. [Google Scholar] [CrossRef]
- Rezgui, A.; Hannachi, Y.; Guibal, E.; Boubaker, T. Biosorption of zinc from aqueous solution by dried activated sludge biomass. Desalination Water Treat. 2014, 56, 2699–2705. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Ramalingam, S.; Abhinaya, R.V.; Kirupha, S.D.; Vidhyadevi, T.; Sivanesan, S. Adsorption equilib-rium, thermodynamics, kinetics, mechanism and process design of zinc (II) ions onto cashew nut shell. Can. J. Chem. Eng. 2012, 90, 973–982. [Google Scholar] [CrossRef]
- Shukla, S.; Pai, R.S. Adsorption of Cu(II), Ni(II) and Zn(II) on dye loaded groundnut shells and sawdust. Sep. Purif. Technol. 2005, 43, 1–8. [Google Scholar] [CrossRef]
- Shukla, S.R.; Pai, R.S.; Shendarkar, A.D. Adsorption of Ni (II), Zn (II) and Fe (II) on modified coir fibers. Sep. Purif. Technol. 2006, 47, 141–147. [Google Scholar] [CrossRef]
- Veli, S.; Alyüz, B. Adsorption of copper and zinc from aqueous solutions by using natural clay. J. Hazard. Mater. 2007, 149, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.W.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, Y.; Li, Z. Biosorption of Zinc from Aqueous Solutions by Rice Bran: Kinetics and Equilibrium Studies. Sep. Sci. Technol. 2006, 41, 747–756. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, X.; Wu Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn (II) removal from aqueous solution. Appl. Clay Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, P.; Wu, D.; Sun, M.; Deng, Y.; Frost, R.L. Effective removal of zinc (II) from aqueous solutions by tricalcium aluminate (C3A). J. Colloid Interface Sci. 2015, 443, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasewar, K.L.; Atif, M.; Prasad, B.; Mishra, I. Batch adsorption of zinc on tea factory waste. Desalination 2009, 244, 66–71. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2012, 20, 358–368. [Google Scholar] [CrossRef]
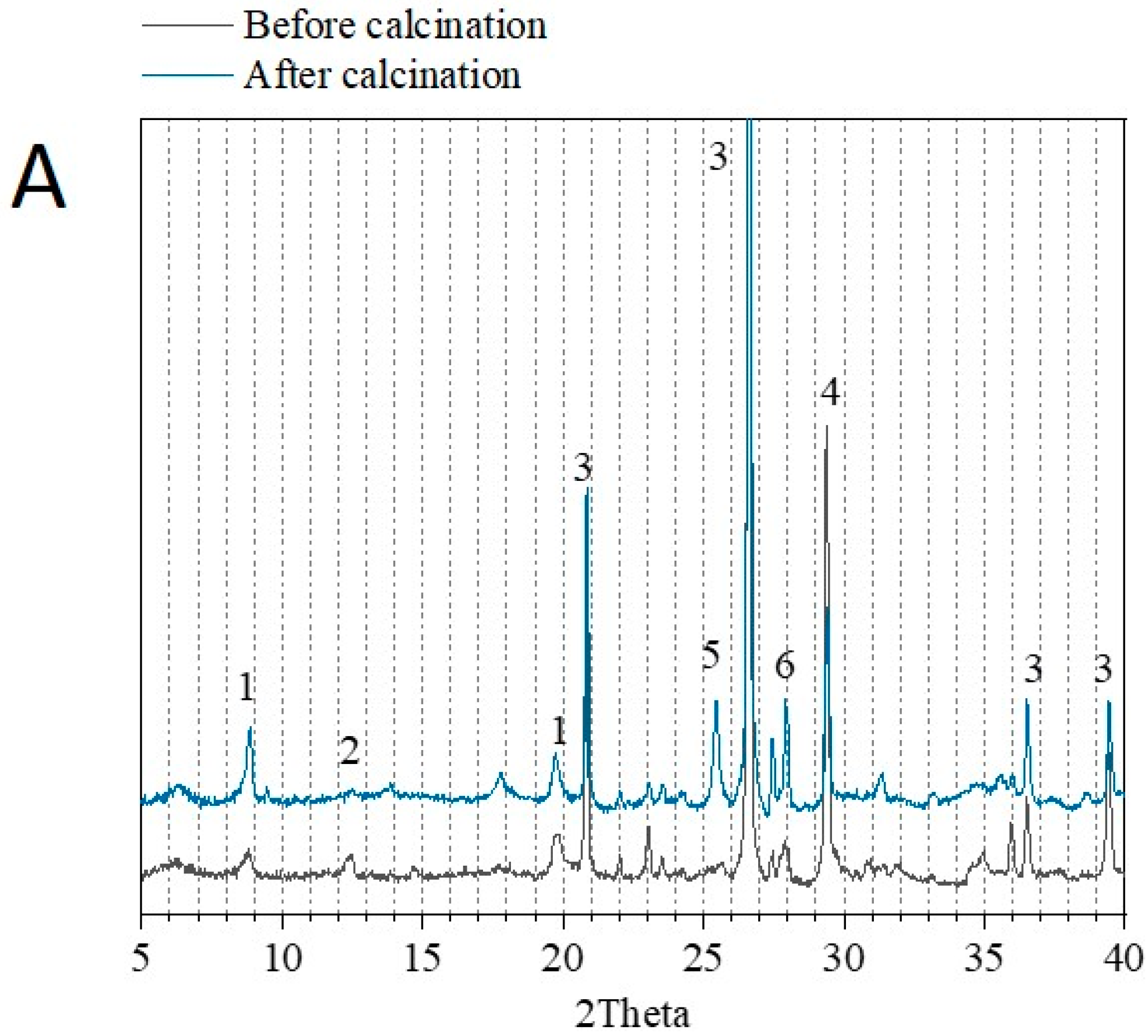
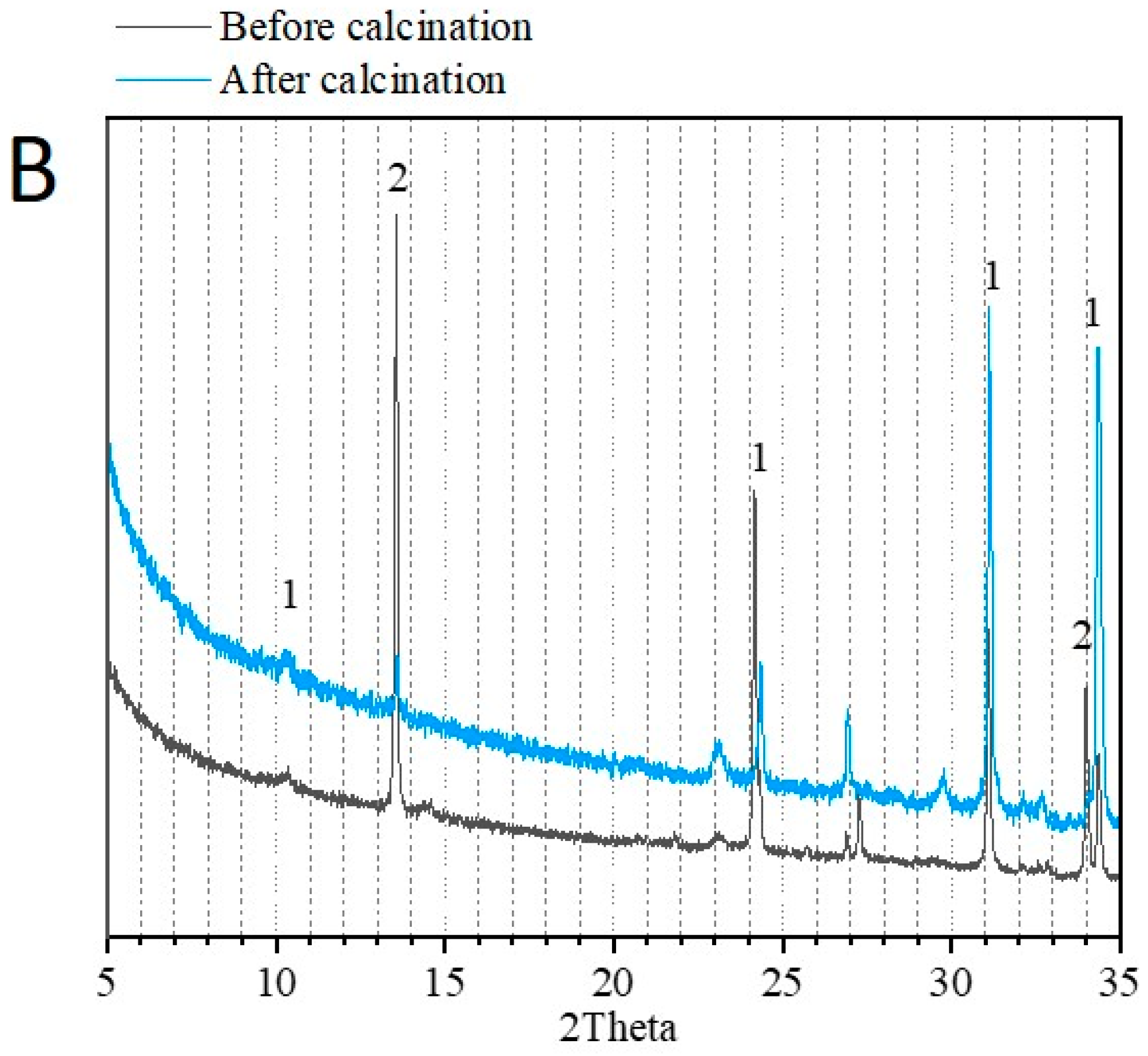
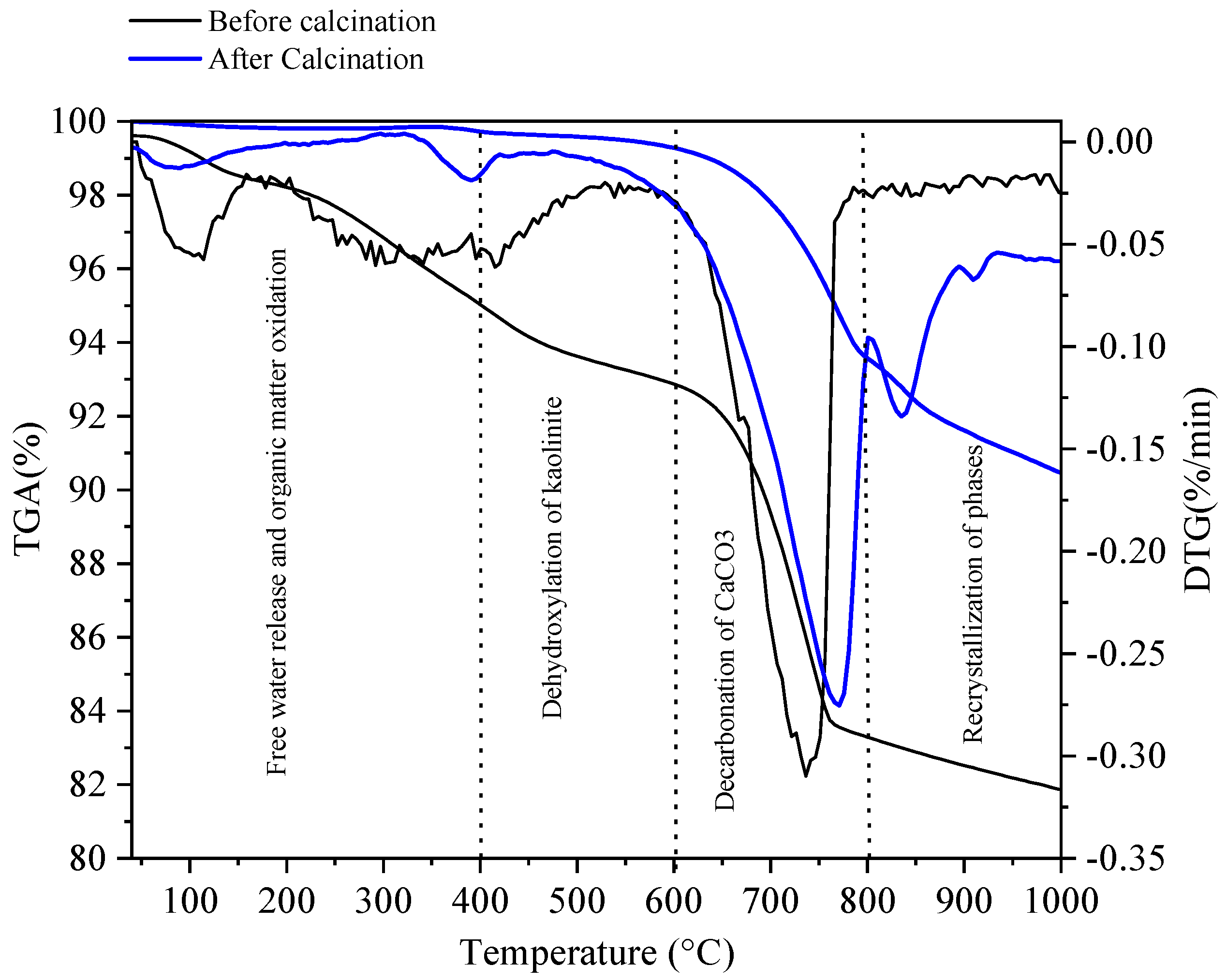

| Before Calcination (Raw Sediment) | After Calcination (FCS750) | ||
|---|---|---|---|
| Chemical composition (% wt) | CaO | 14.65 | 13.05 |
| SiO2 | 54.92 | 57.86 | |
| Al2O3 | 13.36 | 13.97 | |
| Fe2O3 | 7.10 | 6.20 | |
| SO3 | 0.30 | 0.21 | |
| Na2O | 0.96 | 0.86 | |
| K2O | 2.55 | 2.62 | |
| MgO | 1.22 | 1.30 | |
| ZnO | 0.37 | 0.36 | |
| P2O5 | 2.91 | 2.63 | |
| Total | 99.17 | 99.49 | |
| LOI (950 °C) | 27.63 | 13.7 | |
| Physical properties | Density (g cm−3) | 2.43 | 2.63 |
| BET surface (m2 g−1) | 4 38 | 15 59 | |
| Organic matter content (%) | 16.1 | 1.93 | |
| d10 (µm) | 1.24 | 2.63 | |
| d50 (µm) | 11.68 | 14.71 | |
| d90 (µm) | 55.91 | 64.13 |
| Before Calcination | After Calcination | IW | NHW | ||
|---|---|---|---|---|---|
| As | <0.08 | <0.1 | 0.5 | 2 | |
| Ba | 1.4912 | 1.9 | 20 | 100 | |
| Cd | <0.008 | <0.008 | 0.04 | 1 | |
| Cr | <0.03 | 0.37 | 0.5 | 10 | |
| Trace metals (mg kg−1) | Cu | 2.0509 | 0.078 | 2 | 50 |
| Mo | 0.5538 | 1.7 | 0.5 | 10 | |
| Pb | 0.0447 | 0.074 | 0.4 | 10 | |
| Ni | 0.4799 | <0.03 | 0.5 | 10 | |
| Sb | - | <0.05 | 0.06 | 0.7 | |
| Se | 0.1019 | 0.08 | 0.1 | 0.5 | |
| Zn | 7.8518 | <0.05 | 4 | 50 | |
| Anions (mg kg−1) | F | <1 | 8 | 10 | 150 |
| Cl | 262 | 247 | 800 | 15,000 | |
| SO42− | 15,800 | 11,120 | 1000 | 20,000 |
| Kinetic Models | Associated Parameters | Values for FCS750 |
|---|---|---|
| qe exp (µmol g−1) | 13.8 | |
| k1 (min−1) | 22.83 × 10−3 | |
| R2 | 0.947 | |
| Pseudo First Order | qe mod (µmol g−1) | 14.97 |
| χ2 | 9.34 | |
| ARE | 91.55 | |
| k2 (g mmol−1 min−1) | 42.20 × 10−2 | |
| R2 | 0.999 | |
| Pseudo Second Order | qe mod (µmol g−1) | 15.28 |
| χ2 | 13.70 | |
| ARE | 98.9 | |
| kb (g mmol−1 min−1) | 15.24 × 10−3 | |
| R2 | 0.957 | |
| Bangham | qe mod (µmol g−1) | 15.28 |
| χ2 | 13.70 | |
| ARE | 98.9 |
| Isotherms | Associated Parameters | Values for FCS750 at pH 7 | Values for FCS750 at pH 2 |
|---|---|---|---|
| KL (L mmol−1) | 13.51 | 13.48 | |
| qmax (mmol g−1) | 1.35 | 1.37 | |
| Langmuir | R2 | 0.998 | 0.999 |
| KF (mmol g−1) | 1.18 | 1.08 | |
| Freundlich | n | 1.06 | 1.18 |
| R2 | 0.948 | 0.985 | |
| B (J mmol−1) | 0.090 | 0.096 | |
| Temkin | KT | 1.07 | 1.07 |
| R2 | 0.937 | 0.931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, D.C.; Amar, M.; Benzerzour, M.; Kleib, J.; Abriak, N.-E. Flash-Calcined Sediments for Zinc Adsorption. Sustainability 2023, 15, 10230. https://doi.org/10.3390/su151310230
Chu DC, Amar M, Benzerzour M, Kleib J, Abriak N-E. Flash-Calcined Sediments for Zinc Adsorption. Sustainability. 2023; 15(13):10230. https://doi.org/10.3390/su151310230
Chicago/Turabian StyleChu, Duc Chinh, Mouhamadou Amar, Mahfoud Benzerzour, Joelle Kleib, and Nor-Edine Abriak. 2023. "Flash-Calcined Sediments for Zinc Adsorption" Sustainability 15, no. 13: 10230. https://doi.org/10.3390/su151310230






