Novel Synthesis of Carbon Dots from Coconut Wastes and Its Potential as Water Disinfectant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Carbon Dots
2.1.1. Hydrothermal Carbonization
2.1.2. Two Sequential Synthesis Processes
2.2. Synthesis of Surface-Modified CDs
2.2.1. Urea-Doped CDs
2.2.2. PEI-Doped CDs
2.2.3. HMTA-Doped CDs
2.3. Characterization of CDs
2.4. Assessment of Antimicrobial Activity of CDs
Lactate Dehydrogenase (LDH) Assay
2.5. Development of CD-Based Prototypes to Assess the Water Disinfection Potential
2.5.1. Synthesis of CD-Infused Chitosan Beads
2.5.2. Synthesis of CDs Pellets
2.6. Assessing the Water Disinfection Potential of CDs
2.7. Biosafety Studies of Synthesized CDs
2.8. Statistical Analysis
3. Results and Discussion
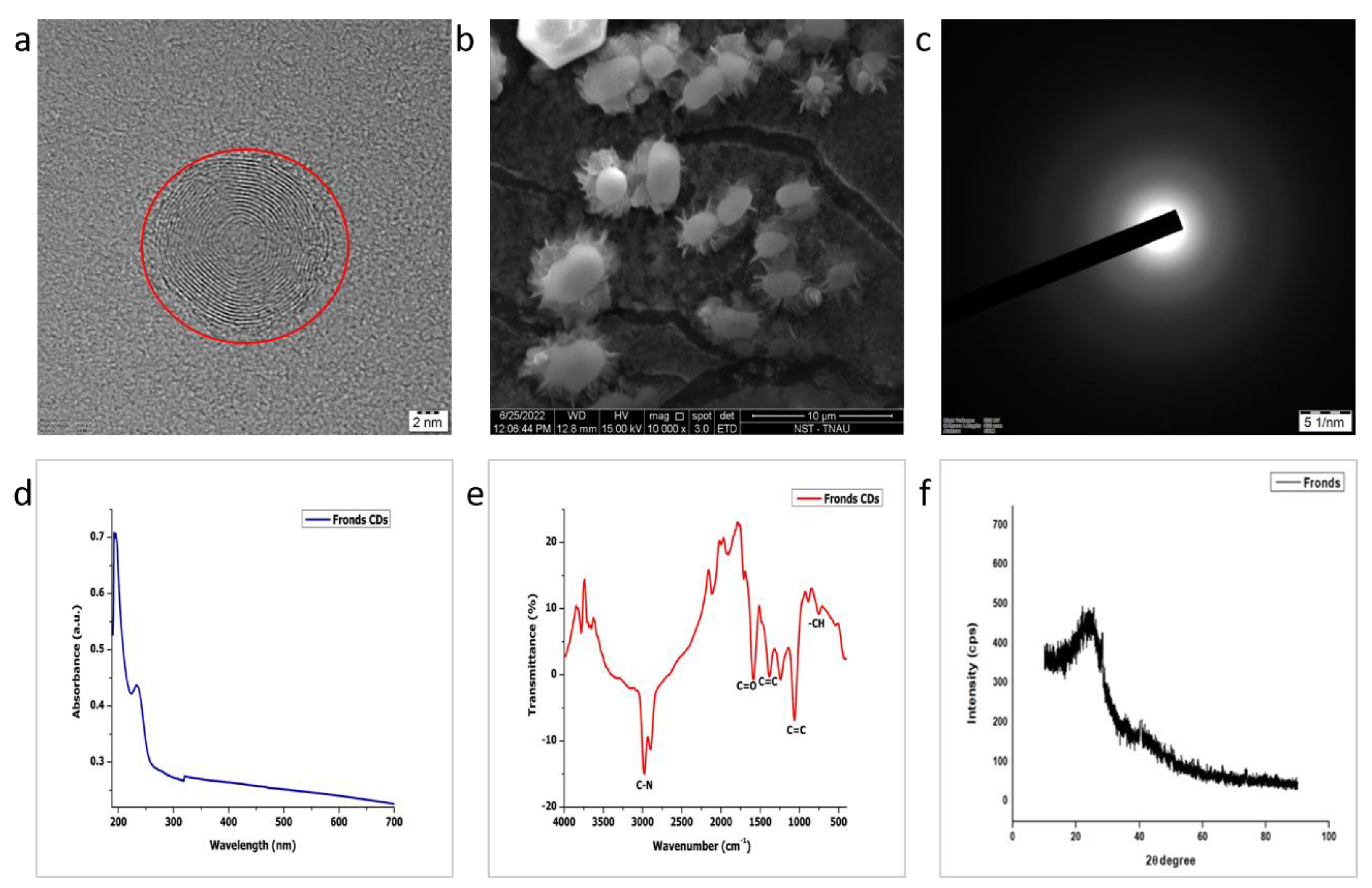
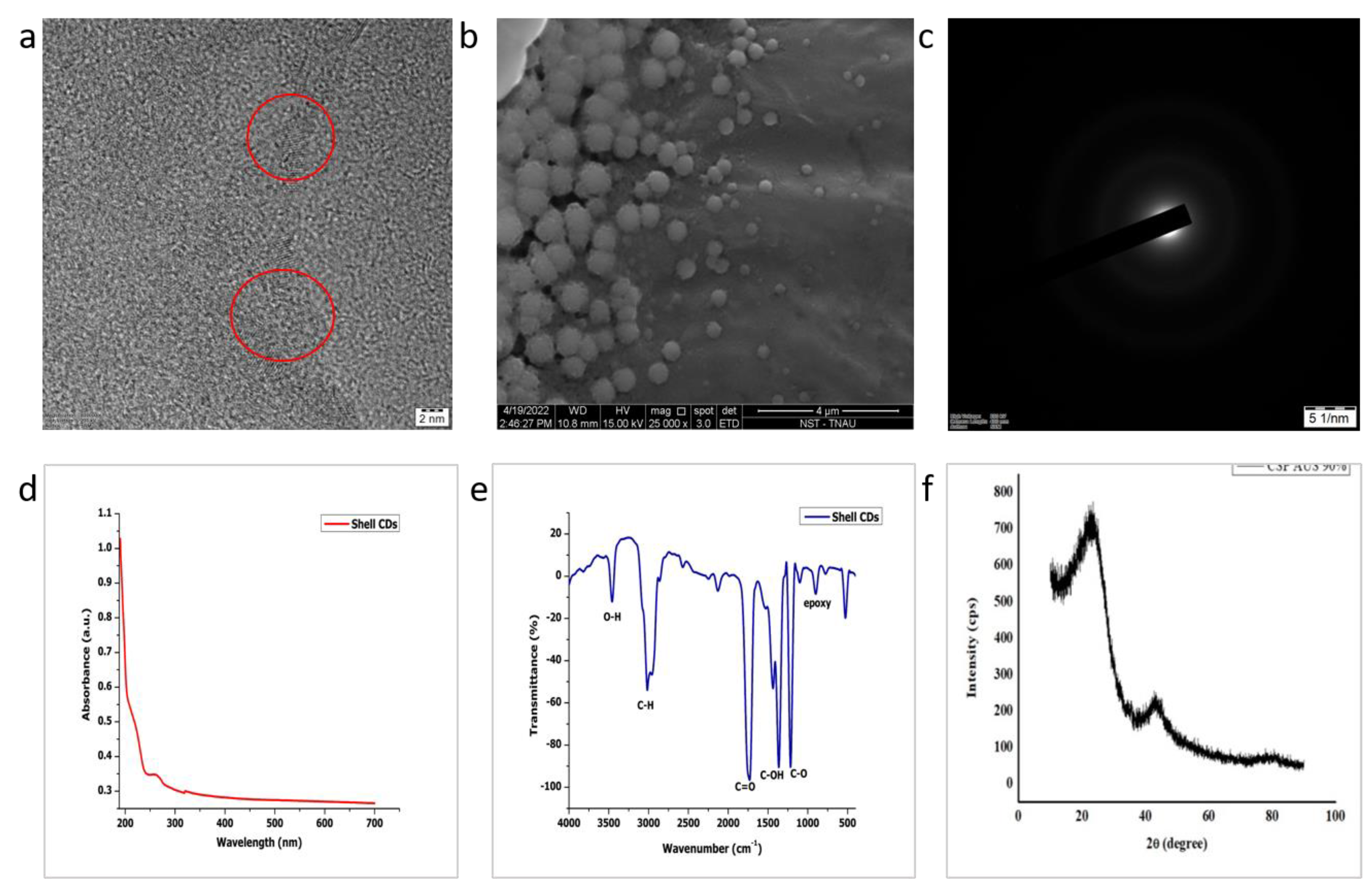
3.1. Size, Morphology, Crystallinity and Stability of CDs
3.2. Optical Studies
3.3. Fourier-Transform IR Spectroscopy
3.4. X-ray Diffraction (XRD)
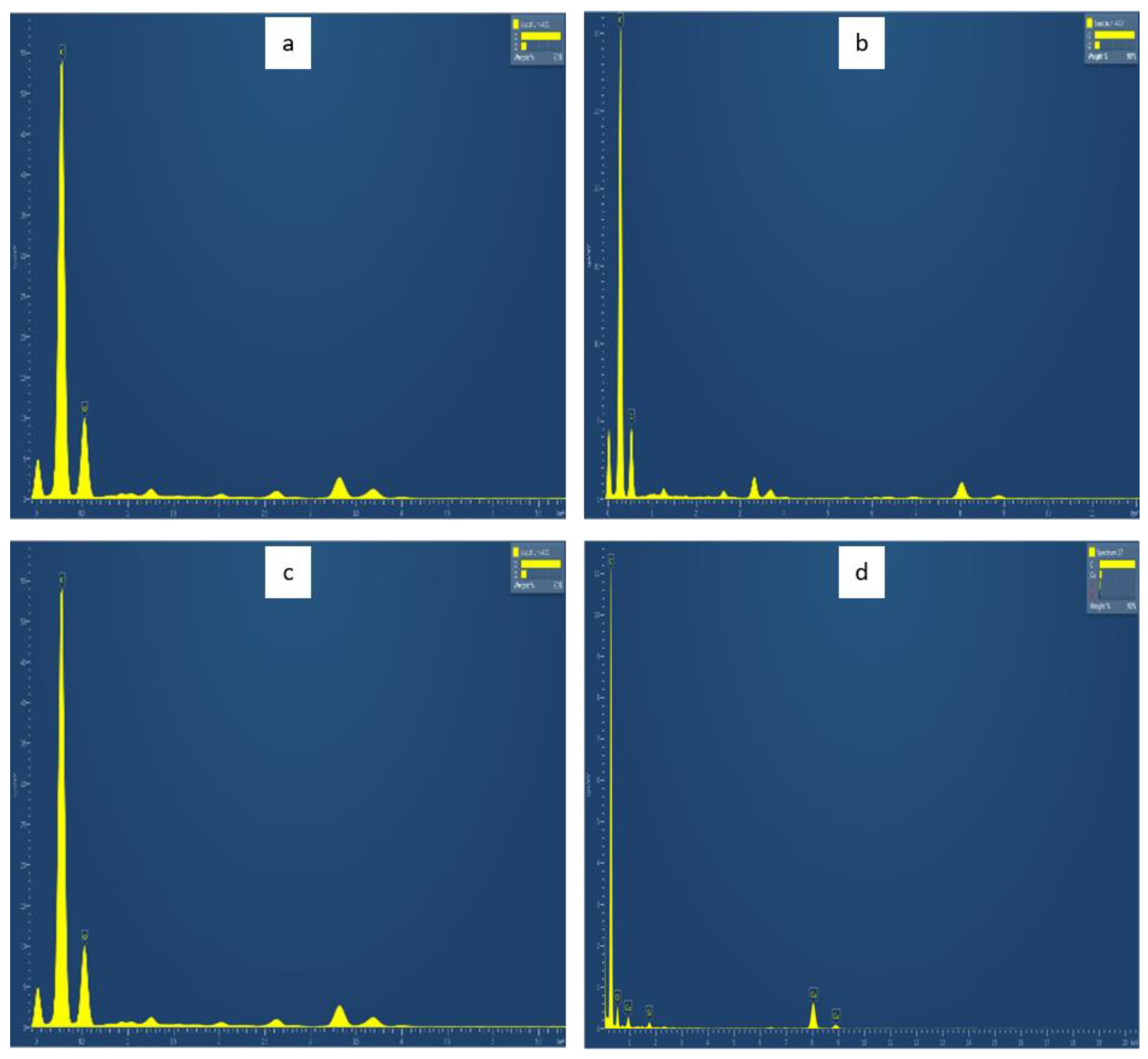
3.5. EDAX (Energy-Dispersive Analysis of X-ray)
3.6. Surface Area and Pore Size
3.7. Antibacterial Activity of Synthesized CDs
3.8. Water Disinfection Potential of CDs
3.9. Biosafety Studies of Synthesized CDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, X.; Chen, G.; Li, Z.; Deng, S.; Xu, N. Quantum-mechanical investigation of field-emission mechanism of a micrometer-long single-walled carbon nanotube. Phys. Rev. Lett. 2004, 92, 106803. [Google Scholar] [CrossRef] [Green Version]
- Mishra, V.; Patil, A.; Thakur, S.; Kesharwani, P. Carbon dots: Emerging theranostic nanoarchitectures. Drug Discov. Today 2018, 23, 1219–1232. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Yin, L.; Liu, Y.; Guo, H.; Lai, J.; Han, Y.; Li, G.; Li, M.; Zhang, J. Full-color fluorescent carbon quantum dots. Sci. Adv. 2020, 6, eabb6772. [Google Scholar] [CrossRef]
- Shen, C.L.; Zang, J.H.; Lou, Q.; Su, L.X.; Li, Z.; Liu, Z.Y.; Dong, L.; Shan, C.X. In-situ embedding of carbon dots in a trisodium citrate crystal matrix for tunable solid-state fluorescence. Carbon 2018, 136, 359–368. [Google Scholar] [CrossRef]
- Odonkor, S.T.; Mahami, T. Escherichia coli as a tool for disease risk assessment of drinking water sources. Int. J. Microbiol. 2020, 2020, 2534130. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.P.; Yang, L. Carbon dots as potent antimicrobial agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef]
- Ghirardello, M.; Ramos-Soriano, J.; Galan, M.C. Carbon dots as an emergent class of antimicrobial agents. Nano Mater. 2021, 11, 1877. [Google Scholar] [CrossRef]
- Chauhan, P.; Dogra, S.; Chaudhary, S.; Kumar, R. Usage of coconut coir for sustainable production of high-valued carbon dots with discriminatory sensing aptitude toward metal ions. Mater. Today Chem. 2020, 16, 100247. [Google Scholar] [CrossRef]
- Chunduri, L.; Kurdekar, A.; Patnaik, S.; Dev, B.V.; Rattan, T.M.; Kamisetti, V.J. Carbon quantum dots from coconut husk: Evaluation for antioxidant and cytotoxic activity. Mater. Focus 2016, 5, 55–61. [Google Scholar] [CrossRef]
- Abinaya, K.; Rajkishore, S.K.; Lakshmanan, A.; Anandham, R.; Dhananchezhiyan, P.; Praghadeesh, M. Synthesis and characterization of carbon dots from coconut shell by optimizing the hydrothermal carbonization process. J. Appl. Nat. Sci. 2021, 13, 1151–1157. [Google Scholar] [CrossRef]
- Rani, U.A.; Ng, L.Y.; Ng, C.Y.; Mahmoudi, E. A review of carbon quantum dots and their applications in wastewater treatment. Adv. Colloid Interface Sci. 2020, 278, 102124. [Google Scholar] [CrossRef]
- Tran, N.A.; Hien, N.Y.; Hoang, N.M.; Dang, H.T.; Huy, D.Q.; Quy, T.V.; Hanh, N.T.; Vu, N.H.; Dao, V.D. Carbon dots in environmental treatment and protection applications. Desalination 2023, 548, 116285. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Wu, F.-G. Carbon Dots for Killing Microorganisms: An Update since 2019. Pharmaceuticals 2022, 15, 1236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Mi, J.; Fu, Y.; Cui, D.; Lü, C. Multiple-cores@shell clustered carbon dots/P25/rGO nanocomposite as robust visible-light photocatalyst for organic pollutant degradation and water disinfection. Appl. Surf. Sci. 2021, 538, 148087. [Google Scholar] [CrossRef]
- Elkodous, M.A.; Gharieb, S.; El-Sayyad; Youssry, S.M.; Hanady, G.N.; Gobara, M.; Elsayed, M.A.; El-Khawaga, A.M.; Kawamura, G.; Tan, W.K.; et al. Carbon-dot-loaded CoxNi1−xFe2O4; x = 0.9/SiO2/TiO2 nanocomposite with enhanced photocatalytic and antimicrobial potential: An engineered nanocomposite for wastewater treatment. Sci. Rep. 2020, 10, 11534. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Sheng, L.; Wang, Z.; Ye, Y.; Zheng, J.; Fan, M.; Zhang, Y.; Sun, X. Carbon dots cooperatively modulating photocatalytic performance and surface charge of O-doped g-C3N4 for efficient water disinfection. J. Colloid Interface Sci. 2023, 631, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.Q.; Yuan, Z.Y.; Ren, T.Z. A facile hydrothermal method for preparation of fluorescent carbon dots on application of Fe3+ and fingerprint detection. Methods Appl. Fluoresc. 2019, 7, 035001. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Edison, T.; Lee, Y.R. Sustainable synthesis of multifunctional carbon dots using biomass and their applications: A mini-review. J. Environ. Chem. Eng. 2021, 9, 105802. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Ye, C.; Fu, W.; Yuan, H.; Hu, P.; Liu, Y. Hexamethylenetetramine: An effective and universal nitrogen-doping reagent to enhance the photoluminescence of carbon nanodots. New J. Chem. 2018, 42, 3519–3525. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, M.; Wang, H.; Wang, B.; Huang, H.; Liu, Y.; Kang, Z. N-doped carbon dots derived from leaves with low toxicity via damaging cytomembrane for broad-spectrum antibacterial activity. Mater. Today Commun. 2020, 24, 101222. [Google Scholar] [CrossRef]
- Zhuang, P.; Li, K.; Li, D.; Qiao, H.; Wang, M.; Sun, J.; Mei, X.; Li, D. Correction: Assembly of Carbon Dots into Frameworks with Enhanced Stability and Antibacterial Activity. Nanoscale Res. Lett. 2021, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of mono-disperse carbon quantum dots from fennel seeds: Photoluminescence analysis using machine learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.B.; Porat, Z.; Gedanken, A. Facile one-step sonochemical synthesis of ultrafine and stable fluorescent C-dots. Ultrason. Sonochem. 2016, 28, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Qiang, S.; Zhang, L.; Li, Z.; Liang, J.; Li, P.; Song, J.; Guo, K.; Wang, Z.; Fan, Q. New Insights into the Cellular Toxicity of Carbon Quantum Dots to Escherichia coli. Antioxidants 2022, 11, 2475. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Rahman, S.; Rostami, M.; Tabasi, Z.A.; Khan, F.; Alodhayb, A.; Zhang, Y. Carbon quantum dot-incorporated chitosan hydrogel for selective sensing of Hg2+ ions: Synthesis, characterization, and density functional theory calculation. ACS Omega 2021, 6, 23504–23514. [Google Scholar] [CrossRef] [PubMed]
- Keiller, B.G.; van Eyk, P.J.; Lane, D.J.; Muhlack, R.; Burton, A.B. Hydrothermal carbonization of Australian saltbush. Energy Fuels 2018, 33, 1157–1166. [Google Scholar] [CrossRef]
- Sawalha, S.; Assali, M.; Nasasrah, A.; Salman, M.; Nasasrah, M.; Jitan, M.; Hilal, H.S.; Zyuod, A.J. Optical properties and photoactivity of carbon nanodots synthesized from olive solid wastes at different carbonization temperatures. RSC Adv. 2022, 12, 4490–4500. [Google Scholar] [CrossRef]
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J. Chem. 2012, 36, 861–864. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, T.G.; Chung, Y.D.; Cho, D.H.; Kim, J.; Kim, K.J.; Yi, Y.; Kim, J.W. Junction formation at the interface of CdS/CuInxGa (1− x) Se2. J. Phys. D Appl. Phys. 2014, 47, 345302. [Google Scholar] [CrossRef]
- Baweja, H.; Jeet, K. Economical and green synthesis of graphene and carbon quantum dots from agricultural waste. Mater. Res. Express 2019, 6, 0850–0858. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Kaur, B.; Mehta, S.J. Potential prospects for carbon dots as a fluorescence sensing probe for metal ions. RSC Adv. 2016, 6, 90526–90536. [Google Scholar] [CrossRef]
- Fatahi, Z.; Esfandiari, N.; Ehtesabi, H.; Bagheri, Z.; Tavana, H.; Ranjbar, Z.; Latifi, H.J. Physicochemical and cytotoxicity analysis of green synthesis carbon dots for cell imaging. Excli J. 2019, 18, 454–466. [Google Scholar]
- Arvapalli, D.M.; Sheardy, A.T.; Alapati, K.C.; Wei, J. High Quantum Yield Fluorescent Carbon Nanodots for detection of Fe (III) Ions and Electrochemical Study of Quenching Mechanism. Talanta 2020, 209, 120538. [Google Scholar] [CrossRef]
- Das, T.; Saikia, B.K.; Dekaboruah, H.; Bordoloi, M.; Neog, D.; Bora, J.J.; Lahkar, J.; Narzary, B.; Roy, S.; Ramaiah, D. Blue-fluorescent and biocompatible carbon dots derived from abundant low-quality coals. J. Photochem. Photobiol. B 2019, 195, 1–11. [Google Scholar] [CrossRef]
- Manoharan, P.; Dhanabalan, S.C.; Alagan, M.; Muthuvijayan, S.; Ponraj, J.S.; Somasundaram, C.K. Facile synthesis and characterisation of green luminescent carbon nanodots prepared from tender coconut water using the acid-assisted ultrasonic route. Micro Nano Lett. 2020, 15, 920–924. [Google Scholar] [CrossRef]
- Chauhan, P.; Mundekkad, D.; Mukherjee, A.; Chaudhary, S.; Umar, A.; Baskoutas, S. Coconut carbon dots: Progressive large-scale synthesis, detailed biological activities and smart sensing aptitudes towards tyrosine. Nanomaterials 2022, 12, 162. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Xu, N.; Lian, H.; Xu, L.; Zhang, W.; Xu, C. From coconut petiole residues to fluorescent carbon dots via a green hydrothermal method for Fe3+ detection. Cellulose 2021, 28, 1647–1661. [Google Scholar] [CrossRef]
- Arvapalli, D.M.; Sheardy, A.T.; Allado, K.; Chevva, H.; Yin, Z.; Wei, J. Design of curcumin loaded carbon nanodots delivery system: Enhanced bioavailability, release kinetics, and anticancer activity. ACS Appl. Biol. Mater. 2020, 3, 8776–8785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, C.; Li, Z.; Ge, J.; Li, C.; Dong, C.; Shuang, S.J. Nitrogen-doped carbon dots as fluorescent probe for detection of curcumin based on the inner filter effect. RSC Adv. 2015, 5, 95054–95060. [Google Scholar] [CrossRef]
- Mathew, S.A.; Praveena, P.; Dhanavel, S.; Manikandan, R.; Senthilkumar, S.; Stephen, A. Luminescent chitosan/carbon dots as an effective nano-drug carrier for neurodegenerative diseases. RSC Adv. 2020, 10, 24386–24396. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Wei, Y.; Yang, Y.; Liu, X.; Chen, Y.; Xu, B. An efficient synthesis and photoelectric properties of green carbon quantum dots with high fluorescent quantum yield. Nanomaterials 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason. Sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R.; Abdala, A.A.; Chaudhary, R.; Singh, N. Photocatalytic degradation of dyes by nanomaterials. Mater. Today 2020, 29, 967–973. [Google Scholar] [CrossRef]
- Devkota, A.; Pandey, A.; Yadegari, Z.; Dumenyo, K.; Taheri, A. Glucosamine/β-alanine carbon dots use as DNA carriers into E. coli cells. Front. Nanotechnol. 2021, 3, 777810. [Google Scholar] [CrossRef]
- Guo, B.; Liu, G.; Hu, C.; Lei, B.; Liu, Y. The structural characteristics and mechanisms of antimicrobial carbon dots: A mini review. Mater. Adv. 2022, 3, 7726–7741. [Google Scholar] [CrossRef]
- Rajkishore, S.K.; Subramanian, K.S.; Natarajan, N.; Gunasekaran, K. Nanotoxicity at various trophic levels: A review. Bioscan 2013, 8, 975–982. [Google Scholar]
- Bing, W.; Sun, H.; Yan, Z.; Ren, J.; Qu, X. Programmed bacteria death induced by carbon dots with different surface charge. Communication 2016, 12, 4713–4718. [Google Scholar] [CrossRef]
- Kuo, W.S.; Chen, H.H.; Chen, S.Y.; Chang, C.Y.; Chen, P.C.; Hou, Y.I.; Shao, Y.T.; Kao, H.F.; Hsu, C.L.L.; Chen, Y.C.; et al. Graphene quantum dots with nitrogen-doped content dependence for highly efficient dual modality photodynamic antimicrobial therapy and bioimaging. Biomaterials 2017, 120, 185–194. [Google Scholar] [CrossRef]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodriguez-Castellon, E.; Bandosz, T.J. S-and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Bose, M.; Mondal, S.; Choudhary, S.; Gangopadhyay, S.; Das, N.C. Zinc and nitrogen ornamented bluish white luminescent carbon dots for engrossing bacteriostatic activity and Fenton based bio-sensor. Mater. Sci. Eng.-C 2018, 88, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Maruthapandi, M.; Saravanan, A.; Luong, J.H.T.; Gedanken, A. Antimicrobial properties of polyaniline and polypyrrole decorated with zinc-doped copper oxide microparticles. Polymers 2020, 12, 1286. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.H.T.; Gedanken, A. Green Synthesis of Multifunctional Carbon Dots with Antibacterial Activities. Nanomaterials 2021, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kang, D. Effect of amino acid-derived nitrogen and/or sulfur doping on the visible-light-driven antimicrobial activity of carbon quantum dots: A comparative study. Chem. Eng. J. 2021, 420, 129990. [Google Scholar] [CrossRef]
- Kung, J.C.; Tseng, I.T.; Chien, C.S.; Lin, S.H.; Wang, C.C.; Shih, C. Microwave assisted synthesis of negative charge carbon dots with potential antibacterial activity against multi-drug resistant bacteria. RSC Adv. 2020, 10, 41202–41208. [Google Scholar] [CrossRef] [PubMed]
- Kurt, S.B.; Sahiner, N. Chitosan based fibers embedding carbon dots with anti-bacterial and fluorescent properties. Polym. Compos. 2021, 42, 872–880. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, C.; Wang, W.; Ji, F.; Cui, Y.; Li, M. Acute toxicity of multi-walled carbon nanotubes, sodium pentachlorophenate, and their complex on earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2014, 103, 29–35. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Li, Q.; Xia, H.; Yang, W.; Wang, R.; Li, Y.; Zhao, H.; Tian, B. Facile synthesis of carbon dots from wheat straw for colorimetric and fluorescent detection of fluoride and cellular imaging. Spectrochim. Acta Part A 2021, 246, 118964. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Wang, M.; Huang, J.; Jiang, X.; Pang, J.; Xu, F.; Zhang, X. Synthesis of N-doped carbon quantum dots from bio-waste lignin for selective irons detection and cellular imaging. Int. J. Biol. Macromol. 2019, 128, 537–545. [Google Scholar] [CrossRef]




| S. No. | Precursor Material | Average Size (nm) | Shape | Zeta Potential (mV) |
|---|---|---|---|---|
| 1. | CDs (Fronds) | 15 | Spherical | −22.4 |
| 2. | CDs (Husk) | 9 | Spherical | −28.8 |
| 3. | CDs (Shell) | 7 | Spherical | −14.8 |
| 4. | Urea-doped CDs (1:1) | 7 | Spherical | −33.3 |
| Elemental Composition | CDs (Fronds) | CDs (Husk) | CDs (Shell) | Urea-Doped CDs |
|---|---|---|---|---|
| Carbon | 90.41 | 91.46 | 95.82 | 94.74 |
| Oxygen | 9.59 | 8.54 | 2.57 | 3.26 |
| CDs | Average Pore Radius (nm) | Total Pore Volume (cc/g) | BET Method (m2 g−1) | Langmuir Method (m2 g−1) |
|---|---|---|---|---|
| CDs (Fronds) | 4.31 | 0.03 | 12.9 | 361 |
| CDs (Husk) | 2.09 | 0.02 | 18.0 | 1478 |
| CDs (Shell) | 1.97 | 0.02 | 139 | 4535 |
| Urea-doped CDs (1:1) | 1.73 | 0.01 | 99.1 | 3653 |
| Treatments | % Cytotoxicity |
|---|---|
| Pristine CDs | 4.89 (±0.24) f |
| PEI-doped CDs (0.5:1) | 1.96 (±0.18) g |
| PEI-doped CDs (1:1) | 1.02 (±0.03) h |
| PEI-doped CDs (1.5:1) | 1.28 (±0.03) h |
| Urea-doped CDs (1:1) | 20.6 (±0.70) a |
| Urea-doped CDs (2:1) | 8.41 (±0.17) c |
| Urea-doped CDs (3:1) | 6.81 (±0.09) d |
| Urea-doped CDs (4:1) | 6.17 (±0.08) e |
| HMTA doped CDs | 14.6 (±0.35) b |
| Treatment | Number of Colonies (×102 CFU/mL) | Efficiency (%) | |
|---|---|---|---|
| Before Filtration | After Filtration | ||
| Pelletized CDs | 5.41 | 3.30 | 40 |
| CD-infused chitosan beads | 5.41 | 2.16 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkishore, S.K.; Devadharshini, K.P.; Sathya Moorthy, P.; Reddy Kiran Kalyan, V.S.; Sunitha, R.; Prasanthrajan, M.; Maheswari, M.; Subramanian, K.S.; Sakthivel, N.; Sakrabani, R. Novel Synthesis of Carbon Dots from Coconut Wastes and Its Potential as Water Disinfectant. Sustainability 2023, 15, 10924. https://doi.org/10.3390/su151410924
Rajkishore SK, Devadharshini KP, Sathya Moorthy P, Reddy Kiran Kalyan VS, Sunitha R, Prasanthrajan M, Maheswari M, Subramanian KS, Sakthivel N, Sakrabani R. Novel Synthesis of Carbon Dots from Coconut Wastes and Its Potential as Water Disinfectant. Sustainability. 2023; 15(14):10924. https://doi.org/10.3390/su151410924
Chicago/Turabian StyleRajkishore, Subramani Krishnaraj, Krishnagounder Padmanaban Devadharshini, Ponnuraj Sathya Moorthy, Vanniya Sreeramulu Reddy Kiran Kalyan, Rajkishore Sunitha, Mohan Prasanthrajan, Muthunalliappan Maheswari, Kizhaeral Sevathapandian Subramanian, Nalliappan Sakthivel, and Ruben Sakrabani. 2023. "Novel Synthesis of Carbon Dots from Coconut Wastes and Its Potential as Water Disinfectant" Sustainability 15, no. 14: 10924. https://doi.org/10.3390/su151410924








