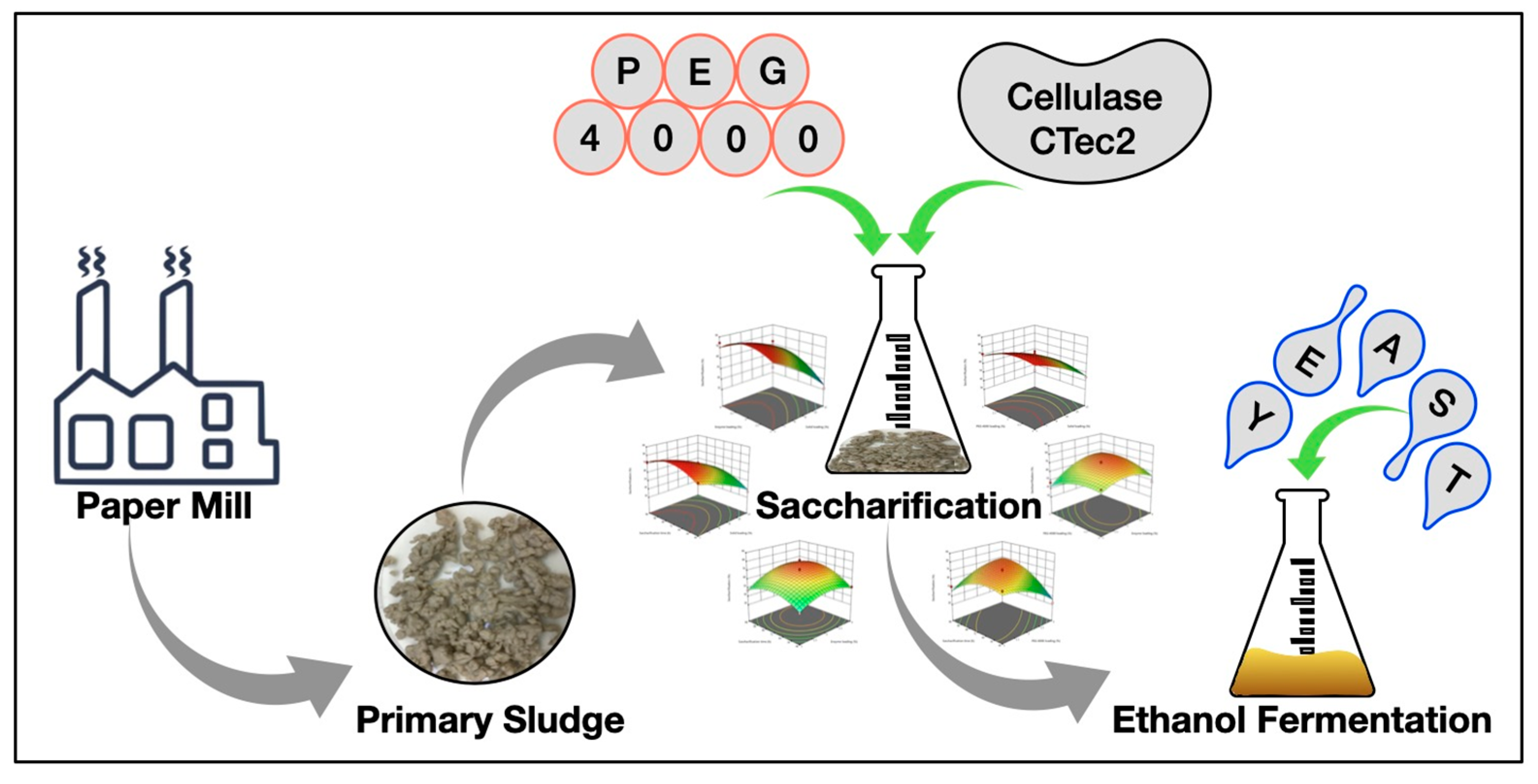
Box–Behnken Design-Based Optimization of the Saccharification of Primary Paper-Mill Sludge as a Renewable Raw Material for Bioethanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate, Enzymes, and Chemicals
2.2. Microorganisms
2.3. Substrate (DPS) Composition
2.4. Enzyme Assay
2.5. Effect of Surfactant and Enzymatic Saccharification
2.6. Saccharification
2.7. BBD-Based Statistical Analysis
2.8. Model Validation
2.9. Yeast Propagation and Ethanol Fermentation
3. Results and Discussion
3.1. Compositional Analysis
3.2. Enzymatic Saccharification
3.2.1. Effect of Non-Ionic Surfactants
3.2.2. Box–Behnken Design
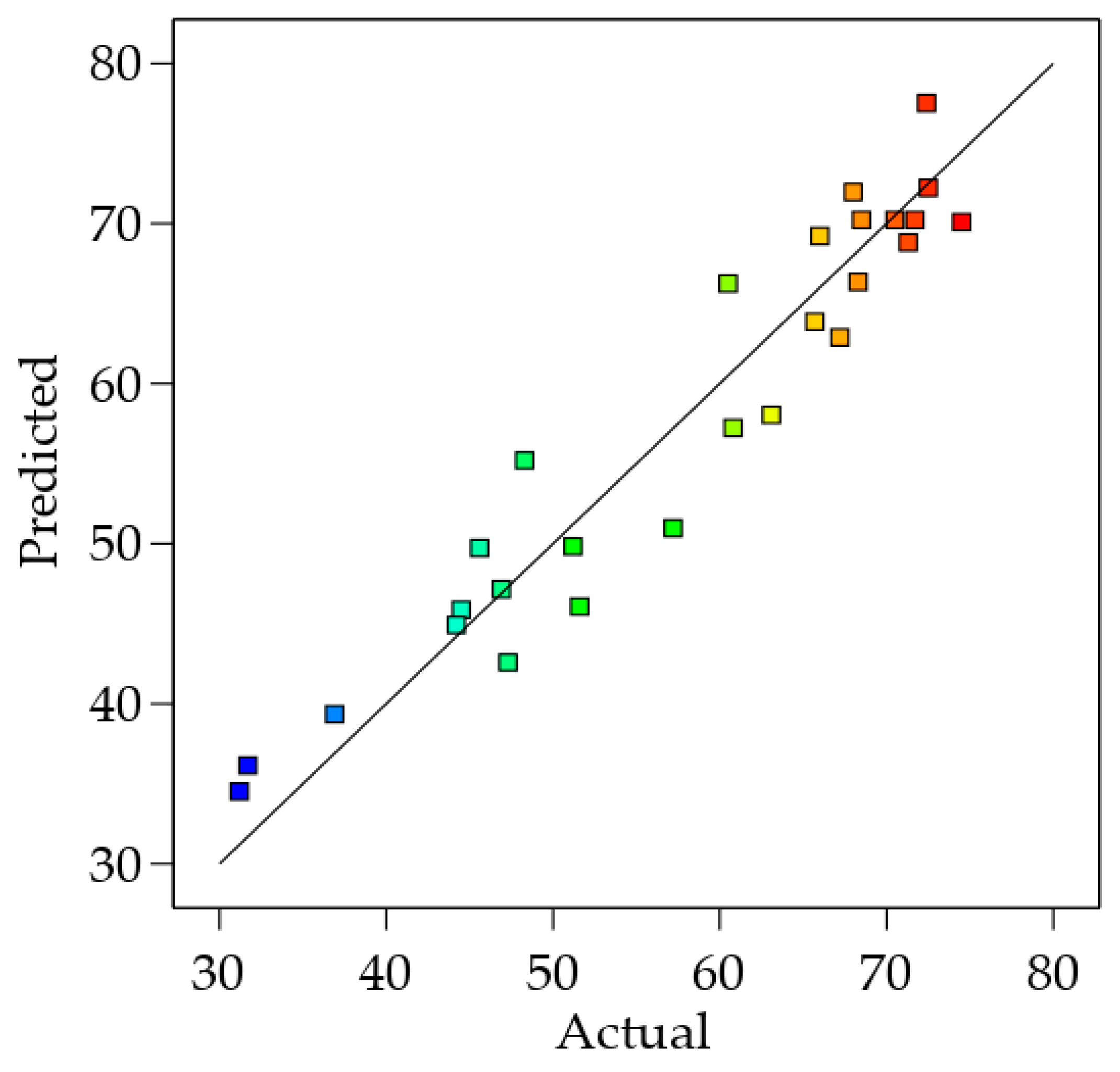
3.2.3. Model Validation
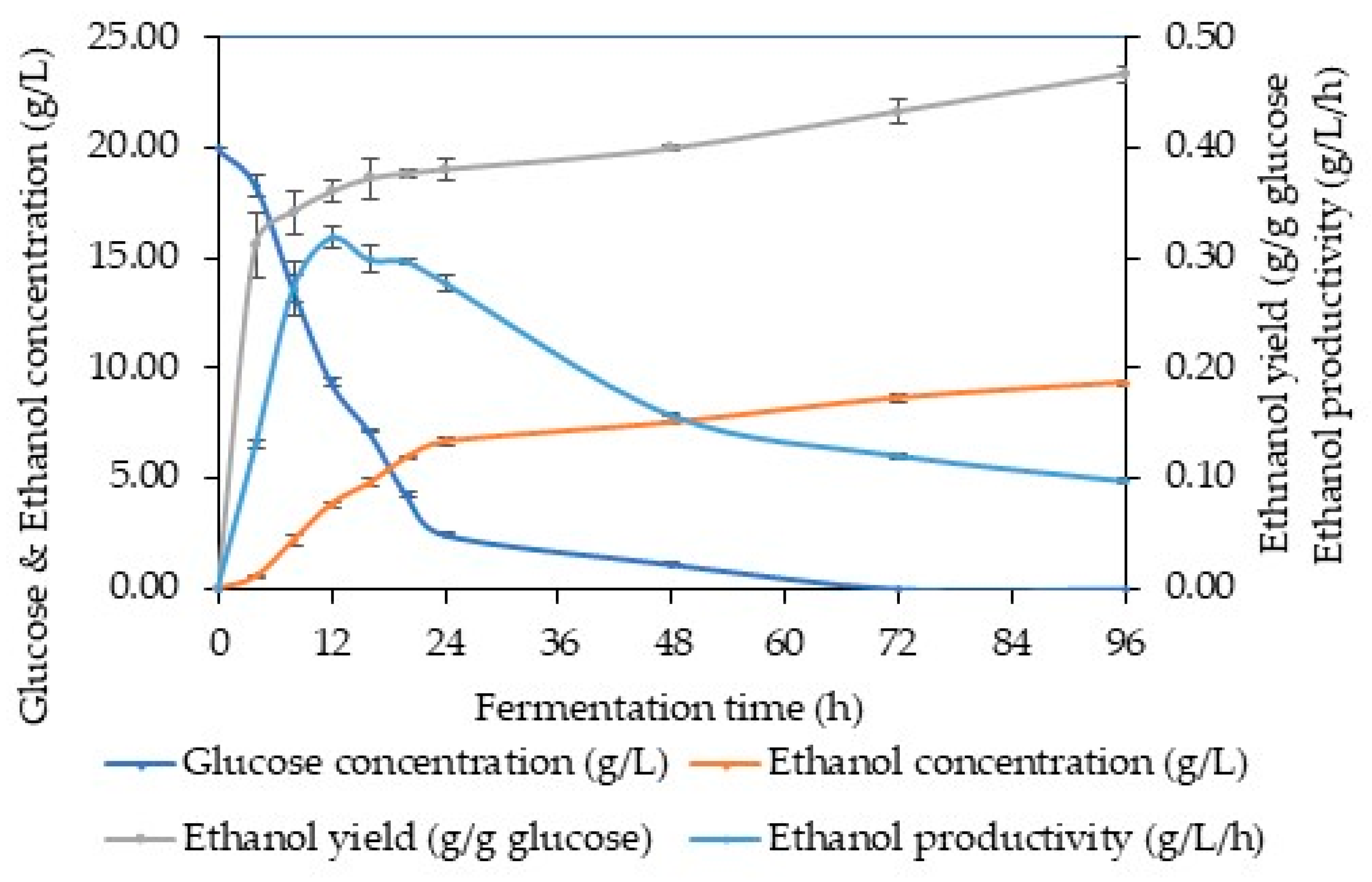
3.3. Ethanol Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Sassi, M.; Galarza, M.X.L. Biofuels and Food Security Future Challenges and Opportunities. Energy-Agro-Food Nexus East Africa 2016, 1, 49–65. [Google Scholar]
- Future Business Insights. The Global Pulp and Paper Market Is Projected to Grow from $354.39 Billion in 2022 to $372.70 Billion by 2029, at a CAGR of 0.72% in Forecast Period 2022–2029; Future Business Insights: Pune, India, 2022. [Google Scholar]
- Statistica. Global Pulp Industry—Statistics & Facts; Statistica: Hamburg, Germany, 2023. [Google Scholar]
- Ramos, M.D.N.; Rangel, A.S.; Azevedo, K.S.; Melo, M.G.B.; Oliveira, M.C.; Watanabe, C.M.U.; Pereira, F.F.; Silva, C.M.; Aguiar, A. Characteristics and treatment of Brazilian pulp and paper mill effluents: A review. Environ. Monit. Assess. 2022, 194, 651. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Generation of Waste in Pulp and Paper Mills. In Management of Pulp and Paper Mill Waste; Springer International Publishing: Cham, Switzerland, 2015; pp. 9–17. [Google Scholar]
- Kang, L.; Wang, W.; Lee, Y.Y. Bioconversion of kraft paper mill sludges to ethanol by SSF and SSCF. Appl. Biochem. Biotechnol. 2010, 161, 53–66. [Google Scholar] [CrossRef] [PubMed]
- MOEF. Tehnical EIA Guidance Manual for Pulp and Paper Industries; MOEF: Hyderabad, India, 2010. [Google Scholar]
- Salam, M.; Pondith, P.C.; Islam, A.; Khan, M.R.; Uddin, M.R.; Islam, M. Conversion of Cellulosic waste into fermentable sugar: Process optimization. J. Chem. Eng. 2014, 28, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, E. Fermentation of Lignocellulosic Hydrolysates: Inhibition and Detoxification. Master’s Thesis, Lund University, Lund, Sweden, 1998. [Google Scholar]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Conversion of C6 and C5 sugars in undetoxified wet exploded bagasse hydrolysates using Scheffersomyces (Pichia) stipitis CBS6054. AMB Express 2013, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Zambare, V.P.; Bhalla, A.; Muthukumarappan, K.; Sani, R.K.; Christopher, L.P. Bioprocessing of agricultural residues to ethanol utilizing a cellulolytic extremophile. Extremophiles 2011, 15, 611–618. [Google Scholar] [CrossRef]
- Birgen, C.; Dürre, P.; Preisig, H.A.; Wentzel, A. Butanol production from lignocellulosic biomass: Revisiting fermentation performance indicators with exploratory data analysis. Biotechnol. Biofuels 2019, 12, 167. [Google Scholar] [CrossRef] [Green Version]
- Torres-Mayanga, P.C.; Lachos-Perez, D.; Mudhoo, A.; Kumar, S.; Brown, A.B.; Tyufekchiev, M.; Dragone, G.; Mussatto, S.I.; Rostagno, M.A.; Timko, M.; et al. Production of biofuel precursors and value-added chemicals from hydrolysates resulting from hydrothermal processing of biomass: A review. Biomass Bioenergy 2019, 130, 105397. [Google Scholar] [CrossRef]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.D.; van Keulen, F.; Ferreira, B.S.; da Fonseca, M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. N. Biotechnol. 2014, 31, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Leu, S.-Y.; Zhu, J.Y. Substrate-Related Factors Affecting Enzymatic Saccharification of Lignocelluloses: Our Recent Understanding. BioEnergy Res. 2013, 6, 405–415. [Google Scholar] [CrossRef]
- Bekele Bayu, A.; Abeto Amibo, T.; Beyan, S.M. Process Optimization for Acid Hydrolysis and Characterization of Bioethanol from Leftover Injera Waste by Using Response Surface Methodology: Central Composite Design. Int. J. Anal. Chem. 2022, 2022, 4809589. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.S.; Milan, T.M.; Tapia-Blácido, D.R. Using Response Surface Methodology (RSM) to optimize 2G bioethanol production: A review. Biomass Bioenergy 2021, 151, 106166. [Google Scholar] [CrossRef]
- Saleh, A.K.; Abdel-Fattah, Y.R.; Soliman, N.A.; Ibrahim, M.M.; El-Sayed, M.H.; Abd El-Aziz, Z.K.; El-Zawawy, W.K. Box-Behnken design for the optimization of bioethanol production from rice straw and sugarcane bagasse by newly isolated Pichia occidentalis strain AS.2. Energy Environ. 2022, 33, 1613–1635. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, S.; Jaiswal, A.K. Artificial neural networks in valorization process modeling of lignocellulosic biomass. Biofuels Bioprod. Biorefining 2022, 16, 1849–1868. [Google Scholar] [CrossRef]
- Zambare, V.P.; Zambare, A.V.; Muthukumarappan, K.; Christopher, L.P. Potential of Thermostable Cellulases in the Bioprocessing of Switchgrass To Ethanol. Bioresources 2011, 6, 2004–2020. [Google Scholar] [CrossRef]
- Zambare, V.; Christopher, L. Optimization of enzymatic hydrolysis of corn stover for improved ethanol production. Energy Explor. Exploit. 2012, 30, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Zambare, V.P.; Zambare, A.V.; Barh, D.; Christopher, L.P. Optimization of enzymatic hydrolysis of prairie cordgrass for improved ethanol production. J. Renew. Sustain. Energy 2012, 4, 33118. [Google Scholar] [CrossRef]
- Qi, B.; Chen, X.; Shen, F.; Su, Y.; Wan, Y. Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Ind. Eng. Chem. Res. 2009, 48, 7346–7353. [Google Scholar] [CrossRef]
- Bacha, E.G. Response surface methodology modeling, experimental validation, and optimization of acid hydrolysis process parameters for nanocellulose extraction. S. Afr. J. Chem. Eng. 2022, 40, 176–185. [Google Scholar] [CrossRef]
- Sasikumar, E.; Viruthagiri, T. Optimization of process conditions using response surface methodology (RSM) for ethanol production from pretreated sugarcane bagasse: Kinetics and modeling. BioEnergy Res. 2008, 1, 239–247. [Google Scholar] [CrossRef]
- Gurram, R.N.; Al-Shannag, M.; Lecher, N.J.; Duncan, S.M.; Singsaas, E.L.; Alkasrawi, M. Bioconversion of paper mill sludge to bioethanol in the presence of accelerants or hydrogen peroxide pretreatment. Bioresour. Technol. 2015, 192, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkasrawi, M.; Al-Othman, A.; Tawalbeh, M.; Doncan, S.; Gurram, R.; Singsaas, E.; Almomani, F.; Al-Asheh, S. A novel technique of paper mill sludge conversion to bioethanol toward sustainable energy production: Effect of fiber recovery on the saccharification hydrolysis and fermentation. Energy 2021, 223, 120018. [Google Scholar] [CrossRef]
- Duncan, S.M.; Alkasrawi, M.; Gurram, R.; Almomani, F.; Wiberley-Bradford, A.E.; Singsaas, E. Paper Mill Sludge as a Source of Sugars for Use in the Production of Bioethanol and Isoprene. Energies 2020, 13, 4662. [Google Scholar] [CrossRef]
- Alkasrawi, M.; Rajangam, A.S.; Tawalbeh, M.; Kafiah, F.; Al-Othman, A.; Al-Asheh, S.; Sun, Q. Techno-economic analysis and a novel assessment technique of paper mill sludge conversion to bioethanol toward sustainable energy production. Int. J. Energy Res. 2020, 44, 12602–12613. [Google Scholar] [CrossRef]
- Zambare, V.P.; Christopher, L.P. Integrated biorefinery approach to utilization of pulp and paper mill sludge for value-added products. J. Clean. Prod. 2020, 274, 122791. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Rajangam, A.S.; Salameh, T.; Al-Othman, A.; Alkasrawi, M. Characterization of paper mill sludge as a renewable feedstock for sustainable hydrogen and biofuels production. Int. J. Hydrogen Energy 2021, 46, 4761–4775. [Google Scholar] [CrossRef]
- Prasetyo, J.; Naruse, K.; Kato, T.; Boonchird, C.; Harashima, S.; Park, E.Y. Bioconversion of paper sludge to biofuel by simultaneous saccharification and fermentation using a cellulase of paper sludge origin and thermotolerant Saccharomyces cerevisiaeTJ14. Biotechnol. Biofuels 2011, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Mohanty, P. Utilization of paper mill sludge as a sustainable source of hydrogen production. Biofuels 2022, 13, 1113–1118. [Google Scholar] [CrossRef]
- Kaur, R.; Tyagi, R.D.; Zhang, X. Review on pulp and paper activated sludge pretreatment, inhibitory effects and detoxification strategies for biovalorization. Environ. Res. 2020, 182, 109094. [Google Scholar] [CrossRef] [PubMed]
- Statista. Leading Pulp for Paper Producing Countries Worldwide in 2021; Statistica: Hamburg, Germany, 2023. [Google Scholar]
- Furszyfer Del Rio, D.D.; Sovacool, B.K.; Griffiths, S.; Bazilian, M.; Kim, J.; Foley, A.M.; Rooney, D. Decarbonizing the pulp and paper industry: A critical and systematic review of sociotechnical developments and policy options. Renew. Sustain. Energy Rev. 2022, 167, 112706. [Google Scholar] [CrossRef]
- TAPPI Test Methods. Carbohydrate composition of extractive-free wood and wood pulp by gas liquid chromatograph. Tech. Assoc. Pulp Pap. Ind. 1984, T249 cm-85, 1–8. [Google Scholar]
- Alhammad, A.; Adewale, P.; Kuttiraja, M.; Christopher, L.P. Enhancing enzyme-aided production of fermentable sugars from poplar pulp in the presence of non-ionic surfactants. Bioprocess Biosyst. Eng. 2018, 41, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kumar, R.; Wyman, C.E. Fast hemicellulose quantification via a simple one-step acid hydrolysis. Biotechnol. Bioeng. 2014, 111, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Adney, B.; Baker, J. Measurement of Cellulase Activities Laboratory; National Renewable Energy Laboratory: Golden, CO, USA, 1996; p. 8. [Google Scholar]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Das, S.; Bhattacharya, A.; Haldar, S.; Ganguly, A.; Gu, S.; Ting, Y.P.; Chatterjee, P.K. Optimization of enzymatic saccharification of water hyacinth biomass for bio-ethanol: Comparison between artificial neural network and response surface methodology. Sustain. Mater. Technol. 2015, 3, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Kanno, A.; Reungsang, A.; Imai, T.; Higuchi, T.; Sekine, M.; Yamamoto, K.; Phummala, K. Optimization of enzymatic hydrolysis for pretreated wood waste by response surface methodology in fermentative hydrogen production. J. Water Environ. Technol. 2015, 13, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Pandiyan, K.; Tiwari, R.; Singh, S.; Nain, P.K.S.; Rana, S.; Arora, A.; Singh, S.B.; Nain, L. Optimization of Enzymatic Saccharification of Alkali Pretreated Parthenium sp. Using Response Surface Methodology. Enzyme Res. 2014, 2014, 764898. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Sprague, M.A.; Griggs, A.J.; Lischeske, J.J.; Stickel, J.J.; Mittal, A.; Wang, W.; Johnson, D.K. Parameter determination and validation for a mechanistic model of the enzymatic saccharification of cellulose-I β. Biotechnol. Prog. 2015, 31, 1237–1248. [Google Scholar] [CrossRef]
- Ladeira Ázar, R.I.S.; Bordignon-Junior, S.E.; Laufer, C.; Specht, J.; Ferrier, D.; Kim, D. Effect of Lignin Content on Cellulolytic Saccharification of Liquid Hot Water Pretreated Sugarcane Bagasse. Molecules 2020, 25, 623. [Google Scholar] [CrossRef] [Green Version]
- Ying, W.; Shi, Z.; Yang, H.; Xu, G.; Zheng, Z.; Yang, J. Effect of alkaline lignin modification on cellulase–lignin interactions and enzymatic saccharification yield. Biotechnol. Biofuels 2018, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Lin, X.; Qiu, X.; Yuan, L.; Li, Z.; Lou, H.; Zhou, M.; Yang, D. Lignin-based polyoxyethylene ether enhanced enzymatic hydrolysis of lignocelluloses by dispersing cellulase aggregates. Bioresour. Technol. 2015, 185, 165–170. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Zhou, Y.; Qin, Y.; Liu, D.; Zhao, X. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and lignin structure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, X.; Zhang, Y.; Li, J. Effect of adding surfactant for transforming lignocellulose into fermentable sugars during biocatalysing. Biotechnol. Bioprocess Eng. 2011, 16, 930–936. [Google Scholar] [CrossRef]
- Sipos, B.; Dienes, D.; Schleicher, Á.; Perazzini, R.; Crestini, C.; Siika-aho, M.; Réczey, K. Hydrolysis efficiency and enzyme adsorption on steam-pretreated spruce in the presence of poly(ethylene glycol). Enzyme Microb. Technol. 2010, 47, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Gunjikar, T.P.; Sawant, S.B.; Joshi, J.B. Shear deactivation of cellulase, exoglucanase, endoglucanase, and β-glucosidase in a mechanically agitated reactor. Biotechnol. Prog. 2001, 17, 1166–1168. [Google Scholar] [CrossRef]
- Wang, W.; Zhuang, X.; Tan, X.; Wang, Q.; Chen, X.; Yu, Q.; Qi, W.; Wang, Z.; Yuan, Z. Dual effect of nonionic surfactants on improving the enzymatic hydrolysis of lignocellulose. Energy Fuels 2018, 32, 5951–5959. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Martinez-Bernal, C.; Pérez-Cobas, Y.; Reyes-Sosa, F.M.; García, B.D. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2017, 244, 48–56. [Google Scholar] [CrossRef]
- Kaar, W.E.; Holtzapple, M.T. Benefits from Tween during enzymic hydrolysis of corn stover. Biotechnol. Bioeng. 1998, 59, 419–427. [Google Scholar] [CrossRef]
- Helle, S.S.; Duff, S.J.B.; Cooper, D.G. Effect of surfactants on cellulose hydrolysis. Biotechnol. Bioeng. 1993, 42, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manaf, S.F.; Indera Luthfi, A.A.; Md Jahim, J.; Harun, S.; Tan, J.P.; Mohd Shah, S.S. Sequential detoxification of oil palm fronds hydrolysate with coconut shell activated charcoal and pH controlled in bioreactor for xylitol production. Chem. Eng. Res. Des. 2022, 179, 90–106. [Google Scholar] [CrossRef]
- Gunawan, E.R.; Basri, M.; Rahman, M.B.A.; Salleh, A.B.; Rahman, R.N.Z.A. Study on response surface methodology (RSM) of lipase-catalyzed synthesis of palm-based wax ester. Enzyme Microb. Technol. 2005, 37, 739–744. [Google Scholar] [CrossRef]
- Fang, H.; Zhao, C.; Song, X.Y. Optimization of enzymatic hydrolysis of steam-exploded corn stover by two approaches: Response surface methodology or using cellulase from mixed cultures of Trichoderma reesei RUT-C30 and Aspergillus niger NL02. Bioresour. Technol. 2010, 101, 4111–4119. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Fan, C.; Zheng, X.; Zhang, W.; Wu, D.; Wang, X.; Kong, H. Optimisation of enzymatic saccharification of wheat straw pre-treated with sodium hydroxide. Sci. Rep. 2021, 11, 23234. [Google Scholar] [CrossRef]
- Portero Barahona, P.; Bastidas Mayorga, B.; Martín-Gil, J.; Martín-Ramos, P.; Carvajal Barriga, E.J. Cellulosic Ethanol: Improving Cost Efficiency by Coupling Semi-Continuous Fermentation and Simultaneous Saccharification Strategies. Processes 2020, 8, 1459. [Google Scholar] [CrossRef]
- Nishimura, H.; Tan, L.; Sun, Z.-Y.; Tang, Y.-Q.; Kida, K.; Morimura, S. Efficient production of ethanol from waste paper and the biochemical methane potential of stillage eluted from ethanol fermentation. Waste Manag. 2016, 48, 644–651. [Google Scholar] [CrossRef]
- Branco, R.; Serafim, L.; Xavier, A. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2018, 5, 4. [Google Scholar] [CrossRef] [Green Version]

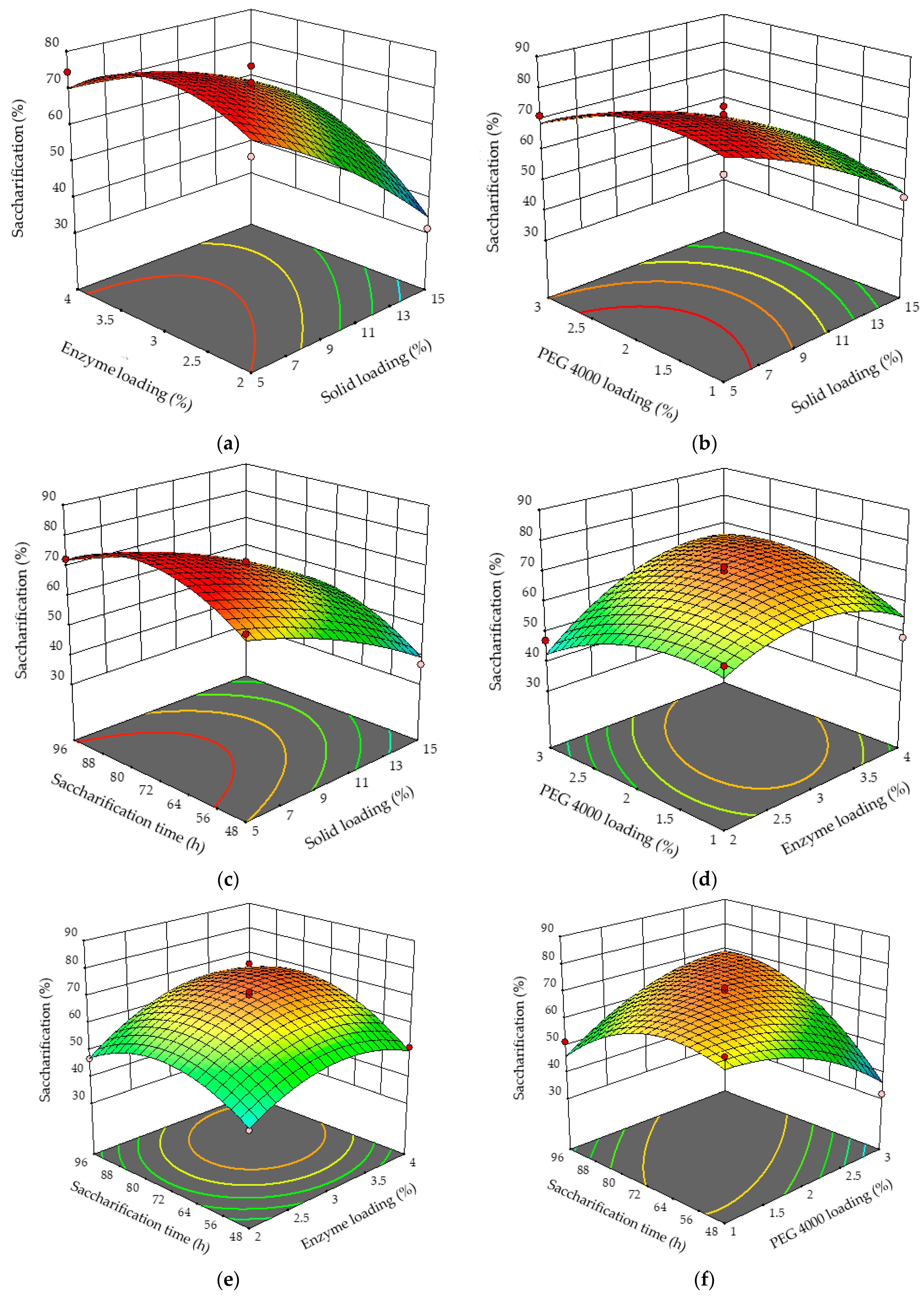
| Factors | Name | Units | Low (−1) | High (+1) |
|---|---|---|---|---|
| X1 | Solid loading | % (w/w *) | 5 | 15 |
| X2 | Enzyme loading | % (w/w *) | 2 | 4 |
| X3 | PEG-4000 loading | % (w/w *) | 1 | 3 |
| X4 | Saccharification time | h | 48 | 96 |
| Components | Composition |
|---|---|
| Moisture | 70.5 ± 3.7% (w/wet w) |
| Solids | 29.5 ± 2.6% (w/wet w) |
| Cellulose (Glucan) | 51.0 ± 3.3% (w/w dry solids) |
| Hemicelluloses | 10.6 ± 1.7% (w/w dry solids) |
| Lignin | 11.0 ± 1.7% (w/w dry solids) |
| Ash | 13.9 ± 2.0% (w/w dry solids) |
| Std. Run | Point Type | Actual Factors | Observed Saccharification (%) | Predicted Saccharification (%) | |||
|---|---|---|---|---|---|---|---|
| X1 (%) * | X2 (%) * | X3 (%) * | X4 (h) | ||||
| 1 | Factorial | 5 | 2 | 2 | 72 | 68.0 | 72.0 |
| 2 | Factorial | 15 | 2 | 2 | 72 | 31.2 | 34.5 |
| 3 | Factorial | 5 | 4 | 2 | 72 | 74.5 | 70.1 |
| 4 | Factorial | 15 | 4 | 2 | 72 | 63.1 | 58.0 |
| 5 | Factorial | 10 | 3 | 1 | 48 | 67.2 | 62.9 |
| 6 | Factorial | 10 | 3 | 3 | 48 | 31.7 | 36.1 |
| 7 | Factorial | 10 | 3 | 1 | 96 | 51.6 | 46.1 |
| 8 | Factorial | 10 | 3 | 3 | 96 | 66.0 | 69.2 |
| 9 | Factorial | 5 | 3 | 2 | 48 | 68.3 | 66.4 |
| 10 | Factorial | 15 | 3 | 2 | 48 | 36.9 | 39.4 |
| 11 | Factorial | 5 | 3 | 2 | 96 | 72.5 | 72.2 |
| 12 | Factorial | 15 | 3 | 2 | 96 | 45.6 | 49.8 |
| 13 | Factorial | 10 | 2 | 1 | 72 | 60.8 | 57.2 |
| 14 | Factorial | 10 | 4 | 1 | 72 | 48.3 | 55.2 |
| 15 | Factorial | 10 | 2 | 3 | 72 | 47.3 | 42.6 |
| 16 | Factorial | 10 | 4 | 3 | 72 | 60.5 | 66.3 |
| 17 | Factorial | 5 | 3 | 1 | 72 | 72.4 | 77.5 |
| 18 | Factorial | 15 | 3 | 1 | 72 | 44.5 | 45.9 |
| 19 | Factorial | 5 | 3 | 3 | 72 | 71.3 | 68.9 |
| 20 | Factorial | 15 | 3 | 3 | 72 | 57.2 | 51.0 |
| 21 | Factorial | 10 | 2 | 2 | 48 | 44.2 | 44.9 |
| 22 | Factorial | 10 | 4 | 2 | 48 | 51.2 | 49.9 |
| 23 | Factorial | 10 | 2 | 2 | 96 | 46.9 | 47.2 |
| 24 | Factorial | 10 | 4 | 2 | 96 | 65.7 | 63.9 |
| 25 | Center | 10 | 3 | 2 | 72 | 70.5 | 70.2 |
| 26 | Center | 10 | 3 | 2 | 72 | 68.5 | 70.2 |
| 27 | Center | 10 | 3 | 2 | 72 | 71.7 | 70.2 |
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 4195.84 | 14 | 299.70 | 9.52 | 0.0002 * |
| X1-Solid loading | 1837.69 | 1 | 1837.69 | 58.37 | <0.0001 * |
| X2-Enzyme loading | 351.00 | 1 | 351.00 | 11.15 | 0.0059 * |
| X3-PEG-4000 loading | 9.72 | 1 | 9.72 | 0.3087 | 0.5887 |
| X4-Saccharification time | 198.45 | 1 | 198.45 | 6.30 | 0.0274 * |
| X1X2 | 161.29 | 1 | 161.29 | 5.12 | 0.0430 * |
| X1X3 | 47.61 | 1 | 47.61 | 1.51 | 0.2424 |
| X1X4 | 5.06 | 1 | 5.06 | 0.1608 | 0.6955 |
| X2X3 | 165.12 | 1 | 165.12 | 5.24 | 0.0409 * |
| X2X4 | 34.81 | 1 | 34.81 | 1.11 | 0.3138 |
| X3X4 | 622.50 | 1 | 622.50 | 19.77 | 0.0008 * |
| X1X1 | 49.61 | 1 | 49.61 | 1.58 | 0.2333 |
| X2X2 | 387.60 | 1 | 387.60 | 12.31 | 0.0043 * |
| X3X3 | 217.60 | 1 | 217.60 | 6.91 | 0.0220 * |
| X4X4 | 561.70 | 1 | 561.70 | 17.84 | 0.0012 * |
| Residual | 377.83 | 12 | 31.49 | ||
| Lack of Fit | 372.60 | 10 | 37.26 | 14.26 | 0.0673 |
| Pure Error | 5.23 | 2 | 2.61 | ||
| Cor Total | 4573.67 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambare, V.; Jacob, S.; Din, M.F.M.; Ponraj, M. Box–Behnken Design-Based Optimization of the Saccharification of Primary Paper-Mill Sludge as a Renewable Raw Material for Bioethanol Production. Sustainability 2023, 15, 10740. https://doi.org/10.3390/su151310740
Zambare V, Jacob S, Din MFM, Ponraj M. Box–Behnken Design-Based Optimization of the Saccharification of Primary Paper-Mill Sludge as a Renewable Raw Material for Bioethanol Production. Sustainability. 2023; 15(13):10740. https://doi.org/10.3390/su151310740
Chicago/Turabian StyleZambare, Vasudeo, Samuel Jacob, Mohd Fadhil Md. Din, and Mohanadoss Ponraj. 2023. "Box–Behnken Design-Based Optimization of the Saccharification of Primary Paper-Mill Sludge as a Renewable Raw Material for Bioethanol Production" Sustainability 15, no. 13: 10740. https://doi.org/10.3390/su151310740






