Evaluation of Ca-Based Sorbents for Gaseous HCl Emissions Adsorption
Abstract
:1. Introduction
2. Materials and Methods
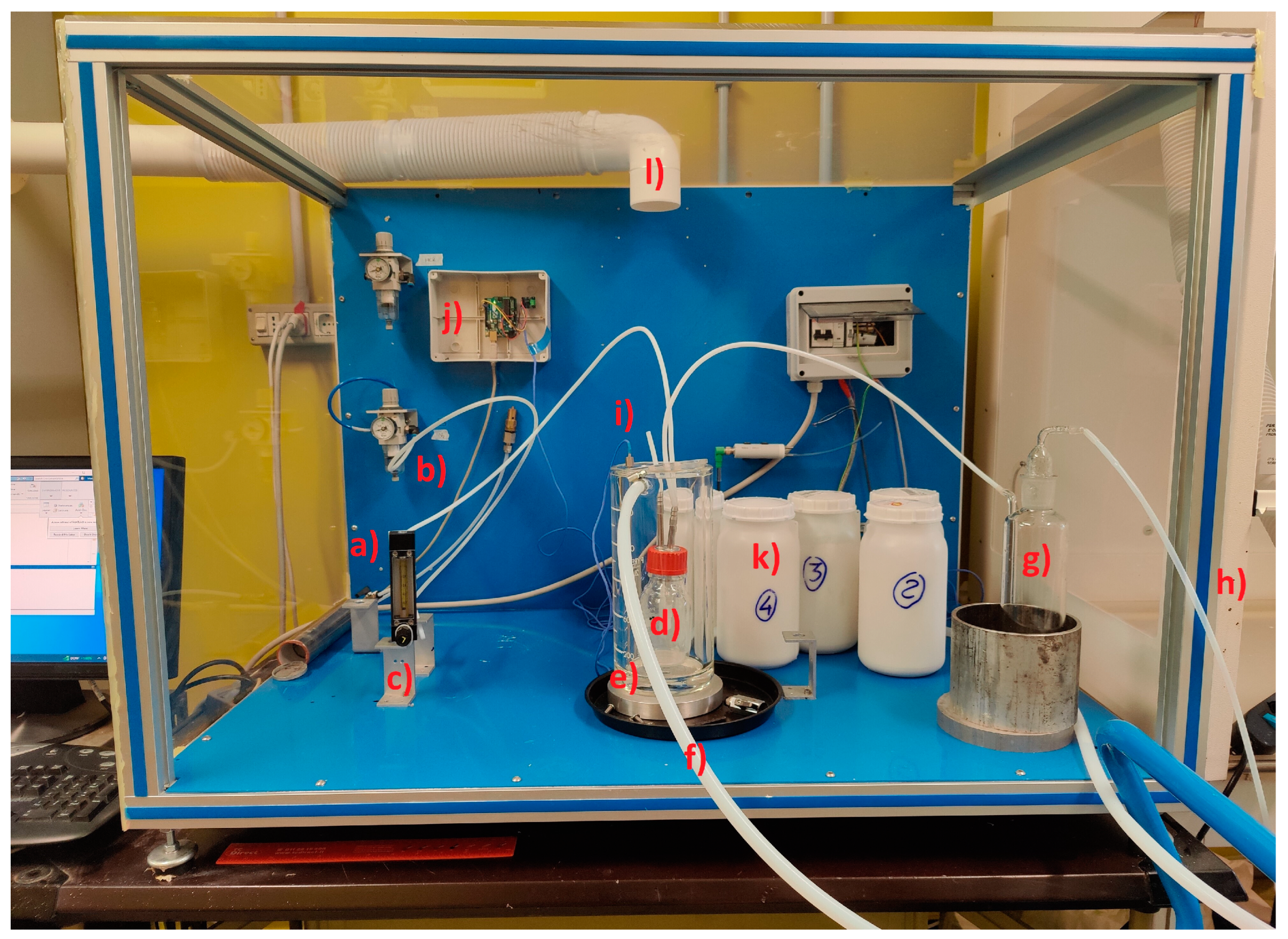
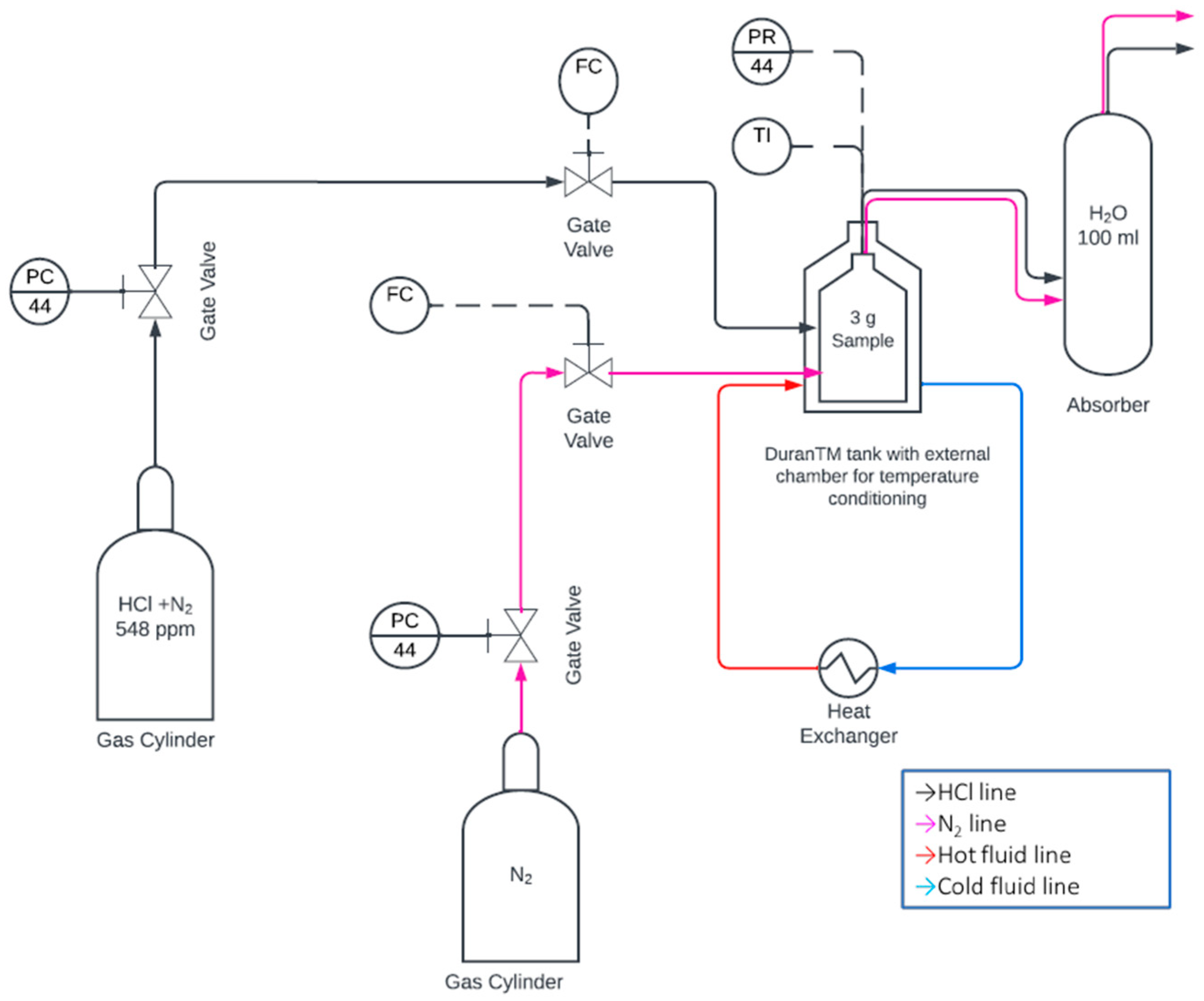
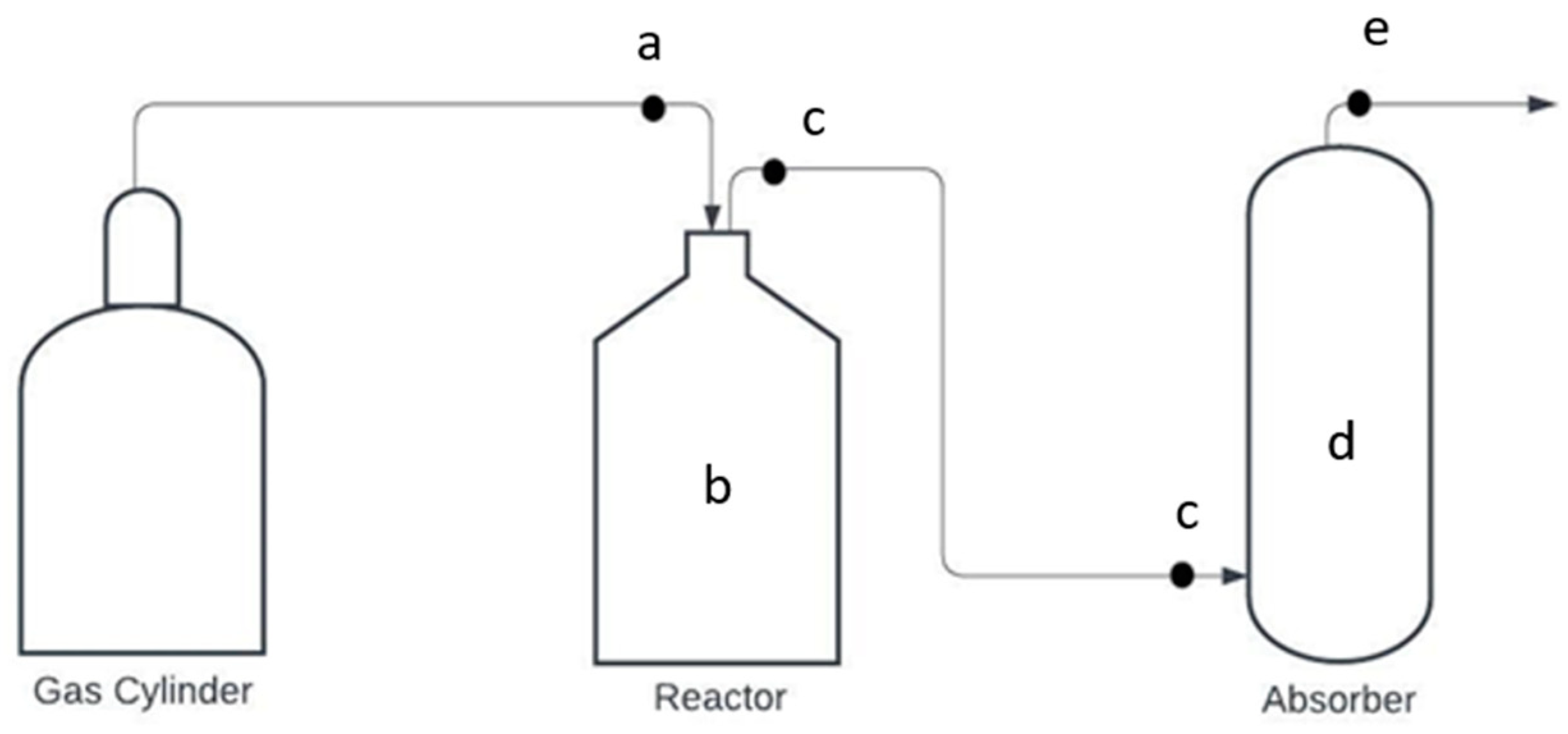
2.1. Experimental Test
2.2. Determination of the Sorption Capacity
3. Results
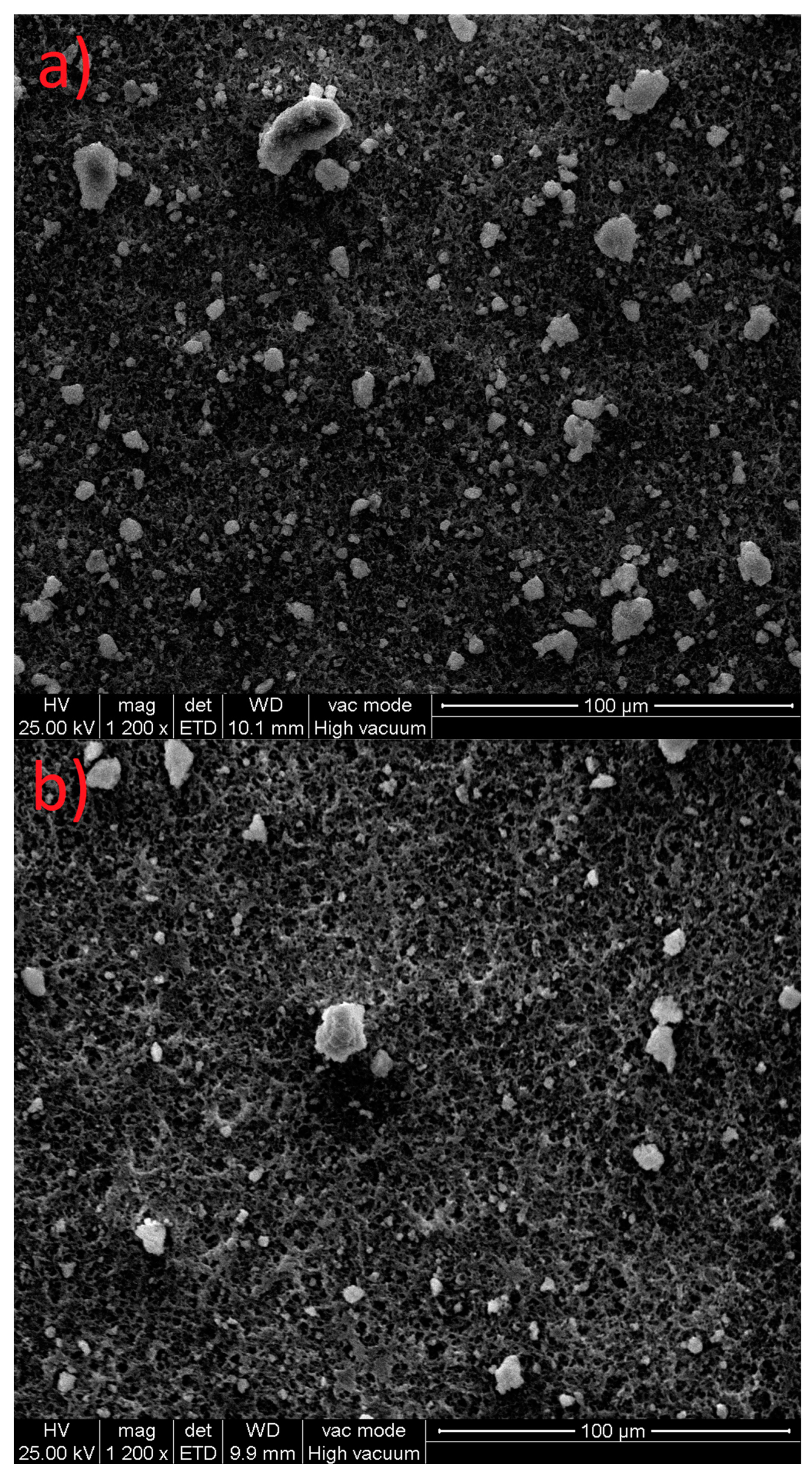
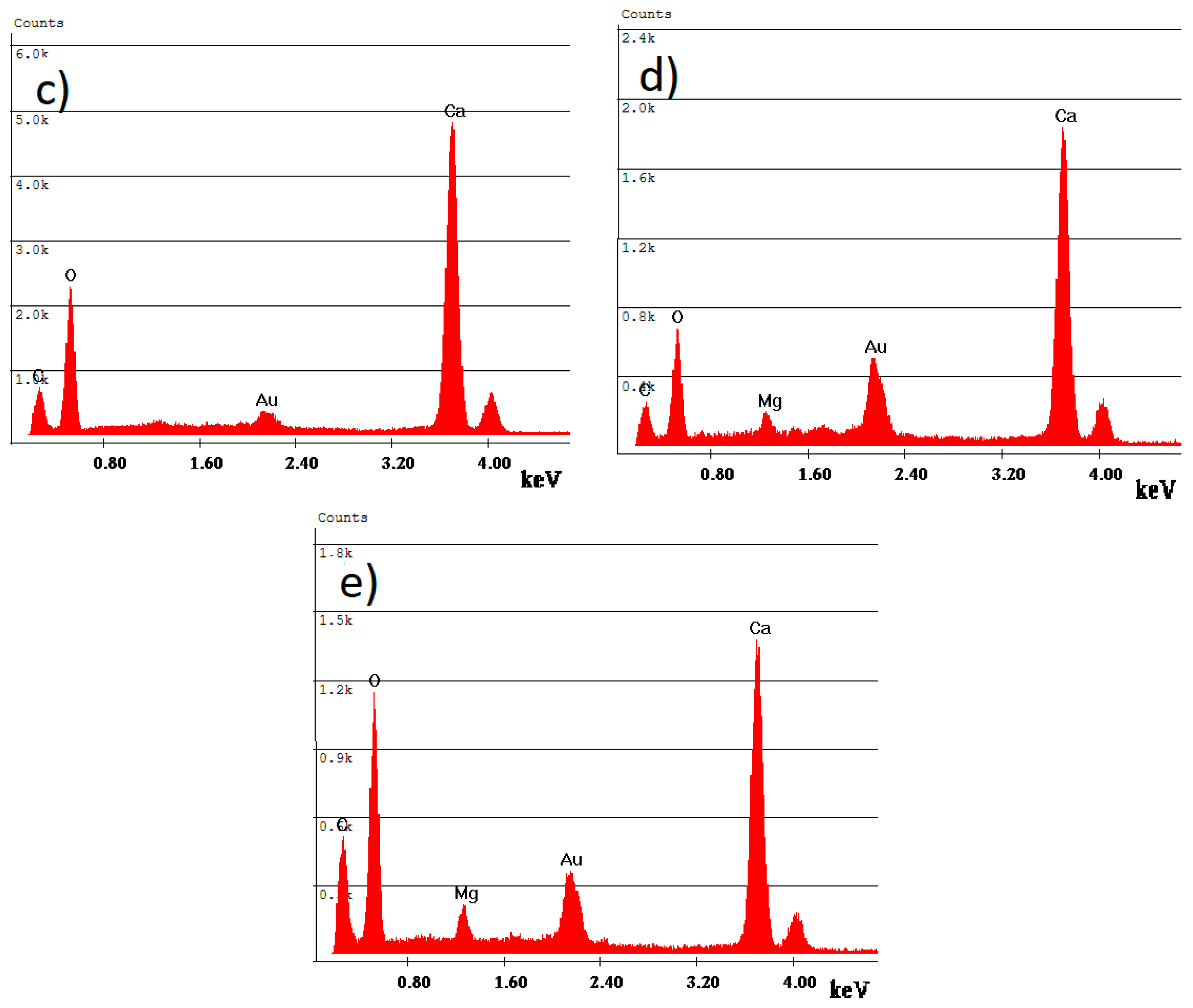
3.1. SEM Analyses
3.2. Sorption Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cole-Hunter, T.; Johnston, F.H.; Marks, G.B.; Morawska, L.; Morgan, G.G.; Overs, M.; Porta-Cubas, A.; Cowie, C.T. The Health Impacts of Waste-to-Energy Emissions: A Systematic Review of the Literature. Environ. Res. Lett. 2020, 15, 123006. [Google Scholar] [CrossRef]
- Neuwahl, F.; Cusano, G.; Gomez Benavides, J.; Holbrook, S.; Roudier, S. Best Available Techniques (BAT) Reference Document for Waste Incineration; EUR 29971 EN; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Dal Pozzo, A.; Antonioni, G.; Guglielmi, D.; Stramigioli, C.; Cozzani, V. Comparison of Alternative Flue Gas Dry Treatment Technologies in Waste-to-Energy Processes. Waste Manag. 2016, 51, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yu, L.; Ma, G.; Mi, H.; Jiao, Y. Residents’ Acceptance towards Waste-to-Energy Facilities: Formation, Diffusion and Policy Implications. J. Clean. Prod. 2021, 287, 125560. [Google Scholar] [CrossRef]
- Ravina, M.; Facelli, A.; Zanetti, M. Halocarbon Emissions from Hazardous Waste Landfills: Analysis of Sources and Risks. Atmosphere 2020, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Jurczyk, M.; Mikus, M.; Dziedzic, K. Flue gas cleaning in municipal Waste-To-Energy plants—Part II. Infrastrukt. I Ekol. Teren. Wiej./Infrastruct. Ecol. Rural Areas 2016, nr IV/2, 1309–1321. [Google Scholar] [CrossRef]
- Kumar, L.; Jana, S.K. Advances in Absorbents and Techniques Used in Wet and Dry FGD: A Critical Review. Rev. Chem. Eng. 2022, 38, 843–880. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Martello, G.; Cozzani, V. Sustainability Assessment of Furnace Versus In-Duct Sorbent Injection to Retrofit Waste-to-Energy Dry Flue Gas Treatment Lines. Chem. Eng. Trans. 2021, 86, 523–528. [Google Scholar] [CrossRef]
- Barba, D.; Brandani, F.; Capocelli, M.; Luberti, M.; Zizza, A. Process Analysis of an Industrial Waste-to-Energy Plant: Theory and Experiments. Process Saf. Environ. Prot. 2015, 96, 61–73. [Google Scholar] [CrossRef]
- Daoudi, M.; Walters, J.K. A Thermogravimetric Study of the Reaction of Hydrogen Chloride Gas with Calcined Limestone: Determination of Kinetic Parameters. Chem. Eng. J. 1991, 47, 1–9. [Google Scholar] [CrossRef]
- Duo, W.; Kirkby, N.F.; Seville, J.P.K.; Clift, R. Alteration with Reaction Progress of the Rate Limiting Step for Solid-Gas Reactions of Ca-Compounds with HCl. Chem. Eng. Sci. 1995, 50, 2017–2027. [Google Scholar] [CrossRef]
- Verdone, N.; De Filippis, P. Reaction Kinetics of Hydrogen Chloride with Sodium Carbonate. Chem. Eng. Sci. 2006, 61, 7487–7496. [Google Scholar] [CrossRef]
- Koech, L.; Rutto, H.; Lerotholi, L.; Everson, R.C.; Neomagus, H.; Branken, D.; Moganelwa, A. Spray Drying Absorption for Desulphurization: A Review of Recent Developments. Clean Technol. Environ. Policy 2021, 23, 1665–1686. [Google Scholar] [CrossRef]
- Bodénan, F.; Deniard, P. Characterization of Flue Gas Cleaning Residues from European Solid Waste Incinerators: Assessment of Various Ca-Based Sorbent Processes. Chemosphere 2003, 51, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Tochinai, M.; Kumagai, S.; Yoshioka, T. Treatment of HCl Gas by Cyclic Use of Mg–Al Layered Double Hydroxide Intercalated with CO32−. Atmos. Pollut. Res. 2020, 11, 290–295. [Google Scholar] [CrossRef]
- Nethe, L.; Uwe, S. Special Lime with High Reactivity for the Absorption of Acid Gas Constituents. ZKG Int. 2008, 61, 1–11. [Google Scholar]
- Borgwarth, R.H.; Bruce, K.R.; Blake, J. EPA Experimental Studies of the Mechanism of Sulphur Capture by Limestone. In Proceedings of the First Joint Symposium on Dry SO2 and Simultaneous SO2/NOx Control Technologies, San Diego, CA, USA, 13–16 November 1984; Report Number EPA600/9-85-020b. U.S. Environmental Protection Agency, Air and Energy Engineering Research Laboratory: Research Triangle Park, NC, USA, 1985. [Google Scholar]
- Pesce, C.; Pesce, G.L.; Molinari, M.; Richardson, A. Effects of Organic Additives on Calcium Hydroxide Crystallisation during Lime Slaking. Cem. Concr. Res. 2021, 139, 106254. [Google Scholar] [CrossRef]
- Liang, S.; Liu, S.; Fan, Z.; Zhang, W.; Guo, M.; Cheng, F.; Zhang, M. Enhanced HCl Removal from CO2-Rich Mixture Gases by CuOx/Na2CO3 Porous Sorbent at Low Temperature: Kinetics and Forecasting. Chem. Eng. J. 2020, 381, 122738. [Google Scholar] [CrossRef]
- Stein, J. The Influence of HCl on S Absorption in the Spray Dry Scrubbing Process. Chem. Eng. J. 2002, 86, 17–23. [Google Scholar] [CrossRef]
- Han, J.W.; Hassoli, N.; Lee, K.S.; Park, S.S.; Kim, K.D.; Kim, H.T.; Park, Y.O. Dry Scrubbing of Gaseous HCl and SO2 with Hydrated Lime in Entrained Mixing Reactor. Powder Technol. 2021, 393, 471–481. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, W.; He, M.; Xiao, X.; Wang, T.; Cheng, S.; Zhang, L. Combined Removal of SO3 and HCl by Modified Ca(OH)2 from Coal-Fired Flue Gas. Sci. Total Environ. 2023, 857, 159466. [Google Scholar] [CrossRef]
- Chin, T.; Yan, R.; Liang, D.T.; Tay, J.H. Hydrated Lime Reaction with HCl under Simulated Flue Gas Conditions. Ind. Eng. Chem. Res. 2005, 44, 3742–3748. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Ortega-Huertas, M.; Hansen, E. Nanostructure and Irreversible Colloidal Behavior of Ca(OH)2: Implications in Cultural Heritage Conservation. Langmuir 2005, 21, 10948–10957. [Google Scholar] [CrossRef] [PubMed]
- Micoli, L.; Bagnasco, G.; Turco, M. HCl Removal from Biogas for Feeding MCFCs: Adsorption on Microporous Materials. Int. J. Hydrogen Energy 2013, 38, 447–452. [Google Scholar] [CrossRef]
- Tan, Z.; Niu, G.; Qi, Q.; Zhou, M.; Wu, B.; Yao, W. Ultralow Emission of Dust, SOx, HCl, and NOx Using a Ceramic Catalytic Filter Tube. Energy Fuels 2020, 34, 4173–4182. [Google Scholar] [CrossRef]
- Wang, Y.; Su, W.; Chen, J.; Xing, Y.; Zhang, H.; Qian, D. A Review of Hydrogen Chloride Removal from Calcium- and Sodium-Based Sorbents. Environ. Sci. Pollut. Res. 2023, 30, 73116–73136. [Google Scholar] [CrossRef]





| Technology | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Boiler/Inline injection of sorbent (or duct sorbent injection, DSI) | A dry sorbent powder is dispersed by injecting it into the effluent gas stream (or combustion chamber). The sorbent reacts with the acid gas to form a solid, which is subsequently removed with dust suppression systems. | Simple technology; minimum amount of auxiliary equipment; low operating costs | Major consumption of sorbent; higher amount of residuals; possible dust pollution of heat exchange surfaces |
| Conditioned dry sorption | Separation of the pollutants is ameliorated by a hydrate shell which is formed around the lime particles and results from the H2O contained in the flue gas. The separation is governed by the dissolution rate of the pollutants in aqueous solution. | Higher efficiency than no conditioned dry sorption | Flue gas must be conditioned to achieve higher relative humidity |
| Semi-dry scrubbing | A spray dryer is used to inject a suspension of lime and water (lime slurry) into the flue gas stream. When the suspension is injected into the flue gas stream, the water component evaporates and only the solid lime particles remain in the flue gas. | 50% less water consumption than wet methods; dry residuals | Worse use of sorbent in comp. to wet methods; Higher investment costs comp. to dry method |
| Wet scrubbing | Makes use of a liquid such as water or an aqueous solution capable of absorbing the acidic compounds present in the gaseous effluent. | Low consumption of sorbents; low sensitivity to fluctuation in flows | Higher complexity; higher corrosion; drop in flue gas temperature; large area needed |
| Flue gas desulphurization (FGD) with seawater | Wet non-regenerative type that makes use of the natural alkalinity of seawater to absorb the acidic contaminants present in the gaseous effluent. | No waste is formed; no reagents or additional chemicals; lower capital and operating costs. | Low efficiency; wet residues. |
| Calcium Hydroxide | Sodium Bicarbonate | ||
|---|---|---|---|
| Ca(OH)2 ⇄ Ca2+ + 2OH− HCl → H+ + Cl− Ca(OH)2 + 2HCl → CaCl2 + 2H2O | (1) | 2NaHCO3 → 2Na+ + HCO3− HCO3− ⇄ CO32− + 2H+ ⇄ CO2 + H2O 2NaHCO3 → Na2CO3 + CO2 + H2O | (4) |
| Ca(OH)2 ⇄ Ca2+ + 2OH− SO2 + 2OH− → SO42− + 2H+ Ca(OH)2 + SO2 + ½O2 → CaSO4 + H2O | (2) | Na2CO3 → 2Na+ + CO32− HCl → H+ + Cl− CO32− + 2H+ ⇄ CO2 + H2O Na2CO3 + 2HCl → 2NaCl + CO2 + H2O | (5) |
| Ca(OH)2 ⇄ Ca2+ + 2OH− CO2 + OH− → HCO3− ⇄ CO32− + H+ Ca(OH)2 + CO2 → CaCO3 + H2O | (3) | Na2CO3 → 2Na+ + CO32− SO2 + 2OH− → SO42− + 2H+ Na2CO3 + SO2 ½O2 → Na2SO4 + CO2 | (6) |
| Sample | Cl Adsorbed (mg/g) | |||
|---|---|---|---|---|
| 5 min | 10 min | 20 min | 30 min | |
| C1 | 0.6466 | 1.2678 | 2.4235 | 3.5965 |
| C2 | 0.6385 | 1.2046 | 2.1851 | 2.8956 |
| C3 | 0.6589 | 1.2989 | 2.4737 | 3.1213 |
| C4 | 0.623 | 1.1960 | 2.181 | 2.9888 |
| C5 | 0.6309 | 1.2098 | 2.2072 | 3.4954 |
| Sample | Linear Fit y = p1x + p2 | |||
|---|---|---|---|---|
| p1 | p2 | R2 | Root Mean Squared Error (RMSE) | |
| C1 | 0.1176 | 0.07268 | 0.997 | 0.048 |
| C2 | 0.09053 | 0.2727 | 0.876 | 0.312 |
| C3 | 0.09802 | 0.3068 | 0.942 | 0.236 |
| C4 | 0.09718 | 0.1873 | 0.981 | 0.113 |
| C5 | 0.1121 | 0.05795 | 0.993 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravina, M.; Marotta, E.; Cerutti, A.; Zanetti, G.; Ruffino, B.; Panepinto, D.; Zanetti, M. Evaluation of Ca-Based Sorbents for Gaseous HCl Emissions Adsorption. Sustainability 2023, 15, 10882. https://doi.org/10.3390/su151410882
Ravina M, Marotta E, Cerutti A, Zanetti G, Ruffino B, Panepinto D, Zanetti M. Evaluation of Ca-Based Sorbents for Gaseous HCl Emissions Adsorption. Sustainability. 2023; 15(14):10882. https://doi.org/10.3390/su151410882
Chicago/Turabian StyleRavina, Marco, Edoardo Marotta, Alberto Cerutti, Giovanna Zanetti, Barbara Ruffino, Deborah Panepinto, and Mariachiara Zanetti. 2023. "Evaluation of Ca-Based Sorbents for Gaseous HCl Emissions Adsorption" Sustainability 15, no. 14: 10882. https://doi.org/10.3390/su151410882
APA StyleRavina, M., Marotta, E., Cerutti, A., Zanetti, G., Ruffino, B., Panepinto, D., & Zanetti, M. (2023). Evaluation of Ca-Based Sorbents for Gaseous HCl Emissions Adsorption. Sustainability, 15(14), 10882. https://doi.org/10.3390/su151410882










