Abstract
While the beneficial physical properties of silicone polymers are exploited in many sustainable applications, the high energy requirement for their synthesis compromises to a degree their sustainability. We report a strategy to mitigate this issue by filling the silicone with inexpensive and renewable starch. Elastomeric materials with covalently grafted starch, utilizing anhydride-modified silicones, permits loading of up to about 75% starch while maintaining many of the properties of the silicone. Alternatively, 50 wt.% starch-filled silicone foams can be prepared simply by mixing powdered starch with a mixture of HSi-functional silicone fluids in the presence of B(C6F5)3. The physical properties of the resulting foams are determined by the quantity of SiH, which controls the final density of the foams (ranging from 0.258–0.875 g mL−1), their Young’s modulus, and their degree of elasticity; both rigid and flexible foams were prepared. Materials with a high natural and renewable material content better adhere to green chemistry principle 7, should enhance the ease of degradation at end of life, and augment the sustainability of these silicone composites.
1. Introduction
Sustainability, among many other things, means that products we make from renewable natural resources will return at end of life to the environment in a form that can be readily converted back to natural materials. That is, sustainability is intimately linked to life cycle. Although nominally prepared from materials readily found in nature, such as sand and methanol, silicone polymers are completely synthetic [1]. Their very unusual properties can be exceptionally beneficial in energy-saving applications ranging from sealants in windows to reinforcements in tires that lead to lower rolling resistance and, therefore, less consumption of the energy source (fossil fuels or battery electricity) for the vehicle [2]. In addition, silicone oils will readily degrade in the environment to CO2, water, and sand, from which the silicones were made in the first place [3,4]. However, silicone polymers have a large energy content because the energetic cost of reducing the SiO bonds in sand is very high; ~2000 °C is required, typically involving greenhouse gas formation (GHG), in addition to the CO2 produced during the reduction of sand [5]. How can the energetic costs of the production of silicones be mitigated, while benefitting from their beneficial properties and their ability to undergo environmental degradation?
The principles of green chemistry articulate that benefit arises from the use of natural materials when creating chemical products (principle 7) [6]. One strategy to exploit this principle involves the incorporation of natural materials as fillers into silicone elastomers. Traditionally, silicone elastomers must be filled with reinforcing agents to improve mechanical properties. Fumed silica is an excellent material for this purpose. However, other materials, including natural materials, can also perform well in this regard. Waste wool was shown to provide comparable reinforcing capabilities to silica if short fibers were used, as well as higher performance with long fibers [7]. Lignin, the polyaromatic materials that reinforces cellulose in trees and other organisms, provided modest reinforcing capacity, but led to improved stability against flames when compared to pure silicone foams [8,9].
In a search for both more accessible and less expensive materials, we turned to starch as a green material [10]. After cellulose, it is one of the most abundant organic chemicals on the planet. Starch is readily available from a variety of different plant species. In addition to its use in the ‘as harvested’ form, it is frequently processed, e.g., degraded using acid and bleached with peroxide, to fulfil both nutritional needs and processing needs, typically for food products. Note, however, that starch is widely used in nonfood applications, such as in the pulp and paper industry [11].
Starch possesses a 2:1 ratio of secondary to primary alcohols as the key functional groups available for synthetic manipulation. Although anomeric centers offer a more reactive aldehydic functional group, there is only one per amylose chain. It was decided at the outset to target the available alcohols on saccharide monomers in (more soluble) starch as linkers to silicones. We report the preparation of starch silicone composites formed from starch alcohols using ester linkages derived from anhydrides, and starch silicone foams from HSi-silicones in a Piers–Rubinsztajn (PR) process.
2. Materials and Methods
2.1. Starting Materials
Tetraethyl orthosilicate, toluene (anhydrous), BaO, DMSO (dimethyl sulfoxide), ligroin (b.p. 60–90 °C), dicyclopentadiene, maleic anhydride, pyridine, BF3˙Et2O, ethyl acetate, magnesium oxide, triflic acid (trifluoromethylsulfonic acid, TfOH), octamethylcyclotetrasiloxane ((Me2SiO)4, D4), anhydrous diethyl ether, activated DARCO 060 charcoal, and Karstedt’s platinum catalyst ((platinum(0)-1,3-divinyl-1,1,3,3,-tetramethyldisiloxane complex) in xylene) were obtained from Sigma Aldrich. Soluble starch was purchased from BDH Inc. Tris(pentafluorophenyl)borane (B(C6F5)3, BCF) was purchased from TCI America. Trimethylsiloxy-terminated polydimethylsiloxane (DMS-T21), α,ω-hydride-terminated poly(dimethylsiloxane) (PDMS-H) DMS-H03 T4-H (2–3 cSt, molar mass ~450 g·mol−1), DMS-H11 T12-H (7–10 cSt, 1050 g·mol−1), DMS-H21 T76-H (100 cSt ~5800 g·mol−1), DMS-H25 T285-H (500 cSt, g·mol−1) and DMS H41 T845-H (10,000 cSt, 62,700 g·mol−1), and MHMH (HMe2SiOSiMe2H), respectively, were purchased from Gelest. Polymethylhydrogensiloxane (DC1107, Me3Si(OSiMeH)nOSiMe3, n was not measured, but is typically 20–60) was received from Dow Corning (now Dow) as a gift. DMSO was refluxed over and subsequently distilled from BaO, discarding the first 5% and the last 10% and stored over 4 Å molecular sieves. Other solvents were purified by distillation before use or passed through an activated alumina column. Otherwise, all chemicals were used as received.
All hydrosilylations were performed neat in an open atmosphere, but other reactions were conducted under a dry nitrogen atmosphere. A stock solution of BCF was prepared by dissolving BCF (0.040 mg, 0.080 mmol) in toluene (0.5 mL) and adding trimethylsiloxyl terminated polydimethylsiloxane (DMS-T21) (7.5 mL) as solvent; the final concentration of BCF in stock solution was 0.005 g/mL (0.010 mmol/mL).
2.2. General Methods
1H-NMR spectra were obtained on a Bruker AC-200 (200.13 MHz), a Bruker AC-300 (300.13 MHz), or a Bruker AM-500 (500.13 MHz) spectrometer. All natural-abundance 13C NMR spectra were obtained on a Bruker AC-200 (at 50.32 MHz), a Bruker AC-300 (at 75.03 MHz), or a Bruker AM-500 (at 125.78 MHz) spectrometer with broad-band proton decoupling. Solid-state 13C-NMR was performed on a Bruker AC-100 (100 MHz). 29Si-NMR spectra were measured on a Bruker AC-300 spectrometer. Infrared spectra (IR) were recorded on a Bio-Rad FfS-40. Mass spectrometry was performed using either electron impact (El) or chemical ionization (Cl) techniques accomplished using a VG ZAB-E mass spectrometer.
The molar masses of polymers were analyzed using a Waters Gel Permeation Chromatograph equipped with a Waters 410 Differential Refractive Index detector. Two Jordi Mixed bed columns in series were utilized with 1,1,1-trichloroethane as solvent flowing at 1.5 mL·min−1. Narrow-molecular-weight polystyrene standards from Polymer Laboratories were used for calibration of the chromatographic system. Differential scanning calorimetry (DSC) measurements were made using a TA Instruments DSC 2910 in a standard aluminum pan purged with nitrogen gas. The pan was placed into the standard cell and heated at 5 °C·min−1 over a temperature range from room temperature to 150 °C.
The volume of silicone starch foams was measured using the water displacement method. A piece of foam of known mass was submerged in water and the increase in apparent volume gave the volume of the foam (rapidly, so that no ingress of water occurred).
Vfoam = Vwater+foam − Vwater.
The density of foams was measured as follows:
Density: d = mfoam/Vfoam.
Young’s moduli were measured using a MACH-1 micromechanical testing instrument (Biomomentum Instruments) equipped with a 17 N multi-axis load cell and 0.5 mm hemispherical indenter using a Poisson ratio of 0.5 and a constant indentation depth of 1.0 mm with a 1 s dwell time. All measurements were conducted at 22 °C and in triplicate; errors are reported as the standard deviation. Prior to measurement, a cross-section was cut using a scalpel so that the top and bottom faces of the sample were both flat and parallel; sample thicknesses of ~2.5 cm were used.
2.3. Preparation of Diels–Alder Adduct: Bicyclo[2.2.l]hept-5-ene-2,3-dicarboxylic-anhydride (the Bicyclic Anhydride) 1
Cyclopentadiene monomer was distilled from dicyclopentadiene (70 mL) at 176 °C. To an ethyl acetate (15 mL) solution of maleic anhydride (3.2 g, 0.033 mol) was added ligroin (10 mL) (requires heating for dissolution). The solution was cooled thoroughly in an ice bath. Cyclopentadiene (3.2 mL, 0.047 mol) was added, and the flask was swirled vigorously before being returned to the ice bath. White spear-shaped crystals formed were obtained by filtration and washed three times with cold ligroin to give 4.1 g of the adduct 1 in 76% yield (m.p. 164–166 °C).
1H-NMR: (CDCl3, 200 MHz) δ = 1.65 (dd, 2H), 3.50 (d (b), m), 4H), 6.28 (t, 2H). 13C-NMR (CDCl3, 50 MHz) δ = 46.1, 47.0, 52.7, 135.5, and 171.3 ppm. IR (KBr pellet) ν = 3602, 2981, 2880, 1854, 1172, 1663, 1466, 1335, 1297, 1232, 1090, 907, 736, and 608 cm−1.
2.4. Preparation of Dimethylsiloxane Anhydride 2
To the bicyclic adduct (4.26 g, 0.026 mol) was added MHMH (5.02 g, 0.013) neat. The mixture was stirred at room temperature under a dry nitrogen atmosphere for 1 min before three drops of Karstedt’s catalyst was added. The mixture was allowed to stir for 24 h at room temperature during which time the clear solution became pale yellow in color. The reaction was monitored by 1H-NMR, and loss of the SiH signal (4.6 ppm) or vinyl protons of the anhydride (6.29 ppm) showed that the reaction went to completion. In some cases, it was necessary to add a small additional amount of catalyst to force the reaction to completion. Anhydrous diethyl ether and activated DARCO 060 charcoal (0.3 g) were added to the mixture. The flask was then heated at reflux for 5 min. After cooling, the charcoal was filtered using cold ether and washed three times with ether. The ether was removed by rotary evaporation. The product was a clear, viscous liquid 2 (7.83 g, 0.011 mol, 84%). We note that alternative anhydride-terminated silicones are now commercially available (Gelest). The platinum efficiently adheres to the charcoal and is, thus, efficiently stripped from the reaction solution; Celite filter aid may also be used for this purpose. Platinum metal can be converted back to chloroplatinic acid [12] and then Karstedt’s catalyst [13] via sequential reaction with aqua regia and then tetramethyldivinyldisiloxane.
1H-NMR (CDCl3, 300 MHz) δ = 0.04, 0.07 (s, 6H), 0.64 (t(br), 1H), 1.54 (m(br), 2H), 1.64 (m(br), 2H), 2.80 (d(br), 2H), 3.38 (m, 2H).13C NMR (CDCl3, 75 MHz) δ = −0.7, 1.1, 25.8, 26.7, 40.8, 41.2, 41.6, 49.6, 52.7, 172.1, and 172.3. 29Si-NMR (CDC13, 250 MHz) δ = −21.8, −20.5 (more intense), −3.2 ppm. Note: The ‘proton-coupled’ silicon NMR was also run, and the terminal Si in the silicone was a septet as opposed to the doublet of septets seen for H–Si–(CH3)2– in the starting material. IR (neat) ν = 2962, 2877, 1859, 1782, 1411, 1261, 1224, 1083 (br), 908, 801, and 591 cm−1.
2.5. Polydimethylsiloxane Anhydride by Redistribution T10-A, T20-A
To 2 (1.0 g, 1.37 mmol) was added D4 (2.99 mL, 9.62 mmol, 7 equiv.). The desired molecular weight was an average molar mass of 1500 g·mol−1. To achieve this, 10–15% excess D4 was added to account for cyclic monomers that would not be incorporated into the final product [14]. The mixture was stirred at room temperature for 1 min before three drops of TfOH (~0.06 mL, ~0.6 mmol) was added. The mixture became cloudy reddish-brown in color after 5 min and was allowed to stir for 4 h before being quenched with excess magnesium oxide. The quenched mixture was left to stir overnight leaving a clear product with the reddish-brown triflate salts adhered to the flask. The silicone was dissolved in hexane and decanted into a 25 mL round-bottom flask. The hexane was removed by rotary evaporation. The mixture was then distilled at reduced pressure (heated to 120 °C, 133 Pa) to remove low-molecular-weight cyclics. The desired product T10-A was obtained as a slightly viscous, clear colorless liquid (1.34 g, 0.89 mmol, 65%) molar mass 750 g·mol−1, with ~10 Me2SiO monomer units in the backbone. The process was repeated with double the quantity of D4 to produce a polymer with molar mass 1450 g·mol−1 T20-A.
T20-A1H-NMR: (CDCl3, 200 MHz) δ = 0.02–0.07 (s, 115H), 0.64 (t (br), 1H), 1.54 (m (br), 2H), 1.64 (m (br), 2H), 2.80 (d (br), 2H), 3.38 (m, 2H). 13C-NMR: (CDCl3, 75 MHz) δ = −0.7, 1.1, 25.8, 26.7, 40.8, 41.2, 41.6, 49.6, 52.7, 172.1, and 172.3. 29Si (CDCl3, 300 MHz) δ = −22.3, −21.8, and 5.2. IR (neat) ν = 2963, 2904, 1860, 1784, 1412, 1261, 1224, 1089 (br), 1021 (br), 908, 865, 800, 701, and 591. Mn = 1450 g·mol−1. Note that all spectroscopic data were identical to the lower-molecular-weight starting material except that the molar mass was higher, as was the integration for the Me2SiO peaks in the 1H-NMR.
2.6. Preparation of Starch/Silicone Composites: General Procedure
To a solution of DMSO containing powdered starch and dimethylaminopyridine (DMAP, 7 mol.% with respect to glucose units in starch) was added T10-A. The mixture was stirred at 80 °C under a dry nitrogen atmosphere for 1 h. The DMSO was then removed by Kugelrohr distillation (70 °C @ 533 Pa). The resulting physical properties for each of the respective starch–silicone composites varied according to the relative ratio between anhydride (A) unit attached to the silicone and glucose (G) units in starch (Table 1, Tables S1 and S2, Supporting Materials). Thermal properties are reported in Table 2.

Table 1.
Reagents used for starch/silicone composites.

Table 2.
Physical properties starch/silicone composites.
2.7. Swelling of Starch/Silicone Composites
A sample of each composite was placed, separately, in a plastic screw-capped vial filled with 1 mL of neutral water or water at pH 9 at 25 °C, water at 100 °C (i.e., placed in a boiling water bath), and deuterated chloroform. After 24 h, the solvent was removed, and the samples were weighed; the wt.% increase is reported (Table 3). In a separate set of tests, a sample of each composite was placed in a vial filled with 1 mL of water or deuterated chloroform; however, this time after 24 h, the solvent was removed, and the sample was left to sit open to the atmosphere and weighed after 24 h of air drying on the lab bench (Table 3).

Table 3.
Swelling test results after 24 h a.
2.8. General Synthesis of Starch/Silicone Foams
A 25 mL plastic syringe was cut (cross-sectionally) near the tip (near the Luer lock). The barrel was retracted to the 25 mL mark. To starch powder (1.295 g, 7.987 mmol) and BCF solution (100 μL, 0.5 mg, 0.001 mmol) was added with thorough mixing using a glass stirring rod; hydride-terminated polydimethylsiloxane T845-H (0.502 g, 0.016 mmol of SiH), T285-H (0.500 g, 0.058 mmol of SiH), and polyhydromethylsiloxane (DC1107) (0.296 g, 4.926 mmol) were added and mixed gently and quickly using glass stirring rod until a few bubbles appeared. The mixture was then allowed to foam and cure. Bubbles formed in 5–10 s after mixing and foams cured (surface tack-free) in 1–30 min depending on the reaction conditions (Table 4 and Table 5).

Table 4.
Reagents used for starch/silicone foams: optimizing formulations.

Table 5.
Reagents used for starch/silicone foams: practical formulations.
3. Results
3.1. Telechelic Silicone Anhydrides
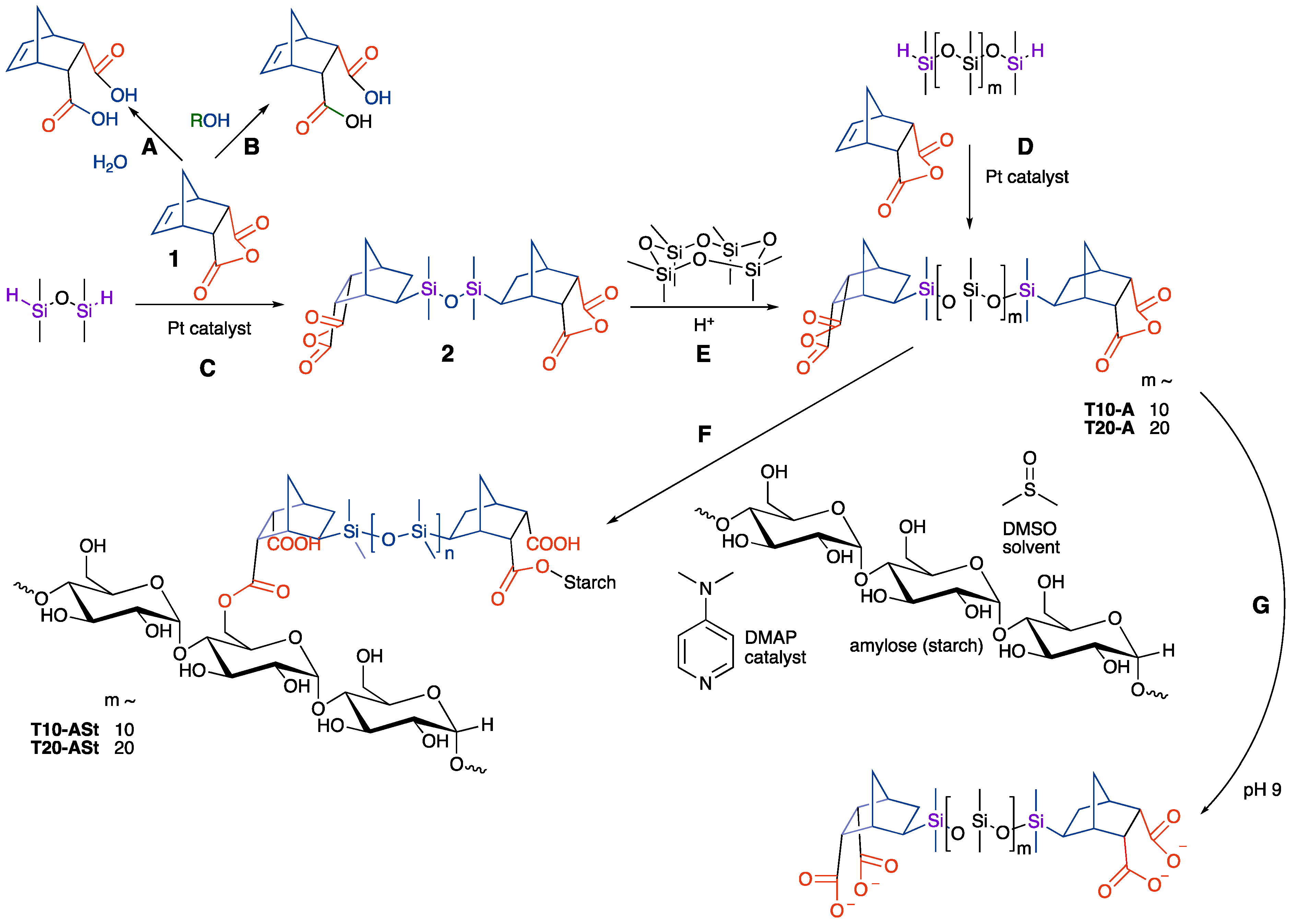
Anhydrides readily react with water to form acids. However, alcohols, including sugars, can kinetically compete with this process to create monoester linkages (Figure 1A,B) [15]. When the anhydride is cyclic, the process is a highly atom-efficient click reaction, as there are no byproducts in the formation of a monoester/monoacid product. Therefore, silicones that carried an anhydride group were sought out.
Figure 1.
(A) Hydrolysis or (B) alcoholysis of a cyclic anhydride. Hydrosilylation of (C) disiloxane or (D) telechelic H-silicone. (E) Ring-opening polymerization (ROP) of silicones to increase molar mass. (F) Starch/silicone block composites via anhydride ring opening. (G) Hydrolysis of untethered T10-A/T20-A.
Hydrosilylation is a very efficient method to create silicon–carbon bonds [16]. Only a few parts per million of platinum catalyst are required to facilitate the process, and use of catalysis supports the principles of green chemistry. A further advantage is that, when making small molecules, the recovery of spent platinum catalyst is straightforward. Normally, the hydrosilylation process is particularly efficient with terminal silicones, but relatively inefficient with internal (1,2-disubstituted olefins) [16], although internal hydrosilylation processes have been reported [17].
Cyclopentadiene, prepared by cracking dicyclopentadiene, was reacted in a Diels–Alder process with maleic anhydride to form 1 (at McMaster, this is a waste product from an undergraduate experiment that can be used and diverted from waste). Hydrosilylation of 1 with the small silicone MHMH (HMe2SiOSiMe2H) led in good yield to adduct 2 (Figure 1C).
In principle, direct hydrosilylation with larger molar mass telechelic silicones would lead to anhydrides with longer silicone chains (Figure 1D). In practice, however, the process was a bit more difficult to manage because of the relatively low density of anhydrides on high-molar-mass compounds. Silicones undergo metathesis reactions in the presence of acid or base [14]. In an alternative approach, therefore, the molar mass of the silicone chain was increased by inserting monomer units using D4 ((Me2SiO)4) in the presence of triflic acid; the length of the chain was simply adjusted by controlling the ratio of D4 to 2. Dianhydrides molar mass ~750 T10-A and 1450 g·mol−1 T20-A were prepared (m ~10 and 20, respectively; Figure 1E. Note: attempts were made to use silicone with molar mass 9000 g·mol−1 m ~116, but the products did not bind well to starch).
3.2. Ester Tethers between Starch and Silicone
The solubilities of starch (water) and silicone (nonpolar organic) are rather different. However, silicones will dissolve in isopropanol, and both can dissolve at low levels in DMSO. Therefore, starch/silicone composites were formed in DMSO at ~70 °C in yields of 55–75% using DMAP (dimethylaminopyridine) as a catalyst (pyridine and BF3˙Et2O were less efficacious catalysts; no reaction was observed in the absence of a catalyst).
Infrared spectroscopy was particularly helpful at demonstrating the presence of the ester peaks in T10-ASt and T20-ASt through new signals observed between 1733 and 1745 cm−1, and, in some cases, C=O shoulders from COOH at 1710 cm−1. As starch content increased, the magnitude of the ester carbonyl stretch diminished, as was to be expected; the 1A:50G composite had carbonyl signals that were comparable to noise. In some cases, depending on reaction time/temperature, residual anhydride could be seen through signals at 1778 and 1854 cm−1.
3.3. Physical Properties of Starch/Silicone Composites
Unsurprisingly, the physical properties of the starch/silicone composites were tied to the relative quantities of silicone and starch. To facilitate comparison, the ratio of saccharide units (G molar mass 158 g·mol−1) is related to anhydride (A linker) concentration. Thus, each anhydride is associated with ~5 D units (Me2SiO) for T10-ASt, and ~10 D units for T20-ASt. The starch content in the composites ranged between about 10 and 90 wt.%. Materials with higher silicone content were typically softer and more flexible (Table 2); the materials ranged from soft gel-like structures to hard elastomers. That is, the starch behaved as a traditional filler.
3.4. Thermal Behavior
Differential scanning calorimetry (DSC) analysis was performed on the starting materials and the respective composites. As expected, starch did not show a glass transition temperature (Table 2). It decomposed at approximately 280 °C. As the ratio of silicone increased, the composite exhibited lower decomposition temperatures until about 230 °C.
Compound T10-ASt with G/A ratio of 1:3 had a glass transition temperature (Tg) of −33.38 °C, which is a substantial increase over the hydride-terminated PDMS starting material that typically exhibits glass transition temperatures around −150 to −100 °C depending on the chain length of the polymer [18]. Silicones have one of the lowest glass transitions temperatures known for polymers, presumably because the silicon–oxygen bonds have considerable torsional mobility [19]. The higher Tg value for the T10-ASt compound when compared to silicone oil can be rationalized when the rigid, bulky anhydride termini are considered. Their size would reduce the overall segmental mobility. Aside from this one silicone-rich sample, Tgs could not be determined.
3.5. Swelling Tests on Starch/Silicone Composites
The solubilities of the starch and silicone constituents are very different, as already noted. The starch utilized in these experiments swells and, at elevated temperatures, dissolves in water. It was expected that the composites would swell to different degrees in both water and CDCl3 depending on the fraction of silicone in the product. The constituents that are not covalently linked in the composite would be expected to leach from the swollen material, leading initially to a weight increase from solvent, but eventually to weight loss with egress of untethered material.
The swelling of the various starch/silicone composites was examined under various types of aqueous stress, including swelling at room temperature up to boiling water, and in CDCl3. Some general trends can be observed (Table 3); there is a correlation between the wt.% saccharide and water uptake, but an inverse correlation with CDCl3 swelling. Composites made with the longer silicone chains swelled better in both solvents than their shorter chain analogues; and, swelling is enhanced at higher pH.
In a separate set of tests, a sample of each composite was placed in a vial filled with 1 mL of either water or deuterated chloroform. After 24 h soaking, in this case, the solvent was removed, and the sample was left to sit open to the atmosphere and weighed after 24 h of air drying on the bench (Table 3). Samples lost mass in both solvents demonstrating the presence of both untethered starch and silicone. The anomalous increase in mass of T10-ASt 20G:1A demonstrates that the drying protocol was insufficient to dehydrate the starch. The starch/silicone materials ‘shrugged off’ exposure to lower levels of water. For example, placing droplets of water on films of the T20-ASt 20G:1A, a soft, translucent plastic-like material, led to a decrease in opacity as the water swelled the starch before a return to the original translucent/opaque state after ~12 h, as excess water evaporated.
3.6. Silyl Ether Tethers between Starch and Silicone: Foams
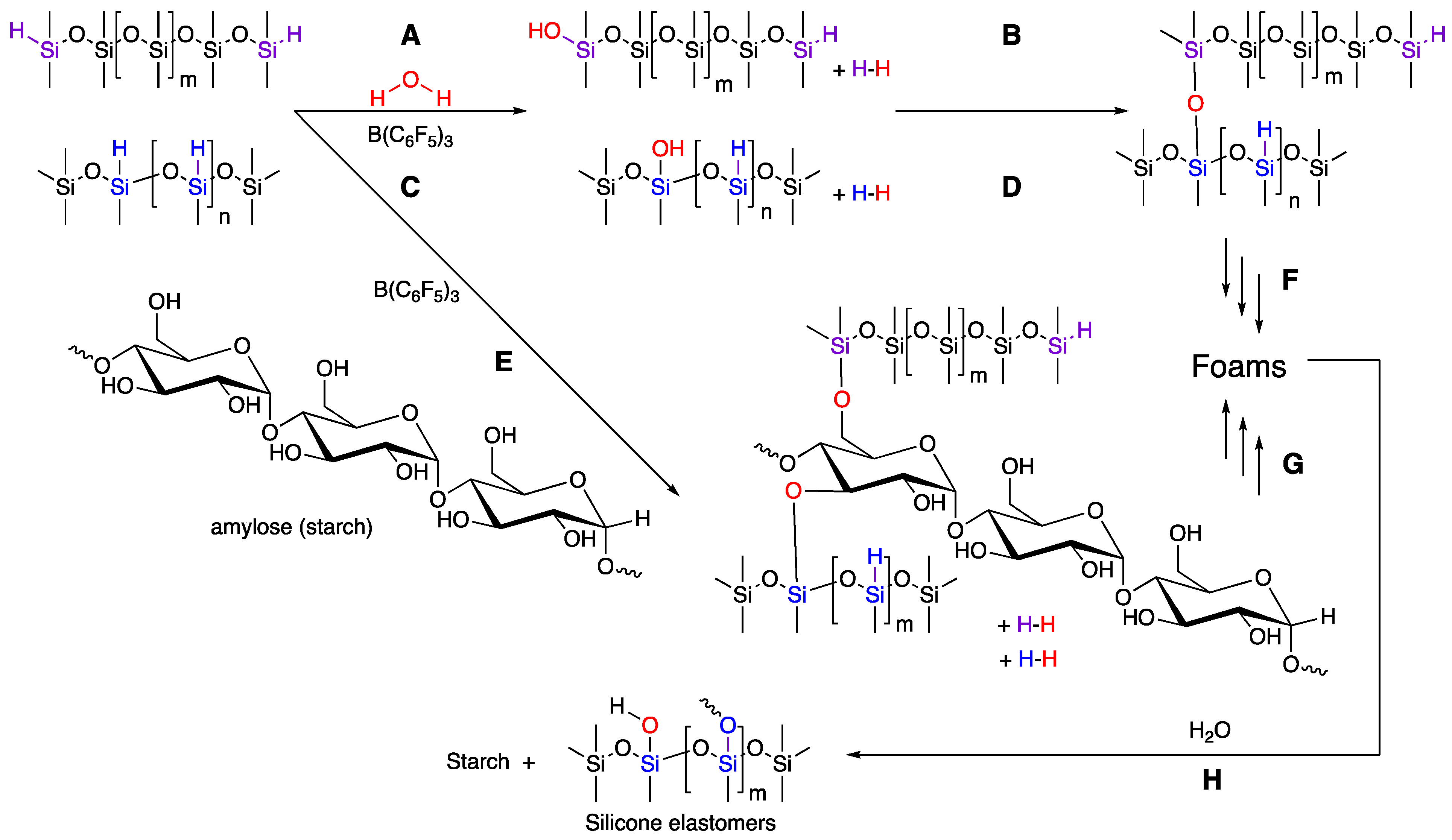
Hydrosilanes are effective reducing agents, particularly when formation of thermodynamically favorable Si–O bonds occurs during the process. For example, carbonyl reduction, leading to silyl ethers, is facile when both transition metal [20] and main group Lewis acid catalysts [21] are employed. Silanes also efficiently react with OH groups, including carboxylic acids, alcohols, and water, to produce a new Si–O bond and H2 as a by-product [20]; when B(C6F5)3 is used as catalyst, this is a type of Piers–Rubinsztajn (PR) reaction [22]. In the former two cases, the product silyl ester or ether, respectively, is subject to hydrolysis, particularly if there is no steric protection for the silicon atom by flanking bulky groups (Figure 2G).
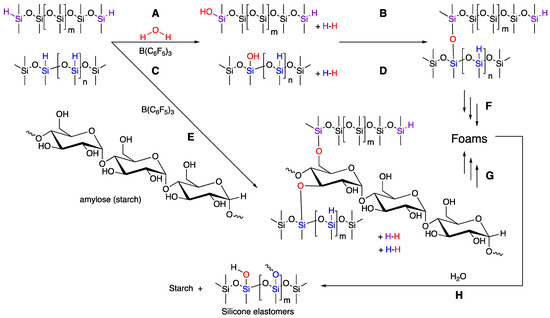
Figure 2.
(A,C) Hydrolysis of HSi-silicones. (B,D) Condensation/crosslinking of silanols. (E) Cross-reaction between his-silicones and starch. (F,G) Continued processes lead to foams. (H) Hydrolysis will eventually convert the foam to silicone elastomer and starch.
As noted above, with rare exceptions, starch is not soluble in the same solvents as silicones. It was of interest to learn if (i) silyl ether bonds would form at the interface between starch and silicone in the absence of solvent, and (ii) whether any resulting Si–O bonds would survive during normal utilization of the product. Therefore, a series of experiments was undertaken with powdered starch and silicones bearing SiH groups, both pendent and telechelic, in the presence of B(C6F5)3 (BCF) a powerful activator for SiH bonds. It was expected that, if reaction occurred, the hydrogen byproduct would act as a blowing agent [8,9].
In the absence of starch, it is possible to blow silicone foams using SiH compound in the presence of alkoxysilanes [23]. However, ambient moisture can also lead to hydrolysis/condensation processes with HSi-silicones [24]. A starting formula was chosen that included a silane crosslinker, and both short- and long-chain telechelic silicones (Figure 2). Control experiments were undertaken without starch, which showed that it was straightforward to blow foams using ambient water in the atmosphere with HSi-silicones alone; both flexible and rigid foams could be prepared with densities ranging from 0.267 to 0.704 g·mL−1 (Table 4).
BCF is expensive; therefore, survey experiments were initially undertaken to determine the minimum concentration needed to create foamed materials. It was found that ~500 ppm (0.5%) was sufficient (Table 4A). One objective of the process is to displace as much silicone as possible with starch, while maintaining useful silicone properties. Above ~50 wt.% starch, the product foams became both more dense and more brittle; thus, it was decided that 50 wt.% was the maximum that could be readily incorporated without significant compromise (Table 4B). This loading of starch was used in all remaining formulations. The ability to tune the foam properties by adding additional SiH groups, which in turn generates more H2 blowing agent, is clear from the data in Table 4C. While keeping the net silicone content the same, significant drops in density of the foams accompanied the use of linear or branched silicones with higher SiH content. These parameters can be used to select formulations with small/medium/long-chain extenders, while controlling the total SiH content, to create foams with varying density, Young’s modulus, and elasticity (Table 4D–F).
There are a variety of properties that are perceived as desirable in foams: density, modulus, stress relaxation, cell size, and the balance between open and closed cell structures. Inspection showed that the foams comprised mostly closed cells with very polydisperse cell sizes. Note that no surfactants were added, which would provide an additional level of control over number of bubbles in the final foam, reduce net bubble size, and narrow the polydispersity.
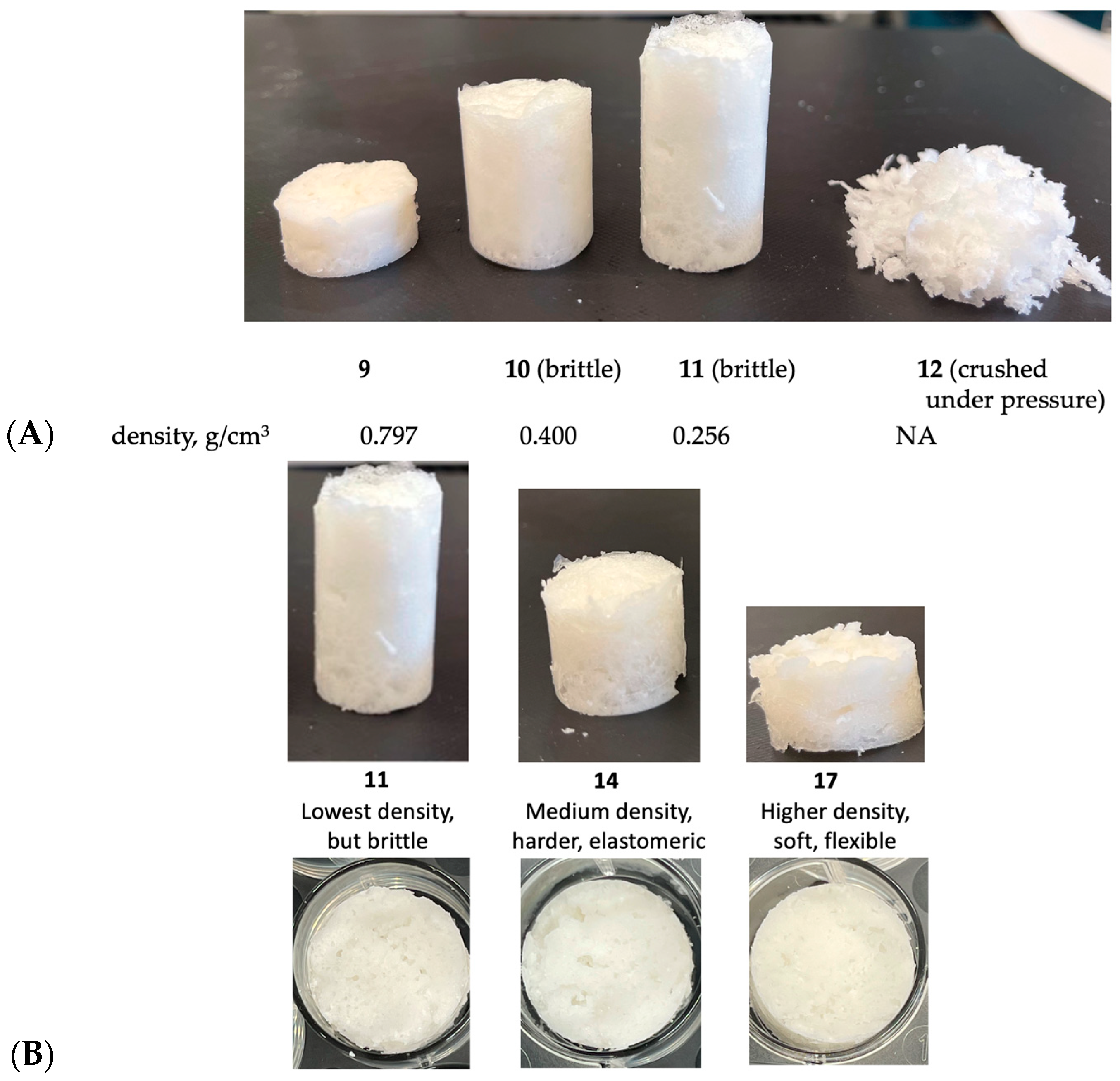
An examination of Table 4 and Table 5 demonstrates the flexibility of the process. Low-density foams require more SiH groups to create hydrogen as a blowing agent. However, one must additionally decide whether the SiH should or should not contribute to crosslink density. At a fixed concentration of crosslinker, longer-chain extenders led to higher-density foams that were more elastic (Figure 3A). It is facile to develop formulations with combinations of different chain extenders to create materials with the desired density and elasticity (Figure 3B). It is interesting to note that the modulus of starch-free and starch-filled silicones is not very different.
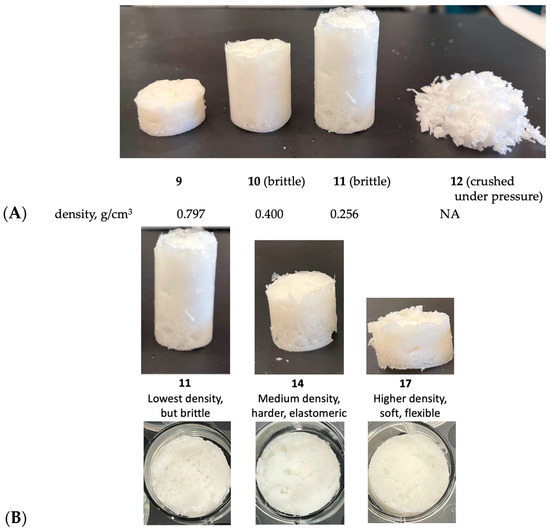
Figure 3.
(A) The same mass of silicone and starch blown into foams whose different properties arise just by varying the HSi content and length of chain extender (starch content was fixed at 50%). (B) Controlling degree of foaming and rigidity from rigid low-density foams to foamed elastomers (side view and top view). For photographs of all foams 1–24, see Table S3.
The silicone foam samples were also surprisingly stable in water. If left in 90 °C water for 3 days, a weight loss of only 3–4% was observed, consistent with the starch particles being fully coated with and bonded to the silicone matrix.
4. Discussion
4.1. Elastomers
4.1.1. Mechanical Properties
The observations here demonstrate that, by varying the molar mass of the silicone and, thus, the crosslink density, as well as the relative quantity of starch to silicone, it is possible to adjust the physical properties from soft gels to extensible elastomers. That is, the starch acts as a crosslinker for the silicone material, even at high loading. The elastomeric materials were formed from a DMSO solution that inevitably will, upon drying, lead to domains of both starch and silicones, accounting for their opacity except for the silicone-rich materials. The process for their manufacture did not permit all anhydrides to graft to starch, resulting in extractables (Table 3). Nevertheless, the process allows for a significant dilution of expensive silicone with inexpensive starch to make materials that exhibit many of the attractive properties of silicones. While yet untested, it is unlikely that these materials will be of much use at temperatures above ~120 °C, as starch will undergo dehydration and then degradation processes in analogy with the Maillard reaction.
4.1.2. Swelling
Water-soluble (non-crosslinked) polymers, such as amylose, absorb water, swell, and concomitantly dissolve (or erode) [25]. While at end of life, this is an excellent attribute, during active use, one would prefer that water modify but not destroy the composite (in this case, a silicone). As the fraction of starch increased in these composites, there was an accompanying increase in the ability to swell water (Table 3), as would be expected. The ability to absorb water was also affected by pH. At higher pH, greater swelling was observed due to the facility with which carboxylate salts sequester water [26]. Carboxylates will be present both because of ring opening of the silicone anhydride materials with starch to form esters and monocarboxylic acids. It is inferred that the latter accounts for the ability of 3G:lA T20-ASt to swell in water so much more than other analogues because of its higher COO− concentrations. Hydrolysis of the anhydride itself can also occur to form dicarboxylates (unproductive hydrolysis, Figure 1G). The composites were surprisingly resistant to boiling in water at starch loadings of 60% or less (Table 3). This indicates that the starch is well grafted to the silicone such that, even after the starch swells in water, the composite maintains its integrity.
There was also a correlation between samples that were richer in the silicone component and their ability to swell in CDCl3. The composites, thus, exhibit the characteristics of both the constituents they contain; they swell in water and chloroform in proportion to the quantity of starch and silicone, respectively, contained within.
It is noted that not all functional groups on the silicone led to bonds with the starch; both unreacted starch and silicone could be extracted from the composites by water and CDCl3, respectively (Table 3), albeit leaving composites with physical properties comparable to the pre-extracted materials in most cases.
4.2. Foams
Silicone foam has a range of uses, including in biomedical devices as scleral buckles [27]. However, the most common utilization is for transportation applications [28] where its resistance to combustion makes it a preferred, if more expensive, alternative to polyurethane foams [29,30]. In commercial silicone foams, (mostly) H2 bubbles are generated by platinum-catalyzed reactions of the OH groups on water, alcohol, or silanols with HSi groups; the cure arises from competitive platinum-catalyzed hydrosilylation. Significant challenges arise when the rate of the cure process is not kinetically matched with the bubble-generating process; if one outcompetes the other, high-density silicone elastomers are formed with only a few bubbles because gases are generated much more quickly or slowly than the cure took place.
The creation of foams from SiH silicones alone is facile in the presence of B(C6F5)3 (Table 4 and Table S1). Foams with very different properties are prepared simply by mixing the desired silicone pre-elastomers with the desired quantity of starch, adding the catalyst and stirring for a couple of seconds. The foaming reaction is rapid and complete within a few minutes, while a full cure takes several hours.
The foam generation process is much easier to practice than Pt-catalyzed systems because of the direct link between cure and bubble generation; mechanistically, the two processes are intimately linked, and the resulting synthesis is very forgiving. The SiH groups react with ambient moisture to generate H2. A crosslink will arise between two SiH groups and one water molecule (Figure 2A/C + B/D). This allows rigid (very highly crosslinked) or flexible (more lightly crosslinked) unfilled elastomers to form around the bubbles. With the addition of starch, each SiH reaction with a surface starch –OH will lead to a crosslink site. Curing should, therefore, be somewhat faster than analogous formulations without starch (one vs. two reactions are required). In some cases, the starch-free systems cured more slowly, but not always, which is consistent with water being an important crosslink former in both starch and starch-free formulations.
The most commonly used reinforcing filler for silicone elastomers and foams is silica, particularly fumed silica, which significantly increases tear strength, modulus, etc. The nominal size of fumed silica particles is <100 nm, while starch particles are typically >10 μm in size. Thus, a better comparison for starch would be precipitated silicas that have comparable (but typically smaller) particle sizes and much higher density (density of starch and precipitated silica is ~1.5 and ~2.0 g·mL−1, respectively; note that precipitated calcium carbonate PCC may also be used as an inexpensive filler in silicones). Normally, it is impossible to incorporate more than about 30 wt.% silica, as the materials become hard to process because of viscosity. By contrast, loading foams with 50 wt.% starch is straightforward in this process, acting even better as a diluent of the silicone. In both cases, the silicone has high affinity with the filler and, additionally, in the case of starch, the starch is covalently linked to the silicone matrix.
The utilization of lignin powder, another natural filler, to reinforce silicone foams has previously been reported. In those cases, a maximum of about 40 wt.% lignin could be incorporated in the foam; greater quantities than that led to highly open structures [8,9]. The reaction with starch is faster and more forgiving. More starch can be incorporated and yet give more robust foams, it is inferred, because of the much higher density of OH groups on starch than lignin.
The large polydispersity of bubbles in the starch composites indicates that some bubbles are trapped rapidly, while others can coalesce as cure continues. It should be possible to use foam stabilizers (typically silicone polyethers) to make closed cell foams with smaller bubbles. However, one of the advantages of the current process is that surfactants, with their own issues of degradation, are not required [31].
4.3. Sustainability
The silicone/starch elastomeric composites required a cosolvent to permit efficient reaction between the two ingredients. Unlike the foams (see below), attempted reactions between silicone anhydrides and starch powder in the absence of a solvent were ineffective. On one hand, DMSO is a wonderful solvent. On the other hand, it is difficult to remove, particularly from polymeric/elastomeric samples, because of its high boiling point. Catalysts are encouraged over stoichiometric reagents according to principle 9 of green chemistry. The DMAP catalyst is highly effective in this and other esterification reactions, but its toxicity and ability to be absorbed through the skin compromise its use, particularly because it will eventually leach from the sample to the environment.
The composites and foams described here can partially replace expensive crosslinked silicone polymers that have an energy tax associated with their preparation The replacement starch is a natural, inexpensive material that dilutes the quantity of silicone needed for a given application. On this basis, the materials better follow green chemistry principle 7 (incorporate natural materials) than traditional silicones. Up to ~75% of the silicone could be replaced by starch in useful elastic composites and up to 50 wt.% in foams. Notably, the processability of silicones with 50 wt.% starch was higher than highly silica-loaded systems. The lower cost of starch than silica is an additional benefit that should encourage adoption. The composites exhibited many of the desirable properties of silicones: ductility, water repellency, and a nice feel to the touch.
Alkoxysilanes found in the foams (starch–O–silicone) are prone to hydrolysis, more prone than esters. Thus, under sufficient aqueous stress, particularly with acidic or basic catalysis, degradation is to be expected to liberate starch and silicone oil. The processes are expected to occur in landfills and in the natural environment. Since the products of hydrolysis will undergo degradation in the environment, this better closes the life cycle of these silicone composites over pure silicone elastomers for which the rate of degradation is not known. This is also associated with better sustainability and green chemistry principle 10.
Silicones bring many beneficial properties to applications ranging from personal care to electronics. In many of these applications, they are coveted for their resistance to various stimuli: water, current, voltage, temperature, oxidation, etc. Placing organic materials within silicones, particularly organic compounds, such as starch that are susceptible to oxidation, will compromise some of those properties. However, these starch/silicone composites should be very suitable for the many other applications that will not experience wild fluctuations in temperature, prolonged exposure to water, or other stresses, particularly when used indoors. The starch is well protected from the outside world by the silicone, as shown in the boiling water experiments, which is the point. Not all silicones are used in critical applications. Compromising some properties by including natural materials for selected applications in certain cases is an important strategy to mitigate the energetic content of silicones and improve the degradation of silicone in the environment, both of which enhance their sustainability.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su15139941/s1: Table S1. Swelling tests; Table S2. IR and NMR data; Table S3. Photos of starch/silicone foams.
Author Contributions
For the elastomer part, conceptualization, M.A.B.; methodology, D.A.V.; formal analysis, M.A.B. and D.A.V.; for the foam part, conceptualization, M.A.B. and Y.C.; methodology, Y.C.; formal analysis, M.A.B. and Y.C.; writing—original draft preparation, M.A.B.; writing—review and editing, M.A.B., D.A.V. and Y.C.; supervision, M.A.B.; project administration, M.A.B.; funding acquisition, M.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Council of Canada.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available data are provided in the article and the Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge the financial support of the Natural Sciences and Engineering Council of Canada. The authors thank Yong-Guen Yu for measuring Young’s moduli of the foams and Dow Corning (now Dow) for the gift of DC-1107.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tomanek, A. Silicones and Industry; Hanser (Wacker Chemie): Munich, Germany, 1991; p. 37. [Google Scholar]
- Energy and Silicones. Available online: https://globalsilicones.org/explore-silicones/benefits-uses/energy/ (accessed on 13 May 2023).
- Lehmann, R.G.; Varaprath, S.; Annelin, R.B.; Arndt, J.L. Degradation of silicone polymer in a variety of soils. Envirn. Toxicol. Chem. 1995, 14, 1299–1305. [Google Scholar] [CrossRef]
- Lehmann, R.G.; Varaprath, S.; Frye, C.L. Degradation of silicone polymers in soil. Envirn. Toxicol. Chem. 1994, 13, 1061–1064. [Google Scholar] [CrossRef]
- Maldonado, S. The Importance of New “Sand-to-Silicon” Processes for the Rapid Future Increase of Photovoltaics. ACS Energy Lett. 2020, 5, 3628–3632. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry Theory and Practice; OUP: Oxford, UK, 2000. [Google Scholar]
- Zheng, S.; D’Angelo, A.; Zell, U.; Chen, Y.; Silverthorne, K.E.C.; Brook, M.A. Naked alpaca wool works better with silicone elastomers. Green Chem. 2021, 23, 7692–7700. [Google Scholar] [CrossRef]
- Zhang, J.; Fleury, E.; Brook, M.A. Foamed lignin-silicone bio-composites by extrusion and then compression molding. Green Chem. 2015, 17, 4647–4656. [Google Scholar] [CrossRef]
- Zhang, J.; Fleury, E.; Chen, Y.; Brook, M.A. Flame retardant lignin-based silicone composites. RSC Adv. 2015, 5, 103907–103914. [Google Scholar] [CrossRef]
- Falua, K.J.; Pokharel, A.; Babaei-Ghazvini, A.; Ai, Y.; Acharya, B. Valorization of Starch to Biobased Materials: A Review. Polymers 2022, 14, 2215. [Google Scholar] [CrossRef] [PubMed]
- Adewale, P.; Yancheshmeh, M.S.; Lam, E. Starch modification for non-food, industrial applications: Market intelligence and critical review. Carbohydr. Polym. 2022, 291, 119590. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.A. Platinum in silicone breast implants. Biomaterials 2006, 27, 3274–3286. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, P.B.; Lappert, M.F.; Warhurst, N.J.W. Synthesis and structure of a rac-tris(divinyldisiloxane)diplatinum(0) complex and its reaction with maleic-anhydride. Angew. Chem. Int. Ed. Engl. 1991, 30, 438–440. [Google Scholar] [CrossRef]
- Brook, M.A. Silicones. In Silicon in Organic, Organometallic and Polymer Chemistry; Wiley: New York, NY, USA, 2000; pp. 256–308. [Google Scholar]
- Hermanson, G.T. Chapter 3—The Reactions of Bioconjugation. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 229–258. [Google Scholar] [CrossRef]
- Marciniec, B.; Gulinski, J.; Urbaniak, W.; Kornetka, Z.W. Comprehensive Handbook on Hydrosilylation Chemistry; Pergamon: Oxford, UK, 1992. [Google Scholar]
- Brook, M.A.; Ragheb, A. Oxidizable coupling agents: Introduction of surface functionality. J. Adhes. 2002, 78, 521–541. [Google Scholar] [CrossRef]
- Clarson, S.J.; Semlyen, J.A. Siloxane Polymers; Prentice Hall: Englewood Cliffs, NJ, USA, 1993. [Google Scholar]
- Allcock, H.R.; Lampe, F.W. Contemporary Polymer Chemistry; Prentice Hall: Englewood Cliffs, NJ, USA, 1981. [Google Scholar]
- Brook, M.A. Hydrosilanes as Reducing Agents. In Silicon in Organic, Organometallic and Polymer Chemistry; Wiley: New York, NY, USA, 2000; pp. 171–188. [Google Scholar]
- Houghton, A.Y.; Hurmalainen, J.; Mansikkamäki, A.; Piers, W.E.; Tuononen, H.M. Direct observation of a borane–silane complex involved in frustrated Lewis-pair-mediated hydrosilylations. Nat. Chem. 2014, 6, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.A. New Control over Silicone Synthesis Using SiH Chemistry: The Piers-Rubinsztajn Reaction. Chem. A Eur. J. 2018, 24, 8458–8469. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.B.; Fawcett, A.S.; McLaughlin, A.J.; Gonzaga, F.; Bender, T.P.; Brook, M.A. Anhydrous formation of foamed silicone elastomers using the Piers-Rubinsztajn reaction. Polymer 2012, 53, 3135–3142. [Google Scholar] [CrossRef]
- Liao, M.; Schneider, A.F.; Laengert, S.E.; Gale, C.B.; Chen, Y.; Brook, M.A. Living synthesis of silicone polymers controlled by humidity. Eur. Polym. J. 2018, 107, 287–293. [Google Scholar] [CrossRef]
- Doelker, E. Cellulose derivatives. In Proceedings of Biopolymers I; Springer: Berlin/Heidelberg, Germany, 1993; pp. 199–265. [Google Scholar]
- Horkay, F.; Tasaki, I.; Basser, P.J. Osmotic Swelling of Polyacrylate Hydrogels in Physiological Salt Solutions. Biomacromolecules 2000, 1, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tsui, I. Scleral Buckle Removal: Indications and Outcomes. Surv. Ophthalmol. 2012, 57, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Landrock, A.H. Handbook of Plastic Foams; Noyes Publications: Park Ridge, NJ, USA, 1995. [Google Scholar]
- Luo, W.; Li, Z.; Luo, H.; Liu, Y.; Xia, G.; Zhu, H.; Zhou, J.; Yu, D.; Zhang, J.; Song, J.; et al. Preparation of Room Temperature Vulcanized Silicone Rubber Foam with Excellent Flame Retardancy. Scanning 2021, 2021, 9976005. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, L.; Yu, Z.; Zhao, Y.; Zhang, Z.X. Lightweight and flame retardant silicone rubber foam prepared by supercritical nitrogen: The influence of flame retardants combined with ceramicizable fillers. Constr. Build. Mater. 2023, 370, 130735. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).