Gaining Profound Knowledge of Cholera Outbreak: The Significance of the Allee Effect on Bacterial Population Growth and Its Implications for Human-Environment Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Related Work
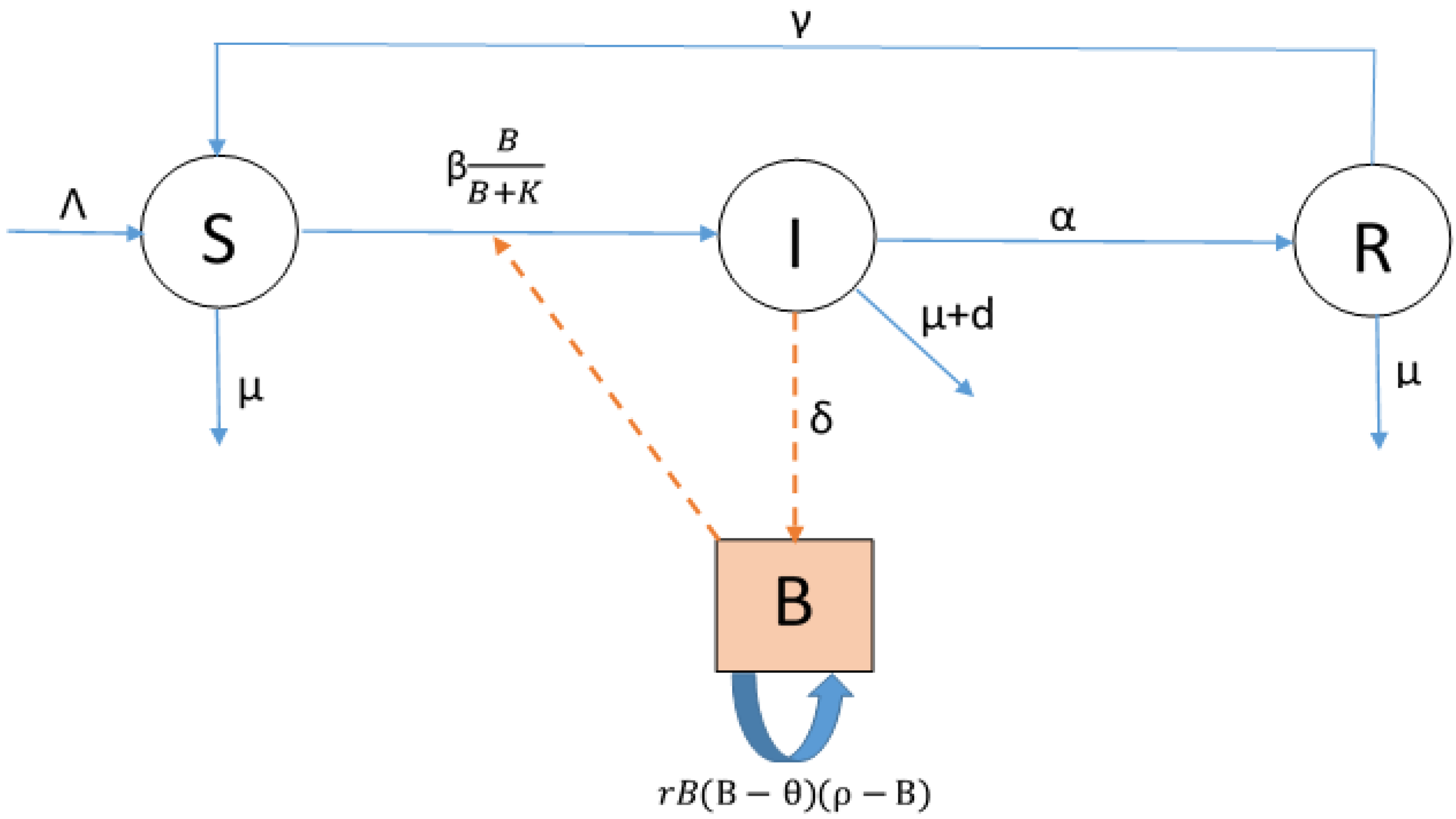
2.2. Model Construction
3. Results
3.1. Sensitivity Analysis
- the parameters , , , , and should significantly affect at least one state variable of model (2);
- is not related to transmission of disease;
- influence of suggests to sensitize population to avoid getting in touch with bacteria;
- influences of and suggest to intensify sanitation campaigns by destroying reservoirs of V. cholera.
| PRCCs and Significance | |||||
|---|---|---|---|---|---|
| Parameters | Range | ||||
| [1–300] | 0.8970 ** | 0.0968 * | 0.0171 | −0.0332 | |
| [10–0.999] | −0.9580 * | −0.2359 ** | 0.1221 ** | −0.1702 ** | |
| [1–1000] | −0.8934 ** | −0.0545 | 0.0885 | −0.1173 * | |
| [10–0.999] | −0.0718 | −0.0255 | 0.0270 | −0.0818 | |
| d | [10–0.999] | 0.0290 | 0.0432 | −0.0676 | −0.0462 |
| r | [10–0.999] | −0.0282 | −0.0180 | 0.0395 | 0.0291 |
| [10–0.999] | −0.0388 | 0.0147 | 0.0577 | −0.0106 | |
| [10–0.999] | 0.1010 | 0.0680 | 0.0530 | 0.0948 | |
| K | [10–10] | 0.0050 | 0.0335 | 0.0092 | −0.0213 |
| [10–10] | −0.0723 | −0.1149 * | 0.0561 | 0.6220 ** | |
| [10–10] | 0.0210 | 0.0628 | 0.0473 | 0.5029 * | |
3.2. Basic Properties
3.2.1. Positivity and Boundedness of Solutions
- Step 1.
- We show that for any initial condition , , the maximal solution of the Cauchy problem associated with system (2) is non-negative.Let and let us show that .Suppose that . At least one of the following conditions is satisfied: , , , or .Suppose . Then from the first equation of model (2),This implies thatIntegrating Equation (6) from 0 to yields:Similarly, one can show that , , and , which is contradictory. Therefore, and consequently, the maximal solution of the Cauchy problem associated to model (2) is non-negative.
- Step 2.
- We then prove that the total population of humans and bacteria satisfies the boundedness property. We first split model (2) into two parts, the human population (i.e., , and ) and the pathogen population (i.e., ).Let . Using the equation of model (2), one can deduce thatThus,where represents the initial value of .The lower limit comes naturally from the fact that the model variables are non-negative () since they monitor human populations.Thus, whenever .Suppose . From the last equation of model (2) and using the fact that for all , one has:where .Note that and is a decreasing in [[. The equation of the tangent of at is given by .It follows that, for , we have:Integrating the above differential inequality yields:where is the initial condition of .Thus, as ,
3.2.2. Positively Invariant Sets
3.3. Existence and Stability of Equilibria
3.3.1. Existence of Disease-Free Equilibria
3.3.2. Stability of Equilibria and Threshold Quantities
- (i)
- If , the DFE is locally stable.
- (ii)
- If , the DFE is locally stable.
- (iii)
- The DFE is always unstable.
3.3.3. Endemic Equilibrium
- either one or three interior equilibria if ,
- either zero or two endemic equilibria if .
4. Discussion
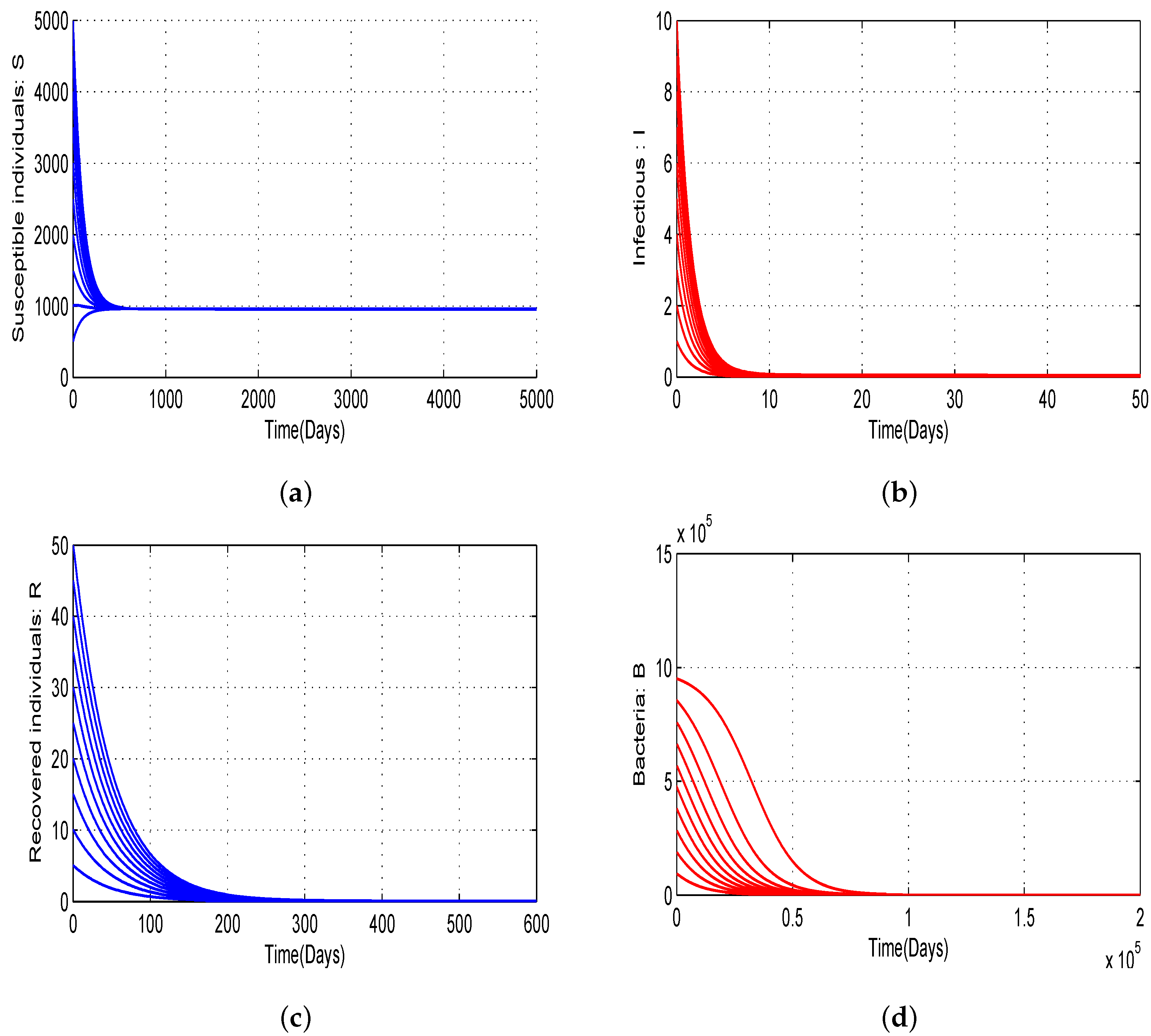
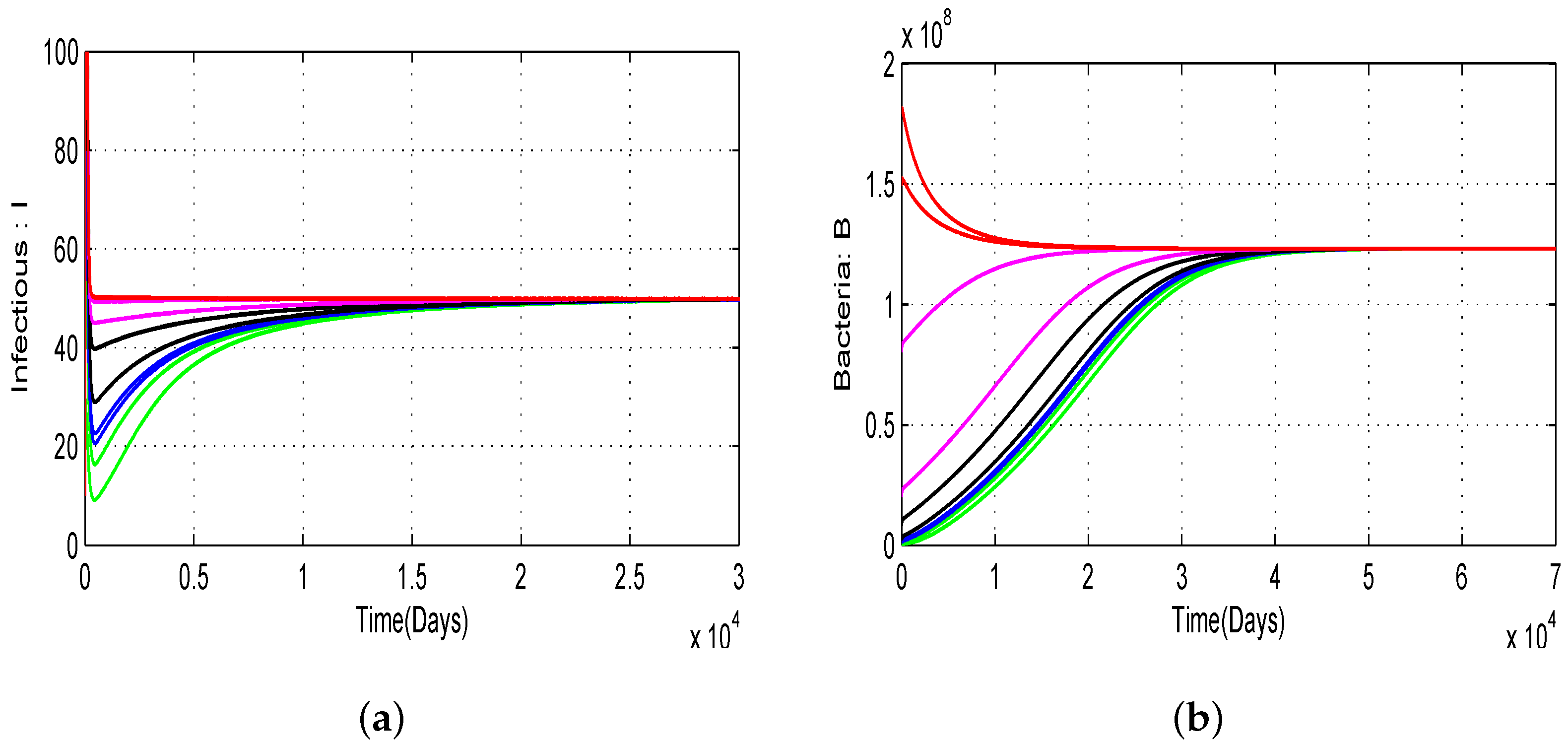
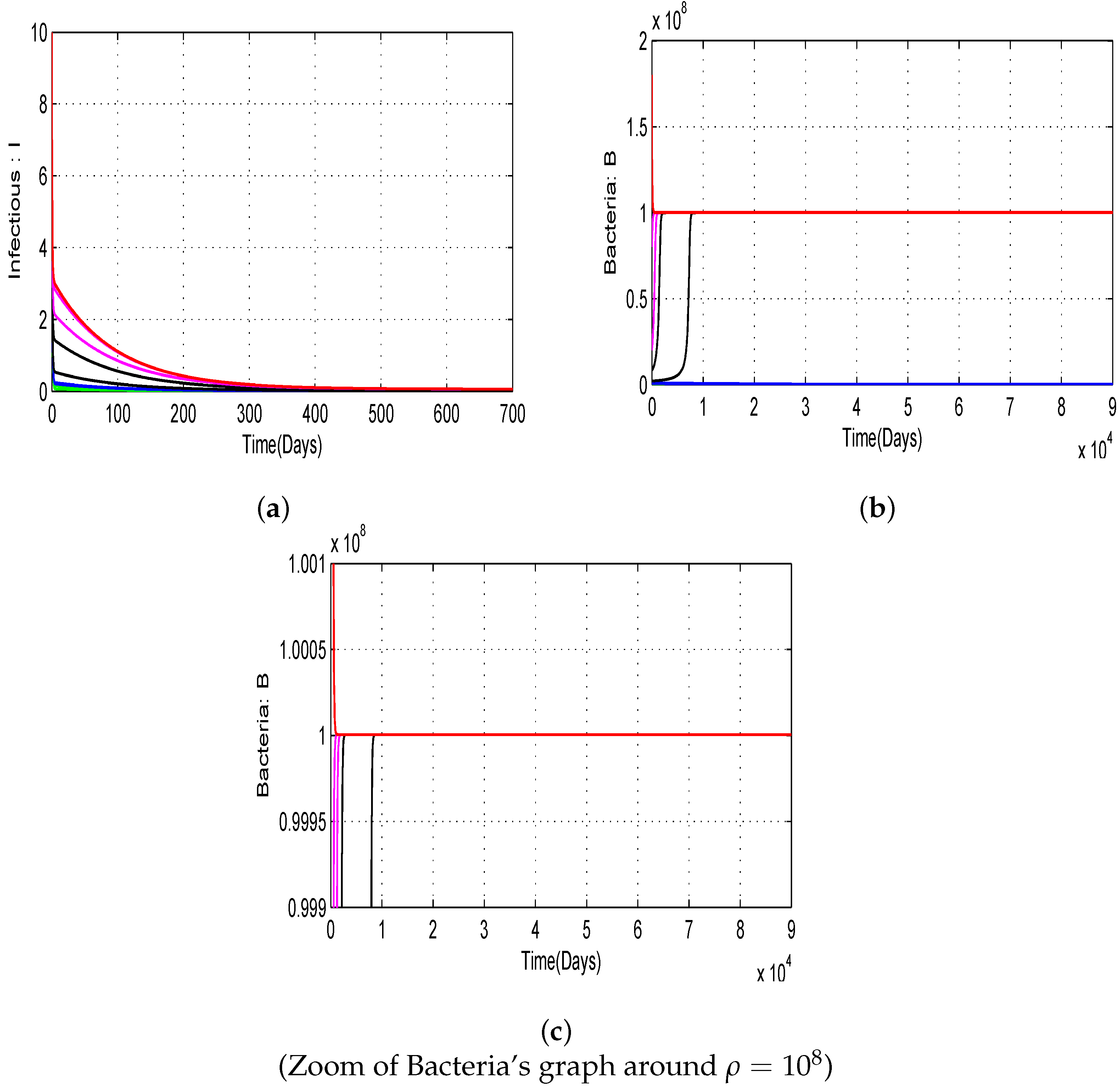
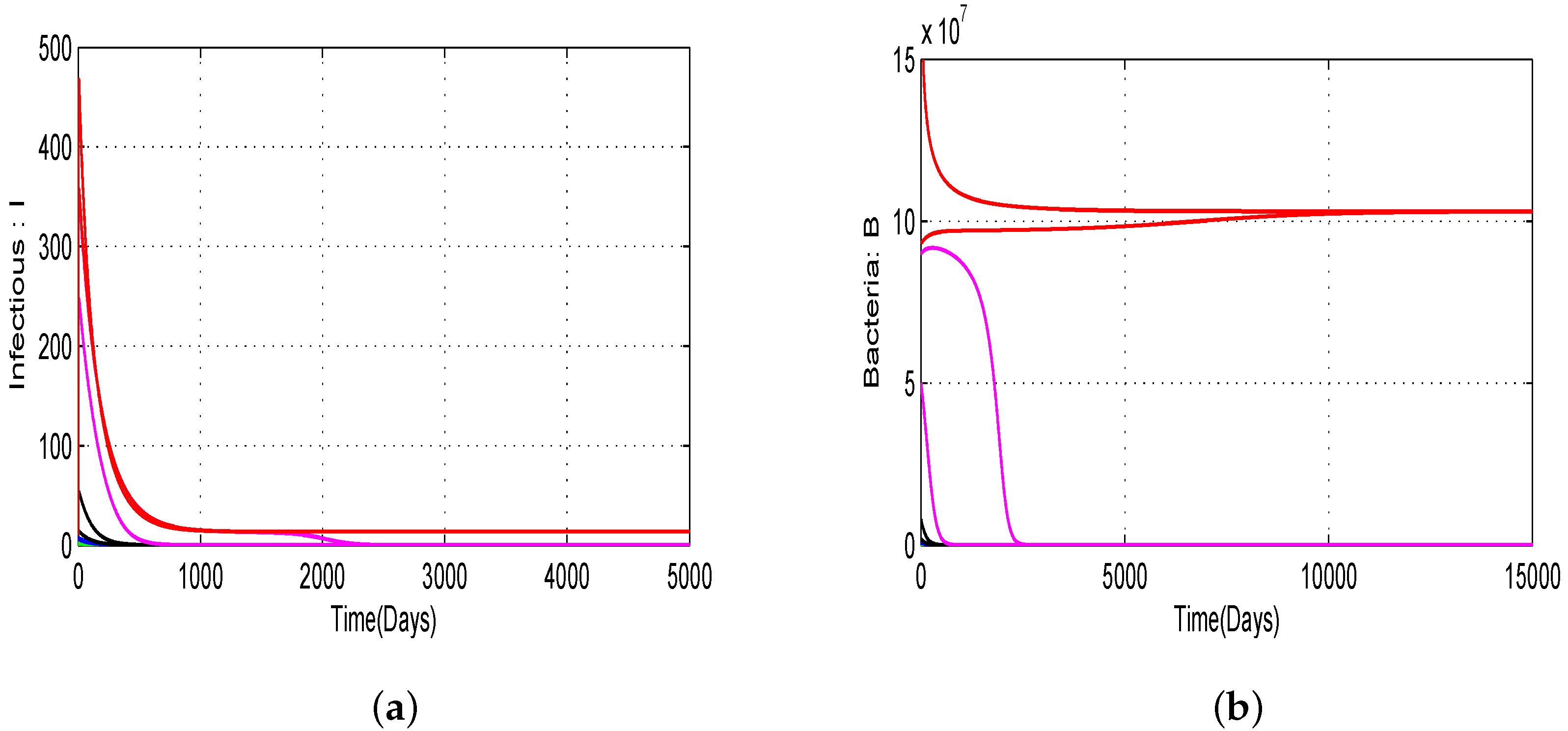
4.1. Numerical Simulations of the Proposed Model with Variation of Threshold Quantities
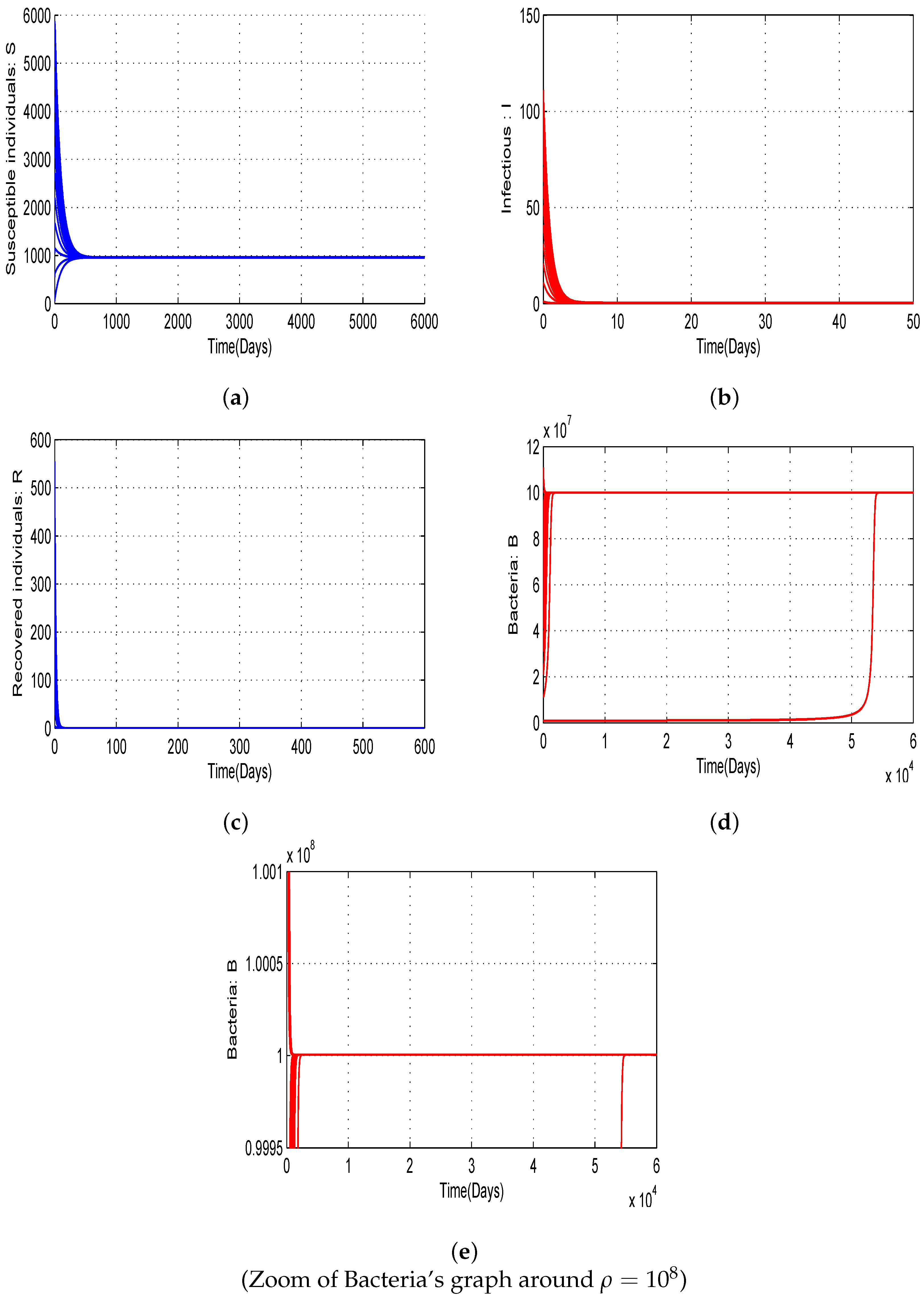
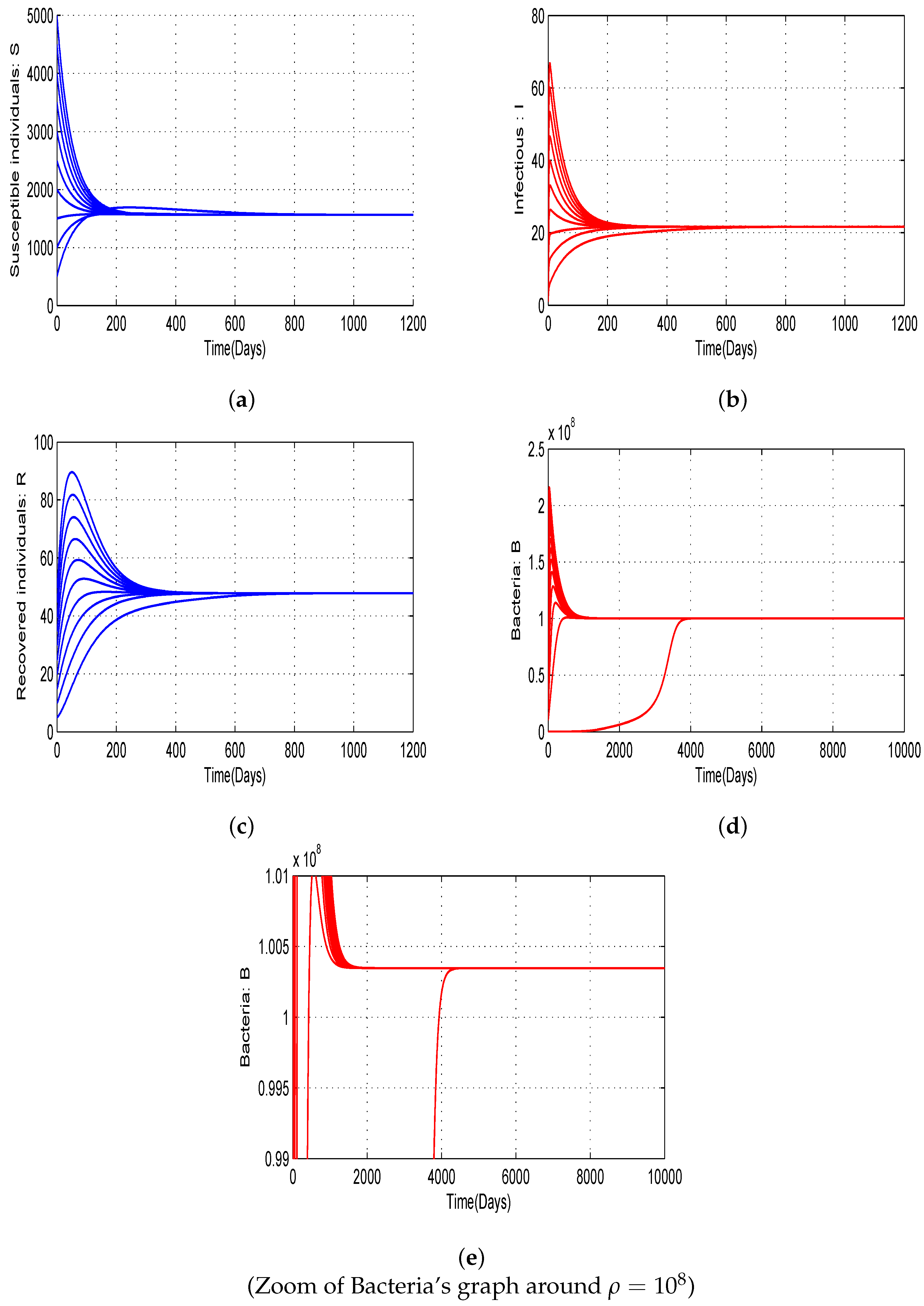
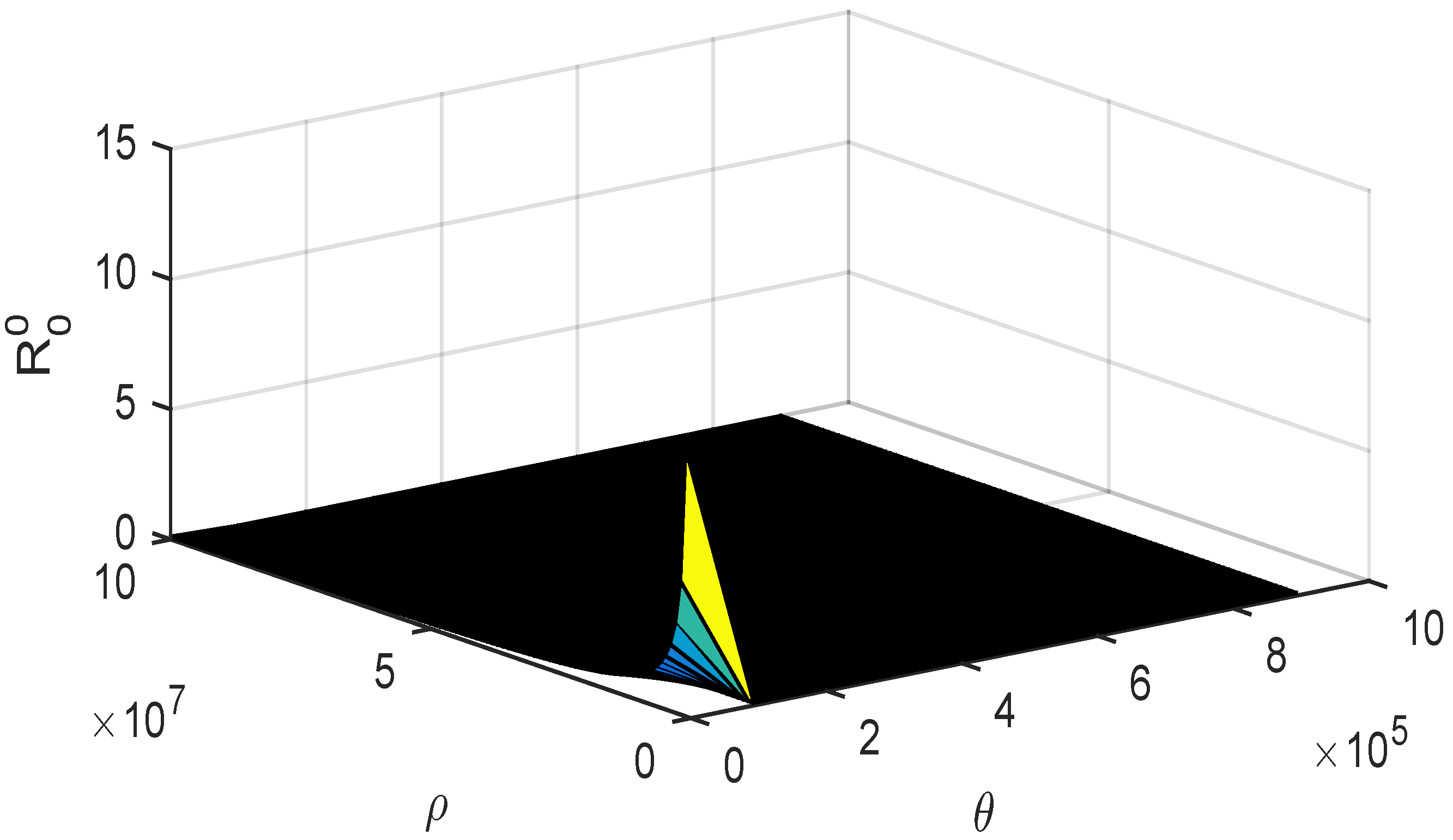
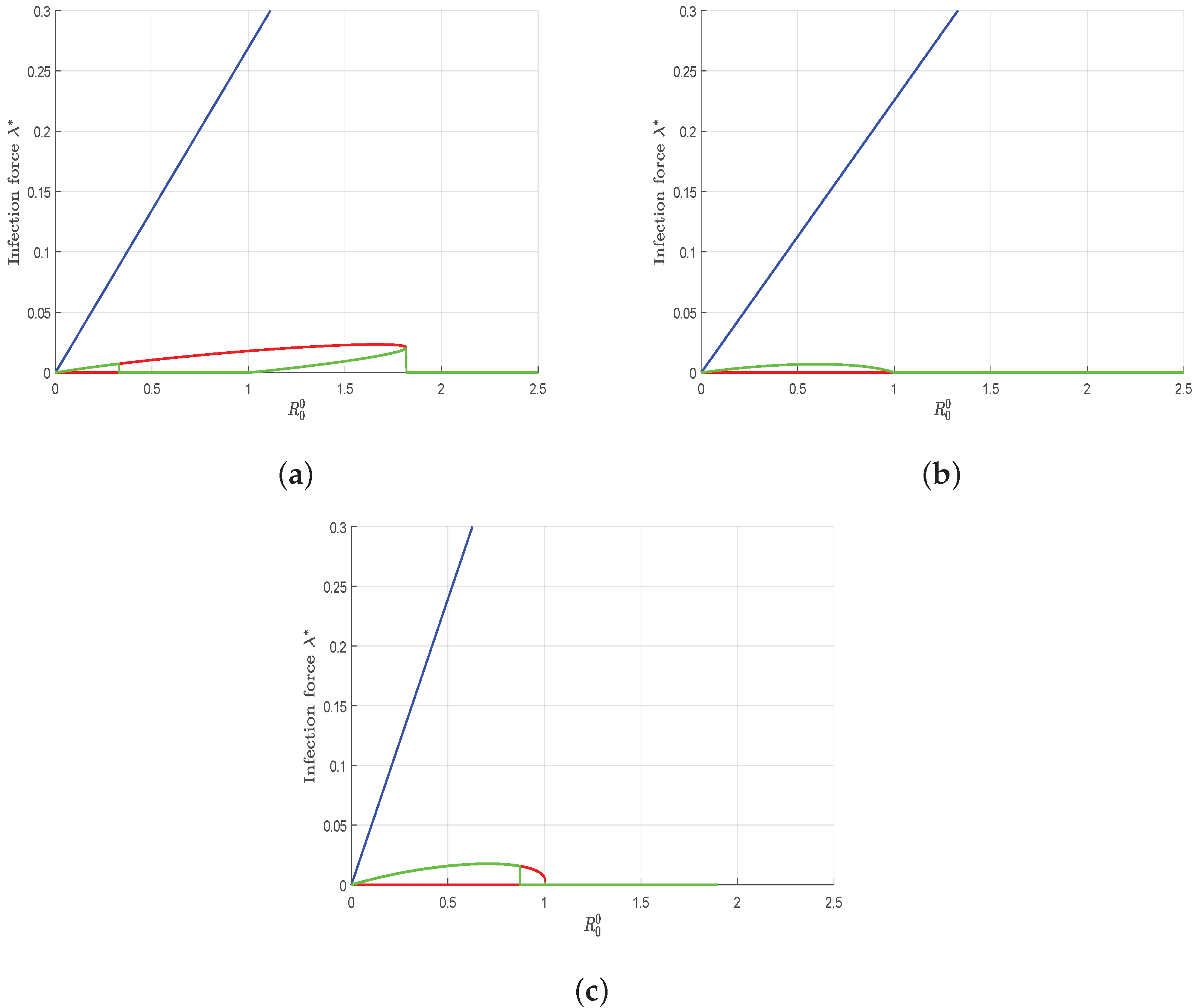
4.2. Numerical Simulations of Threshold Quantities with Variation of Allee Parameters and and Bifurcation
- The curves obtained highlight the importance of emphasizing that the risk of disease outbreaks cannot be neglected for any value of .
- Additionally, as approaches , the probability of epidemic outbreaks significantly increases.

5. Conclusions
- The model exhibits three disease-free equilibria related to three different real situations.
- The dynamics of the proposed model are determined by the threshold quantity .
- The phenomenon of bi-stability is observed, with backward and forward bifurcation.
- This research demonstrates that the Allee effect provides a robust framework for characterizing fluctuations in bacterial populations and the onset of cholera outbreaks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFE | Disease-Free Equilibrium |
| GAS | Global Asymptotic Stability |
| LHS | Latin Hypercube Sampling |
| PRCC | Partial Rank Correlation Coefficients |
| SIR | Susceptible Infected Recovered |
| VNC | Viable but Non-Culturable |
Appendix A. Calculation of Persistence Threshold for Disease-Free Equilibrium Qρ
Appendix B. Proof of Lemma 4
Appendix C. Proof of Theorem 4
- 1.
- is the linearization matrix of model (A4) around the equilibrium 0 with Φ evaluated at 0. Zero is a simple eigenvalue of A and other eigenvalues of A have negative real parts;
- 2.
- Matrix A has a right eigenvector u and a center eigenvector v (each corresponding to the zero eigenvalue). Let be the component of f andthen, the local dynamics of the system around the equilibrium point 0 is totally determined by the signs of a and b.
- 1.
- , . When with , 0 is locally asymptotically stable and there exists a positive unstable equilibrium; when , 0 is unstable and there exists a negative, locally asymptotically stable equilibrium;
- 2.
- , . When with , 0 is unstable; when , 0, is locally asymptotically stable equilibrium, and there exists a positive unstable equilibrium;
- 3.
- , . When with , 0 is unstable and there exists a locally asymptotically stable negative equilibrium; when , 0 is stable, and a positive unstable equilibrium appears;
- 4.
- , . When Φ changes from negative to positive, 0 changes its stability from stable to unstable. Correspondingly a negative unstable equilibrium becomes positive and locally asymptotically stable.
- Eigenvectors of
- For the case when , it can be shown that the Jacobian of model (A2) has a right eigenvector (corresponding to the zero eigenvalue), given by , whereSimilarly, the components of the center eigenvectors of (corresponding to the zero eigenvalue), denoted by , are given by:
- Computation of b
- For the sign of b, it can be shown that the associated non-vanishing partial derivatives of f are:It follows that:
- Computation of a
- Then, it follows that:
Appendix D. Calculation of Persistence Threshold for Endemicity
Appendix E. Proof of Theorem 5
- Eigenvectors of
- For the case when , it can be shown that the Jacobian of model (A8) has a right eigenvector (corresponding to the zero eigenvalue), given by , whereSimilarly, the components of the center eigenvectors of (corresponding to the zero eigenvalue), denoted by , are given by:
- Computation of b:
- For the sign of b, it can be shown that the associated non-vanishing partial derivatives of f are:It follows that:
- Computation of a:
- Then, it follows that:
References
- World Health Organization, Cholera. Available online: https://www.who.int/news-room/fact-sheets/detail/cholera. (accessed on 30 March 2023).
- Lipp, E.K.; Huq, A.; Colwell, R.R. Effects of global climate on infectious disease: The cholera model. Clin. Microbiol. Rev. 2002, 15, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Drasar, B.S.; Sack, R.B. The aquatic environment as a reservoir of Vibrio cholerae: A review. J. Diarrhoeal Dis. Res. 1993, 11, 197–206. [Google Scholar] [PubMed]
- Kolaye, G.; Bowong, S.; Houe, R.; Aziz-Alaoui, M.A.; Cadivel, M. Mathematical assessment of the role of environmental factors on the dynamical transmission of cholera. Commun. Nonlinear Sci. Numer. Simul. 2019, 67, 203–222. [Google Scholar] [CrossRef]
- Codeço, C.T. Endemic and epidemic dynamics of cholera: The role of the aquatic reservoir. BMC Infect. Dis. 2001, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Hartley, D.M.; Morris, J.G., Jr.; Smith, D.L. Hyperinfectivity: A critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 2006, 3, e7. [Google Scholar] [CrossRef] [PubMed]
- Kolaye, G.; Damakoa, I.; Bowong, S.; Houe, R.; Békollè, D. Theoretical assessment of the impact of climatic factors in a Vibrio cholerae model. Acta Biotheor. 2018, 66, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Kolaye, G.; Damakoa, I.; Bowong, S.; Houe, R.; Békollè, D. A mathematical model of cholera in a periodic environment with control actions. Int. J. Biomath. 2020, 13, 2050025. [Google Scholar] [CrossRef]
- Colwell, R.; Brayton, P.; Grimes, D.; Roszak, D.; Huq, S.; Palmer, L. Viable but non-culturable Vibrio cholerae and related pathogens in the environment: Implications for release of genetically engineered microorganisms. Nat. Biotechnol. 1985, 3, 817–820. [Google Scholar] [CrossRef]
- Roszak, D.; Colwell, R. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 1987, 51, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Campus de Microbiologie Medicale, VIBRIO. Available online: http://www.microbes-edu.org/etudiant/vibrio.html (accessed on 18 December 2022).
- Cuzin, L.; Delpierre, C. Épidémiologie des maladies infectieuses. EMC Mal. Infect. 2005, 2, 157–162. [Google Scholar] [CrossRef]
- Statistiques Mondiales. Available online: http://www.statistiques-mondiales.com/cameroun.htm (accessed on 18 December 2022).
- Bayleyegn, Y.N. Mathematical Analysis of a Model of Cholera Transmission Dynamics. Master’s Thesis, African Institute for Mathematical Sciences (AIMS), Cape Town, South Africa, 2009. [Google Scholar]
- King, A.A.; Ionides, E.L.; Pascual, M.; Bouma, M.J. Inapparent infections and cholera dynamics. Nature 2008, 454, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, N.; Hyman, J.M.; Cushing, J.M. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol. 2008, 70, 1272–1296. [Google Scholar] [CrossRef] [PubMed]
- Blower, S.M.; Dowlatabadi, H. Sensitivity and uncertainty analysis of complex models of disease transmission: An HIV model, as an example. Int. Stat. Rev./Rev. Int. Stat. 1994, 62, 229–243. [Google Scholar] [CrossRef]
- Marino, S.; Hogue, I.B.; Ray, C.J.; Kirschner, D.E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 2008, 254, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, J.C.; Sallet, G. Global asymptotic stability for the disease free equilibrium for epidemiological models. C. R. Math. 2005, 341, 433–438. [Google Scholar] [CrossRef]
- Kamgang, J.C. Contribution à la stabilisation des systèmes mécaniques: Contribution à l’étude de la stabilité des modèles épidémiologiques. Ph.D. Thesis, Université Paul Verlaine, Metz, France, 2003. [Google Scholar]
- Van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Chavez, C.; Song, B. Dynamical models of tuberculosis and their applications. Math. Biosci. Eng 2004, 1, 361–404. [Google Scholar] [CrossRef] [PubMed]
- Bouma, M.J.; Pascual, M. Seasonal and interannual cycles of endemic cholera in Bengal 1891–1940 in relation to climate and geography. In Proceedings of the The Ecology and Etiology of Newly Emerging Marine Diseases; Porter, J., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 147–156. [Google Scholar] [CrossRef]
- Carr, J. Applications of Centre Manifold Theory; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 35. [Google Scholar]



| Definition | Symbol | Estimated | Source |
|---|---|---|---|
| Recruitment rate | 10 day | Assumed | |
| Bacteria ingestion rate | 0.0001 person day | Assumed | |
| Human population death rate | day | [13] | |
| Bacteria shedding rate | 70 cells/(mL day) | [14] | |
| Half-saturation constant | K | cells/person/mL/day | Assumed |
| Cholera related death | d | 0.6 year | Assumed |
| Loss of immunity rate | 0.01 day | Assumed | |
| Recovery rate | 0.045 day | [15] | |
| Growth rate of Vibrios | r | 1 × 10 day | Assumed |
| Carrying capacity bacterial population | 1 × 10 cell/mL | Assumed | |
| Allee threshold bacterial population | 1 × 10 cell/mL | Assumed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolaye Guilsou, G.; Aziz-Alaoui, M.-A.; Houé Ngouna, R.; Archimede, B.; Bowong, S. Gaining Profound Knowledge of Cholera Outbreak: The Significance of the Allee Effect on Bacterial Population Growth and Its Implications for Human-Environment Health. Sustainability 2023, 15, 10384. https://doi.org/10.3390/su151310384
Kolaye Guilsou G, Aziz-Alaoui M-A, Houé Ngouna R, Archimede B, Bowong S. Gaining Profound Knowledge of Cholera Outbreak: The Significance of the Allee Effect on Bacterial Population Growth and Its Implications for Human-Environment Health. Sustainability. 2023; 15(13):10384. https://doi.org/10.3390/su151310384
Chicago/Turabian StyleKolaye Guilsou, Gabriel, Moulay-Ahmed Aziz-Alaoui, Raymond Houé Ngouna, Bernard Archimede, and Samuel Bowong. 2023. "Gaining Profound Knowledge of Cholera Outbreak: The Significance of the Allee Effect on Bacterial Population Growth and Its Implications for Human-Environment Health" Sustainability 15, no. 13: 10384. https://doi.org/10.3390/su151310384
APA StyleKolaye Guilsou, G., Aziz-Alaoui, M.-A., Houé Ngouna, R., Archimede, B., & Bowong, S. (2023). Gaining Profound Knowledge of Cholera Outbreak: The Significance of the Allee Effect on Bacterial Population Growth and Its Implications for Human-Environment Health. Sustainability, 15(13), 10384. https://doi.org/10.3390/su151310384







