Peach–Potato Aphid Myzus persicae: Current Management Strategies, Challenges, and Proposed Solutions
Abstract
:1. Introduction
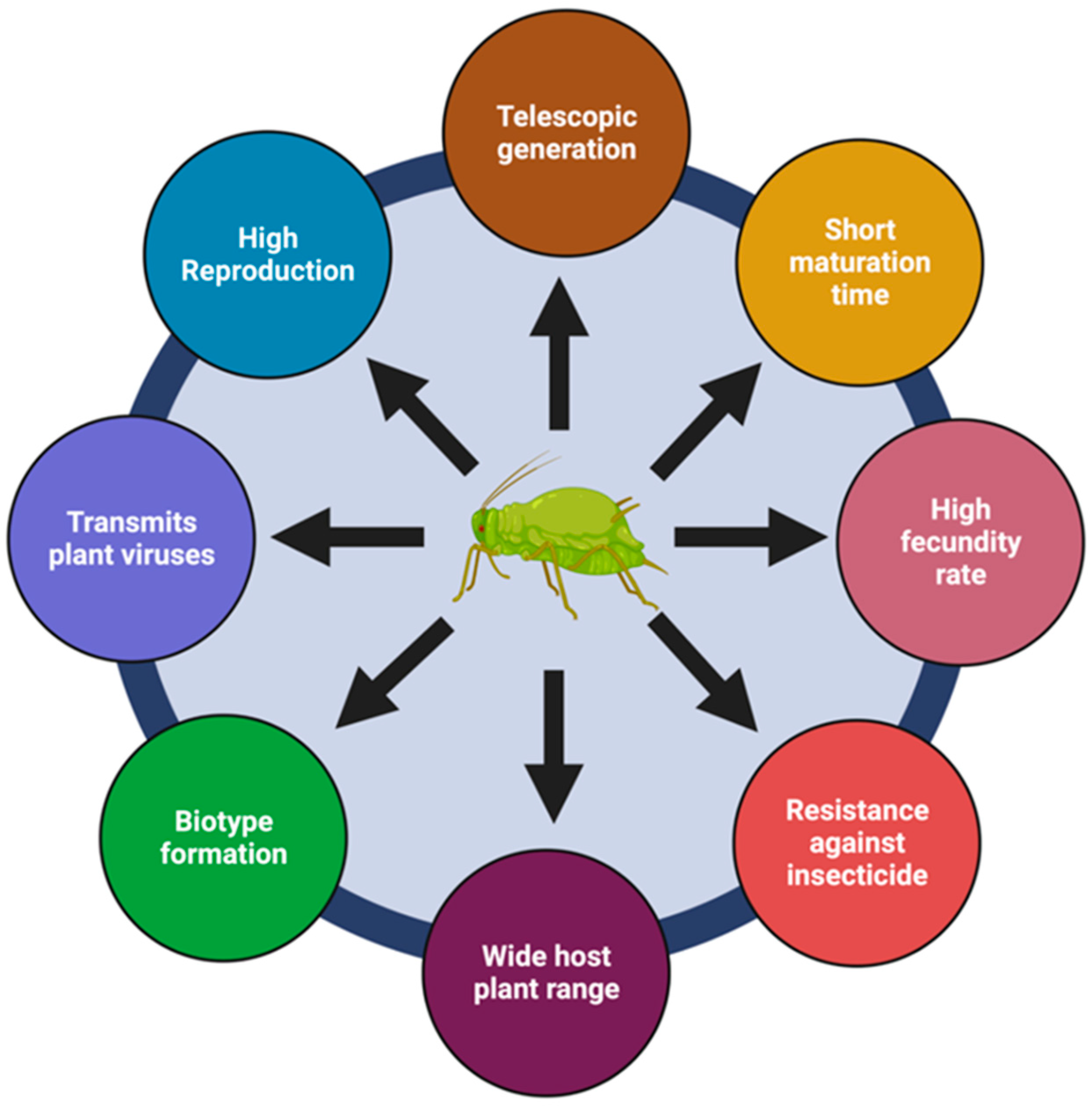
2. Pest Traits Influence Control Strategies
3. Suppression of Plant Defence
4. Economic Importance of Peach–Potato Aphid
5. Current Management Practices
6. Challenges with Current Management Practices
6.1. Development of Insecticidal Resistance
6.2. Adverse Effect on Non-Target Organisms
6.3. Elicitation of Plant Defence: An Underutilized Tool
6.4. Underutilisation of Cultural Practices
6.5. Lack of Resistant Cultivars
7. Potential Biological Control Agents
7.1. Predators and Parasitoids
7.2. Entomopathogenic Bacteria
7.3. Entomopathogenic Fungi
7.4. Entomopathogenic Viruses (EPVs)
7.5. Entomopathogenic Nematodes
8. Sustainable Strategies
8.1. Augmentative Release of Biocontrol Agents
8.2. Resistant Plant Varieties
8.3. Natural Compounds as Plant Defence Elicitor
8.4. Intercropping Companion Plants
9. Proposed Integrated Pest Management for Myzus persicae
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Emden, H.F.; Harrington, R. Aphids as Crop Pests; Cabi: New York, NY, USA, 2017. [Google Scholar]
- Dedryver, C.A.; Le Ralec, A.; Fabre, F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. Comptes Rendus Biol. 2010, 333, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Covaci, A.D.; Roberts, J.M.; Sobhy, I.S.; Kirk, W.D.J.; Bruce, T.J.A. Effects of cis-Jasmone Treatment of Brassicas on Interactions with Myzus persicae Aphids and Their Parasitoid Diaeretiella rapae. Front. Plant Sci. 2021, 12, 711896. [Google Scholar] [CrossRef] [PubMed]
- Goggin, F.L. Plant–aphid interactions: Molecular and ecological perspectives. Curr. Opin. Plant Biol. 2007, 10, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Musser, R.O.; Vogel, H.; Hum-Musser, S.M.; Thaler, J.S. Molecular, biochemical, and organismal analyses of tomato plants simultaneously attacked by herbivores from two feeding guilds. J. Chem. Ecol. 2010, 36, 1043–1057. [Google Scholar] [CrossRef] [Green Version]
- Van Emden, H.F.; Eastop, V.F.; Hughes, R.D.; Way, M.J. The ecology of Myzus persicae. Annu. Rev. Entomol. 1969, 14, 197–270. [Google Scholar] [CrossRef]
- Vorburger, C.; Lancaster, M.; Sunnucks, P. Environmentally related patterns of reproductive modes in the aphid Myzus persicae and the predominance of two ‘superclones’ in Victoria, Australia. Mol. Ecol. 2003, 12, 3493–3504. [Google Scholar] [CrossRef]
- Capinera, J. Handbook of Vegetable Pests; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide; John Wiley & Sons Ltd.: New York, NY, USA, 2000. [Google Scholar]
- Holman, J. Host Plant Catalog of Aphids: Palaearctic Region; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Ali, J. The Chemical Ecology of a Model Aphid Pest, Myzus persicae, and Its Natural Enemies; Keele University: Edinburgh, UK, 2022. [Google Scholar]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Edde, P.A. Field Crop Arthropod Pests of Economic Importance; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’ s Herbaceous; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 1–134. [Google Scholar]
- Naga, K.C.; Buckseth, T.; Subhash, S.; Bairwa, A.; Verma, G.; Kumar, R.; Malik, K.; Sharma, S.; Chakrabarti, S.K. Transmission efficiency of Potato leaf roll virus (PLRV) by potato aphid Aulacorthum solani and green peach aphid Myzus persicae. Indian J. Entomol. 2020, 82, 68–71. [Google Scholar] [CrossRef]
- Capinera, J.L. Green Peach Aphid, Myzus persicae (Sulzer) (Insecta: Hemiptera: Aphididae); University of Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, EDIS: Panama City, FL, USA, 2001. [Google Scholar]
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. B Biol. Sci. 2002, 269, 455–460. [Google Scholar] [CrossRef]
- Qi, Y.-H.; He, Y.-J.; Wang, X.; Zhang, C.-X.; Chen, J.-P.; Lu, G.; Li, J.-M. Physical contact transmission of Cucumber green mottle mosaic virus by Myzus persicae. PLoS ONE 2021, 16, e0252856. [Google Scholar] [CrossRef]
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host–vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Hoffmann, K.H. Aphid honeydew: Rubbish or signaler. In Biology and Ecology of Aphids; CRC Press: Boca Raton, FL, USA, 2016; pp. 199–220. [Google Scholar]
- Erdos, Z.; Chandler, D.; Bass, C.; Raymond, B. Controlling insecticide resistant clones of the aphid, Myzus persicae, using the entomopathogenic fungus Akanthomyces muscarius: Fitness cost of resistance under pathogen challenge. Pest Manag. Sci. 2021, 77, 5286–5293. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Liu, Y.-Q.; Song, W.-M.; Chen, D.-Y.; Chen, F.-Y.; Chen, X.-Y.; Chen, Z.-W.; Ge, S.-X.; Wang, C.-Z.; Zhan, S. An effector from cotton bollworm oral secretion impairs host plant defense signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 14331–14338. [Google Scholar] [CrossRef] [Green Version]
- Van Emden, H.F.; Harrington, R. Aphids as Crop Pests, 1st ed.; van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2007. [Google Scholar]
- Kallure, G.S.; Kumari, A.; Shinde, B.A.; Giri, A.P. Characterized constituents of insect herbivore oral secretions and their influence on the regulation of plant defenses. Phytochemistry 2022, 193, 113008. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, B.W.; Schroeder, F.C.; Jander, G. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J. 2008, 54, 1015–1026. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Will, T.; van Bel, A.J.E. Physical and chemical interactions between aphids and plants. J. Exp. Bot. 2006, 57, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Elzinga, D.A.; De Vos, M.; Jander, G. Suppression of Plant Defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mol. Plant-Microbe Interact. 2014, 27, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S. Brassica-aphid interaction: Challenges and prospects of genetic engineering for integrated aphid management. Physiol. Mol. Plant Pathol. 2019, 108, 101442. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Schwartzberg, E.G.; Tumlinson, J.H. Aphid honeydew alters plant defence responses. Funct. Ecol. 2014, 28, 386–394. [Google Scholar] [CrossRef]
- Jaouannet, M.; Rodriguez, P.A.; Thorpe, P.; Lenoir, C.J.G.; MacLeod, R.; Escudero-Martinez, C.; Bos, J.I.B. Plant immunity in plant–aphid interactions. Front. Plant Sci. 2014, 5, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, J.I.B.; Prince, D.; Pitino, M.; Maffei, M.E.; Win, J.; Hogenhout, S.A. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010, 6, e1001216. [Google Scholar] [CrossRef] [PubMed]
- Nalam, V.; Louis, J.; Shah, J. Plant defense against aphids, the pest extraordinaire. Plant Sci. 2019, 279, 96–107. [Google Scholar] [CrossRef]
- Pitino, M.; Hogenhout, S.A. Aphid protein effectors promote aphid colonization in a plant species-specific manner. Mol. Plant-Microbe Interact. 2013, 26, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Silva-Sanzana, C.; Zavala, D.; Moraga, F.; Herrera-Vásquez, A.; Blanco-Herrera, F. Oligogalacturonides Enhance Resistance against Aphids through Pattern-Triggered Immunity and Activation of Salicylic Acid Signaling. Int. J. Mol. Sci. 2022, 23, 9753. [Google Scholar] [CrossRef]
- Hewer, A.; Becker, A.; van Bel, A.J.E. An aphid’s Odyssey–the cortical quest for the vascular bundle. J. Exp. Biol. 2011, 214, 3868–3879. [Google Scholar] [CrossRef] [Green Version]
- Ali, J. The Peach Potato Aphid (Myzus persicae): Ecology and Management; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Hemming, D.; Bell, J.; Collier, R.; Dunbar, T.; Dunstone, N.; Everatt, M.; Eyre, D.; Kaye, N.; Korycinska, A.; Pickup, J. Likelihood of extreme early flight of Myzus persicae (Hemiptera: Aphididae) across the UK. J. Econ. Entomol. 2022, 115, 1342–1349. [Google Scholar] [CrossRef]
- Stevens, M.; McGrann, G.; Clark, B.; Authority, H. Turnip Yellows Virus (Syn Beet Western Yellows Virus): An Emerging Threat to European Oilseed Rape Production; HGCA: Cape Town, South Africa, 2008. [Google Scholar]
- Sharma, S.; Sood, A.K.; Ghongade, D.S. Assessment of losses inflicted by the aphid, Myzus persicae (Sulzer) to sweet pepper under protected environment in north western Indian Himalayan region. Phytoparasitica 2022, 50, 51–62. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; de Queiroz, V.T.; Rondelli, V.M.; Costa, A.V.; Marcelino, T.d.P.; Pratissoli, D. Insecticidal activity of citronella grass essential oil on Frankliniella schultzei and Myzus persicae. Ciência Agrotecnol. 2013, 37, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Nancarrow, N.; Aftab, M.; Hollaway, G.; Rodoni, B.; Trebicki, P. Symptomless turnip yellows virus infection causes grain yield loss in lentil and field pea: A three-year field study in south-eastern Australia. Front. Plant Sci. 2022, 13, 1049905. [Google Scholar] [CrossRef]
- London, H.; Saville, D.J.; Merfield, C.N.; Olaniyan, O.; Wratten, S.D. The ability of the green peach aphid (Myzus persicae) to penetrate mesh crop covers used to protect potato crops against tomato potato psyllid (Bactericera cockerelli). PeerJ 2020, 8, e9317. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Shi, L.; Shen, G.; He, L. Insecticide resistance monitoring and metabolic mechanism study of the green peach aphid, Myzus persicae (Sulzer)(Hemiptera: Aphididae), in Chongqing, China. Pestic. Biochem. Physiol. 2016, 132, 21–28. [Google Scholar] [CrossRef]
- Annis, B.; Tamaki, G.; Berry, R.E. Seasonal occurrence of wild secondary hosts of the green peach aphid, Myzus persicae (Sulzer), in agricultural systems in the Yakima Valley. Environ. Entomol. 1981, 10, 307–312. [Google Scholar] [CrossRef]
- Nampeera, E.L. Management of Green Peach Aphid, Myzus persicae in Amaranth Using Host Plant Resistance and Seed Treatment; JKUAT-CoANRE: Kumasi, Ghana, 2022. [Google Scholar]
- Tamaki, G. Weeds in orchards as important alternate sources of green peach aphids in late spring. Environ. Entomol. 1975, 4, 958–960. [Google Scholar] [CrossRef]
- Tamaki, G.; Fox, L. Weed species hosting viruliferous green peach aphids, vector of beet western yellows virus. Environ. Entomol. 1982, 11, 115–117. [Google Scholar] [CrossRef]
- Sidauruk, L.; Sipayung, P. Population of Myzus persicae (Sulzer) and insect diversity on intercropping potatoes with other plants which planting at different time. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 12018. [Google Scholar]
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A brief profile of a new butenolide insecticide. Pest Manag. Sci. 2015, 71, 850–862. [Google Scholar] [CrossRef] [Green Version]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef] [Green Version]
- Deguine, J.-P.; Aubertot, J.-N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.G.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- Wu, X.F.; Song, C.M. The resistance of Myzuss persicae (Sulzer) against Omethoate in Tobacco fields of Yunnan. J. Gansu Agric. Univ. 2007, 6, 102–105. [Google Scholar]
- Rawat, N.; Singh, R.; Sharma, P.L. Evaluation of some insecticides against the green peach aphid, Myzus persicae (sulzer)(hemiptera: Aphididae). Indian J. Entomol. 2013, 75, 113–117. [Google Scholar]
- Gibson, R.W.; Rice, A.D.; Sawicki, R.M. Effects of the pyrethroid deltamethrin on the acquisition and inoculation of viruses by Myzus persicae. Ann. Appl. Biol. 1982, 100, 49–54. [Google Scholar] [CrossRef]
- Foster, S.P.; Denholm, I.; Devonshire, A.L. The ups and downs of insecticide resistance in peach-potato aphids (Myzus persicae) in the UK. Crop Prot. 2000, 19, 873–879. [Google Scholar] [CrossRef]
- Faraone, N.; Hillier, N.K.; Cutler, G.C. Plant essential oils synergize and antagonize toxicity of different conventional insecticides against Myzus persicae (Hemiptera: Aphididae). PLoS ONE 2015, 10, e0127774. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Q.; Agarwal, M.; Al-Obaidi, R.; Wang, P.; Ren, Y. Evaluation of aphicidal effect of essential oils and their synergistic effect against Myzus persicae (Sulzer) (Hemiptera: Aphididae). Molecules 2021, 26, 3055. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Dardouri, T.; Gautier, H.; Ben Issa, R.; Costagliola, G.; Gomez, L. Repellence of Myzus persicae (Sulzer): Evidence of two modes of action of volatiles from selected living aromatic plants. Pest Manag. Sci. 2019, 75, 1571–1584. [Google Scholar] [CrossRef] [Green Version]
- Andorno, A.V.; López, S.N. Biological control of Myzus persicae (Hemiptera: Aphididae) through banker plant system in protected crops. Biol. Control 2014, 78, 9–14. [Google Scholar] [CrossRef]
- Cabral, S.; Soares, A.O.; Garcia, P. Predation by Coccinella undecimpunctata L. (Coleoptera: Coccinellidae) on Myzus persicae Sulzer (Homoptera: Aphididae): Effect of prey density. Biol. Control 2009, 50, 25–29. [Google Scholar] [CrossRef]
- Paschapur, A.; Subbanna, A.; Singh, A.K.; Jeevan, B.; Stanley, J.; Rajashekhar, H.; Mishra, K.K. Unraveling the Importance of Metabolites from Entomopathogenic Fungi in Insect Pest Management. In Microbes for Sustainable Lnsect Pest Management; Springer: Berlin/Heidelberg, Germany, 2021; pp. 89–120. [Google Scholar]
- Bamisile, B.S.; Akutse, K.S.; Siddiqui, J.A.; Xu, Y. Model application of entomopathogenic fungi as alternatives to chemical pesticides: Prospects, challenges, and insights for next-generation sustainable agriculture. Front. Plant Sci. 2021, 12, 741804. [Google Scholar] [CrossRef]
- Déla, M.A.; Koffivi, K.G.; Komina, A.; Arnaud, A.; Philippe, G.; Adolé, G.I. Evaluation of neem leaves-based preparations as insecticidal agents against the green peach aphid, Myzus persicae (Sternorrhyncha: Aphididae). Afr. J. Agric. Res. 2014, 9, 1344–1352. [Google Scholar]
- Grechi, I.; Ould-Sidi, M.-M.; Hilgert, N.; Senoussi, R.; Sauphanor, B.; Lescourret, F. Designing integrated management scenarios using simulation-based and multi-objective optimization: Application to the peach tree–Myzus persicae aphid system. Ecol. Modell. 2012, 246, 47–59. [Google Scholar] [CrossRef]
- Conboy, N.J.A.; McDaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Gatehouse, A.M.R.; Tosh, C.R. Volatile organic compounds as insect repellents and plant elicitors: An integrated pest management (IPM) strategy for glasshouse whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2020, 46, 1090–1104. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2006, 120, 175–188. [Google Scholar] [CrossRef]
- Dent, D.; Binks, R.H. Insect Pest Management; Cabi: New York, NY, USA, 2020. [Google Scholar]
- Margaritopoulos, J.T.; Kati, A.N.; Voudouris, C.C.; Skouras, P.J.; Tsitsipis, J.A. Long-term studies on the evolution of resistance of Myzus persicae (Hemiptera: Aphididae) to insecticides in Greece. Bull. Entomol. Res. 2021, 111, 1–16. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Wise, J.C. The Arthropod Pesticide Resistance Database; Michigan State University: East Lansing, MI, USA, 2021. [Google Scholar]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Bass, C.; Nauen, R. The molecular mechanisms of insecticide resistance in aphid crop pests. Insect Biochem. Mol. Biol. 2023, 156, 103937. [Google Scholar] [CrossRef]
- Kaleem Ullah, R.M.; Gao, F.; Sikandar, A.; Wu, H. Insights into the Effects of Insecticides on Aphids (Hemiptera: Aphididae): Resistance Mechanisms and Molecular Basis. Int. J. Mol. Sci. 2023, 24, 6750. [Google Scholar] [CrossRef]
- Devonshire, A.L.; Moores, G.D. A carboxylesterase with broad substrate specificity causes organophosphorus, carbamate and pyrethroid resistance in peach-potato aphids (Myzus persicae). Pestic. Biochem. Physiol. 1982, 18, 235–246. [Google Scholar] [CrossRef]
- Field, L.M.; Devonshire, A.L.; Forde, B.G. Molecular evidence that insecticide resistance in peach-potato aphids (Myzus persicae Sulz.) results from amplification of an esterase gene. Biochem. J. 1988, 251, 309–312. [Google Scholar] [CrossRef] [Green Version]
- Andrews, M.C.; Callaghan, A.; Bass, C.; Williamson, M.S.; Field, L.M.; Moores, G.D. A Single Amino Acid Substitution Found in Pirimicarb-Insensitive Acetylcholinesterase of the Peach-Potato Aphid, Myzus persicae (Sulzer); Cholinergic Mech Funct Dysfunction: St. Moritz, Switzerland, 2002. [Google Scholar]
- Nabeshima, T.; Kozaki, T.; Tomita, T.; Kono, Y. An amino acid substitution on the second acetylcholinesterase in the pirimicarb-resistant strains of the peach potato aphid, Myzus persicae. Biochem. Biophys. Res. Commun. 2003, 307, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Foster, S.P.; Williamson, M.S.; Denholm, I. Characterization of the M918T sodium channel gene mutation associated with strong resistance to pyrethroid insecticides in the peach-potato aphid, Myzus persicae (Sulzer). Bull. Entomol. Res. 2008, 98, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.; Caddoux, L.; Brazier, C.; Bertho, C.; Bertolla, P.; Micoud, A.; Roy, L. Uncommon associations in target resistance among French populations of Myzus persicae from oilseed rape crops. Pest Manag. Sci. 2011, 67, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Torres, D.; Devonshire, A.L.; Williamson, M.S. Molecular studies of knockdown resistance to pyrethroids: Cloning of domain II sodium channel gene sequences from insects. Pestic. Sci. 1997, 51, 265–270. [Google Scholar] [CrossRef]
- Panini, M.; Dradi, D.; Marani, G.; Butturini, A.; Mazzoni, E. Detecting the presence of target-site resistance to neonicotinoids and pyrethroids in Italian populations of Myzus persicae. Pest Manag. Sci. 2014, 70, 931–938. [Google Scholar] [CrossRef]
- Anthony, N.; Unruh, T.; Ganser, D.; Ffrench-Constant, R. Duplication of the Rdl GABA receptor subunit gene in an insecticide-resistant aphid, Myzus persicae. Mol. Gen. Genet. MGG 1998, 260, 165–175. [Google Scholar] [CrossRef]
- Singh, K.S.; Cordeiro, E.M.G.; Troczka, B.J.; Pym, A.; Mackisack, J.; Mathers, T.C.; Duarte, A.; Legeai, F.; Robin, S.; Bielza, P. Global patterns in genomic diversity underpinning the evolution of insecticide resistance in the aphid crop pest Myzus persicae. Commun. Biol. 2021, 4, 847. [Google Scholar] [CrossRef]
- Puinean, A.M.; Foster, S.P.; Oliphant, L.; Denholm, I.; Field, L.M.; Millar, N.S.; Williamson, M.S.; Bass, C. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010, 6, e1000999. [Google Scholar] [CrossRef] [Green Version]
- Bass, C.; Puinean, A.M.; Andrews, M.; Cutler, P.; Daniels, M.; Elias, J.; Paul, V.L.; Crossthwaite, A.J.; Denholm, I.; Field, L.M. Mutation of a nicotinic acetylcholine receptor β subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. BMC Neurosci. 2011, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Troczka, B.J.; Singh, K.S.; Zimmer, C.T.; Vontas, J.; Nauen, R.; Hayward, A.; Bass, C. Molecular innovations underlying resistance to nicotine and neonicotinoids in the aphid Myzus persicae. Pest Manag. Sci. 2021, 77, 5311–5320. [Google Scholar] [CrossRef]
- Panini, M.; Chiesa, O.; Troczka, B.J.; Mallott, M.; Manicardi, G.C.; Cassanelli, S.; Cominelli, F.; Hayward, A.; Mazzoni, E.; Bass, C. Transposon-mediated insertional mutagenesis unmasks recessive insecticide resistance in the aphid Myzus persicae. Proc. Natl. Acad. Sci. USA 2021, 118, e2100559118. [Google Scholar] [CrossRef]
- Theiling, K.M.; Croft, B.A. Pesticide side-effects on arthropod natural enemies: A database summary. Agric. Ecosyst. Environ. 1988, 21, 191–218. [Google Scholar] [CrossRef]
- Biondi, A.; Mommaerts, V.; Smagghe, G.; Vinuela, E.; Zappala, L.; Desneux, N. The non-target impact of spinosyns on beneficial arthropods. Pest Manag. Sci. 2012, 68, 1523–1536. [Google Scholar] [CrossRef]
- Stanley, J.; Preetha, G.; Stanley, J. Pesticide Toxicity to Non-Target Organisms; Springer: Berlin/Heidelberg, Germany, 2016; Volume 502. [Google Scholar]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-effects of pesticides on non-target insects in agriculture: A mini-review. Sci. Nat. 2022, 109, 17. [Google Scholar] [CrossRef]
- Kampfraath, A.A.; Giesen, D.; Van Gestel, C.A.M.; Le Lann, C. Pesticide stress on plants negatively affects parasitoid fitness through a bypass of their phytophage hosts. Ecotoxicology 2017, 26, 383–395. [Google Scholar] [CrossRef]
- Ulber, B.; Klukowski, Z.; Williams, I.H. Impact of insecticides on parasitoids of oilseed rape pests. In Biocontrol-Based Integrated Management of Oilseed Rape Pests; Springer: Berlin/Heidelberg, Germany, 2010; pp. 337–355. [Google Scholar]
- El-Wakeil, N.; Gaafar, N.; Sallam, A.; Volkmar, C. Side effects of insecticides on natural enemies and possibility of their integration in plant protection strategies. In Agricultural and Biological Sciences “Insecticides—Development of Safer and More Effective Technologies; IntechOpen: Rijeka, Croatia, 2013; pp. 1–54. [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Müller, C. Impacts of sublethal insecticide exposure on insects—Facts and knowledge gaps. Basic Appl. Ecol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Shoeb, M.A. Effect of some insecticides on the immature stages of the egg parasitoid Trichogramma evanescens West.(Hym., Trichogrammatidae). Egypt. Acad. J. Biol. Sci. A Entomol. 2010, 3, 31–38. [Google Scholar] [CrossRef]
- Desneux, N.; Fauvergue, X.; Dechaume-Moncharmont, F.-X.; Kerhoas, L.; Ballanger, Y.; Kaiser, L. Diaeretiella rapae limits Myzus persicae populations after applications of deltamethrin in oilseed rape. J. Econ. Entomol. 2005, 98, 9–17. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef] [Green Version]
- Birkett, M.A.; Campbell, C.A.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, I.S.; Woodcock, C.M.; Powers, S.J.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. cis-Jasmone Elicits Aphid-Induced Stress Signalling in Potatoes. J. Chem. Ecol. 2017, 43, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, T.J.A.; Martin, J.L.; Pickett, J.A.; Pye, B.J.; Smart, L.E.; Wadhams, L.J. cis-Jasmone treatment induces resistance in wheat plants against the grain aphid, sitobion avenae (Fabricius) (Homoptera: Aphididae). Pest Manag. Sci. 2003, 59, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Bayram, A.; Tonğa, A. cis-Jasmone treatments affect pests and beneficial insects of wheat (Triticum aestivum L.): The influence of doses and plant growth stages. Crop Prot. 2018, 105, 70–79. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils from Foeniculum vulgare Miller as a safe environmental insecticide against the aphid Myzus persicae Sulzer. Environ. Sci. Pollut. Res. 2018, 25, 10904–10910. [Google Scholar] [CrossRef]
- Hamouda, A.B.; Boussadia, O.; Khaoula, B.; Laarif, A.; Braham, M. Studies on insecticidal and deterrent effects of olive leaf extracts on Myzus persicae and Phthorimaea operculella. J. Entomol. Zool. Stud. 2015, 3, 294–297. [Google Scholar]
- Erdoğan, P.; Yıldırım, A. Insecticidal activity of three different plant extracts on the green peach aphid [(Myzus persicae Sulzer)(Hemiptera: Aphididae)]. J. Entomol. Res. Soc. 2016, 18, 27–35. [Google Scholar]
- Madanat, H.M.; Al Antary, T.M.; Zarqa, M. Toxicity of six ethanol plant extracts against the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae). Fresen. Environ. Bull. 2016, 25, 706–718. [Google Scholar]
- Abate, T.; van Huis, A.; Ampofo, J.K.O. Pest management strategies in traditional agriculture: An African perspective. Annu. Rev. Entomol. 2000, 45, 631–659. [Google Scholar] [CrossRef]
- Brader, L. Integrated pest control in the developing world. Annu. Rev. Entomol. 1979, 24, 225–254. [Google Scholar] [CrossRef]
- Smit, N.; Matengo, L.O. Farmers’ cultural practices and their effects on pest control in sweet potato in South Nyanza, Kenya. Int. J. Pest Manag. 1995, 41, 2–7. [Google Scholar] [CrossRef]
- Grechi, I.; Sauge, M.; Sauphanor, B.; Hilgert, N.; Senoussi, R.; Lescourret, F. How does winter pruning affect peach tree–Myzus persicae interactions? Entomol. Exp. Appl. 2008, 128, 369–379. [Google Scholar] [CrossRef]
- Dardouri, T.; Gautier, H.; Costagliola, G.; Gomez, L. How French marigold (Tagetes patula L.) volatiles can affect the performance of green peach aphid. IOBC-WPRS Bull. 2017, 123, 71–78. [Google Scholar]
- Dáder, B.; Legarrea, S.; Moreno, A.; Plaza, M.; Carmo-Sousa, M.; Amor, F.; Viñuela, E.; Fereres, A. Control of insect vectors and plant viruses in protected crops by novel pyrethroid-treated nets. Pest Manag. Sci. 2015, 71, 1397–1406. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.; Palix, R.; Kamal, A.; Deletre, E.; Bonafos, R.; Simon, S.; Ngouajio, M. A repellent net as a new technology to protect cabbage crops. J. Econ. Entomol. 2013, 106, 1699–1706. [Google Scholar] [CrossRef]
- Mpumi, N.; Machunda, R.S.; Mtei, K.M.; Ndakidemi, P.A. Selected insect pests of economic importance to Brassica oleracea, their control strategies and the potential threat to environmental pollution in Africa. Sustainability 2020, 12, 3824. [Google Scholar] [CrossRef]
- Tetreault, H.M.; Grover, S.; Scully, E.D.; Gries, T.; Palmer, N.A.; Sarath, G.; Louis, J.; Sattler, S.E. Global responses of resistant and susceptible sorghum (Sorghum bicolor) to sugarcane aphid (Melanaphis sacchari). Front. Plant Sci. 2019, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, D.L.; Campbell, B.C. Chemical basis of host-plant resistance to aphids. Plant. Cell Environ. 1987, 10, 353–361. [Google Scholar]
- Klingler, J.; Creasy, R.; Gao, L.; Nair, R.M.; Calix, A.S.; Jacob, H.S.; Edwards, O.R.; Singh, K.B. Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol. 2005, 137, 1445–1455. [Google Scholar] [CrossRef] [Green Version]
- Greenslade, A.F.C.; Ward, J.L.; Martin, J.L.; Corol, D.I.; Clark, S.J.; Smart, L.E.; Aradottir, G.I. Triticum monococcum lines with distinct metabolic phenotypes and phloem-based partial resistance to the bird cherry–oat aphid Rhopalosiphum padi. Ann. Appl. Biol. 2016, 168, 435–449. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.M.; Chuang, W. Plant resistance to aphid feeding: Behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag. Sci. 2014, 70, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Bosque-Perez, N.A.; Buddenhagen, I.W. The development of host-plant resistance to insect pests: Outlook for the tropics. In Proceedings of the 8th International Symposium on Insect-Plant Relationships; Springer: Berlin/Heidelberg, Germany, 1992; pp. 235–249. [Google Scholar]
- Tagu, D.; Klingler, J.P.; Moya, A.; Simon, J.-C. Early progress in aphid genomics and consequences for plant–aphid interactions studies. Mol. Plant-Microbe Interact. 2008, 21, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; Van Lenteren, J.C. The status of biological control and recommendations for improving uptake for the future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Acheampong, S.; Gillespie, D.R.; Quiring, D. Survey of parasitoids and hyperparasitoids (Hymenoptera) of the green peach aphid, Myzus persicae and the foxglove aphid, Aulacorthum solani (Hemiptera: Aphididae) in British Columbia. J. Entomol. Soc. Br. Columbia 2012, 109, 12–22. [Google Scholar]
- Mohammed, A.A.; Hatcher, P.E. Combining entomopathogenic fungi and parasitoids to control the green peach aphid Myzus persicae. Biol. Control 2017, 110, 44–55. [Google Scholar] [CrossRef]
- Clavijo Mccormick, A.; Gershenzon, J.; Unsicker, S.B. Little peaks with big effects: Establishing the role of minor plant volatiles in plant–insect interactions. Plant. Cell Environ. 2014, 37, 1836–1844. [Google Scholar] [CrossRef]
- Xu, H.; Turlings, T.C.J. Plant volatiles as mate-finding cues for insects. Trends Plant Sci. 2018, 23, 100–111. [Google Scholar] [CrossRef]
- Ahmed, Q.; Agarwal, M.; Alobaidi, R.; Zhang, H.; Ren, Y. Response of Aphid Parasitoids to Volatile Organic Compounds from Undamaged and Infested Brassica oleracea with Myzus persicae. Molecules 2022, 27, 1522. [Google Scholar] [CrossRef]
- López-Isasmendi, G.; Alvarez, A.E.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C. Aphicidal activity of Bacillus amyloliquefaciens strains in the peach-potato aphid (Myzus persicae). Microbiol. Res. 2019, 226, 41–47. [Google Scholar] [CrossRef]
- Paliwal, D.; Hamilton, A.J.; Barrett, G.A.; Alberti, F.; Van Emden, H.; Monteil, C.L.; Mauchline, T.H.; Nauen, R.; Wagstaff, C.; Bass, C. Identification of novel aphid-killing bacteria to protect plants. Microb. Biotechnol. 2022, 15, 1203–1220. [Google Scholar] [CrossRef]
- Akbar, M.F.; Rana, H.U.; Perveen, F. Management of cauliflower aphid (Myzus persicae (Sulzer) Aphididae: Hemiptera) through environment friendly bioinsecticides. Pak. Entomol 2014, 36, 25–30. [Google Scholar]
- Priest, F.G.; Goodfellow, M.; Shute, L.A.; Berkeley, R.C.W. Bacillus amyloliquefaciens sp. nov., nom. rev. Int. J. Syst. Bacteriol. 1987, 37, 69–71. [Google Scholar] [CrossRef]
- MERTZ, F.P.; Yao, R.C. Saccharopolyspora spinosa sp. nov. isolated from soil collected in a sugar mill rum still. Int. J. Syst. Evol. Microbiol. 1990, 40, 34–39. [Google Scholar] [CrossRef]
- Torres-Quintero, M.C.; Arenas-Sosa, I.; Hernández-Velázquez, V.M.; Suárez-Rodríguez, R.; Peña-Chora, G. Characterization of Bacillus thuringiensis (Bacillaceae) strains pathogenic to Myzus persicae (Hemiptera: Aphididae). Florida Entomol. 2016, 99, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Mora, M.A.E.; Rouws, J.R.C.; Fraga, M.E. Occurrence of entomopathogenic fungi in Atlantic forest soils. Microbiol. Discov. 2016, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.W.; Hajek, A.E. Entomopathogenic fungi as bioinsecticides. In Frontiers in Industrial Mycology; Springer: Berlin/Heidelberg, Germany, 1992; pp. 144–159. [Google Scholar]
- Khachatourians, G.G.; Qazi, S.S. Entomopathogenic fungi: Biochemistry and molecular biology. In Human and Animal Relationships; Springer: Berlin/Heidelberg, Germany, 2008; pp. 33–61. [Google Scholar]
- De Barros, N.M.; Fronza, E.; Bertholdo-Vargas, L.R. Use of fungi as biopesticides. Biopestic. Handb. 2015, 247–281. [Google Scholar]
- Yun, H.-G.; Kim, D.-J.; Gwak, W.-S.; Shin, T.-Y.; Woo, S.-D. Entomopathogenic fungi as dual control agents against both the pest Myzus persicae and phytopathogen Botrytis cinerea. Mycobiology 2017, 45, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Manoussopoulos, Y.; Mantzoukas, S.; Lagogiannis, I.; Goudoudaki, S.; Kambouris, M. Effects of three strawberry entomopathogenic fungi on the prefeeding behavior of the aphid Myzus persicae. J. Insect Behav. 2019, 32, 99–108. [Google Scholar] [CrossRef]
- Jaber, L.R.; Araj, S.-E. Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol. Control 2018, 116, 53–61. [Google Scholar] [CrossRef]
- Lee, W.W.; Shin, T.Y.; Bae, S.M.; Woo, S.D. Screening and evaluation of entomopathogenic fungi against the green peach aphid, Myzus persicae, using multiple tools. J. Asia. Pac. Entomol. 2015, 18, 607–615. [Google Scholar] [CrossRef]
- Ali, S.; Farooqi, M.A.; Sajjad, A.; Ullah, M.I.; Qureshi, A.K.; Siddique, B.; Waheed, W.; Sarfraz, M.; Asghar, A. Compatibility of entomopathogenic fungi and botanical extracts against the wheat aphid, Sitobion avenae (Fab.)(Hemiptera: Aphididae). Egypt. J. Biol. Pest Control 2018, 28, 97. [Google Scholar] [CrossRef]
- Clifton, E.H.; Jaronski, S.T.; Coates, B.S.; Hodgson, E.W.; Gassmann, A.J. Effects of endophytic entomopathogenic fungi on soybean aphid and identification of Metarhizium isolates from agricultural fields. PLoS ONE 2018, 13, e0194815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saranya, S.; Ushakumari, R.; Jacob, S.; Philip, B.M. Efficacy of different entomopathogenic fungi against cowpea aphid, Aphis craccivora (Koch). J. Biopestic. 2010, 3, 138. [Google Scholar]
- Der Geest, V. Can plants use entomopathogens as bodyguards? Ecol. Lett. 2000, 3, 228–235. [Google Scholar]
- Van Munster, M.; Janssen, A.; Clérivet, A.; Van den Heuvel, J. Can plants use an entomopathogenic virus as a defense against herbivores? Oecologia 2005, 143, 396–401. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Chen, D.; Wang, X.; Fan, X.; Liu, X. Potato virus Y-infected tobacco affects the growth, reproduction, and feeding behavior of a vector aphid, Myzus persicae (Hemiptera: Aphididae). Appl. Entomol. Zool. 2015, 50, 239–243. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Infection of host plants by Cucumber mosaic virus increases the susceptibility of Myzus persicae aphids to the parasitoid Aphidius colemani. Sci. Rep. 2015, 5, 10963. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, Y.; Donkersley, P.; Dong, Y.; Chen, X.; Zang, Y.; Xu, P.; Ren, G. Preference of the aphid Myzus persicae (Hemiptera: Aphididae) for tobacco plants at specific stages of potato virus Y infection. Arch. Virol. 2019, 164, 1567–1573. [Google Scholar] [CrossRef]
- Li, Y.; Zhen, S.; Shan, S.; Sun, B.; Li, J.; Hu, F.; Cui, Q.; Zhang, L.; Gu, X.; Cheng, W. Modulation of above-belowground plant-herbivore interactions by entomopathogenic nematodes. Appl. Soil Ecol. 2020, 148, 103479. [Google Scholar] [CrossRef]
- Helms, A.M.; Ray, S.; Matulis, N.L.; Kuzemchak, M.C.; Grisales, W.; Tooker, J.F.; Ali, J.G. Chemical cues linked to risk: Cues from below-ground natural enemies enhance plant defences and influence herbivore behaviour and performance. Funct. Ecol. 2019, 33, 798–808. [Google Scholar] [CrossRef]
- Kamali, S.; Javadmanesh, A.; Stelinski, L.L.; Kyndt, T.; Seifi, A.; Cheniany, M.; Zaki-Aghl, M.; Hosseini, M.; Heydarpour, M.; Asili, J. Beneficial worm allies warn plants of parasite attack below-ground and reduce above-ground herbivore preference and performance. Mol. Ecol. 2022, 31, 691–712. [Google Scholar] [CrossRef]
- Johnson, S.N.; Rasmann, S. Root-feeding insects and their interactions with organisms in the rhizosphere. Annu. Rev. Entomol. 2015, 60, 517–535. [Google Scholar] [CrossRef]
- Park, H.W.; Kim, H.H.; Cho, M.R.; Kang, T.J.; Ahn, S.-J.; Jeon, S.W.; Choo, H.Y. Infectivity of entomopathogenic nematode Steinernema carpocapsae Pocheon strain (Nematoda: Steinernematidae) on the green peach aphid Myzus persicae (Hemiptera: Aphididae) and its parasitoids. Biocontrol Sci. Technol. 2013, 23, 637–645. [Google Scholar] [CrossRef]
- Eizi, Y. Augmentative Biological Control in Greenhouses in Japan; CABI Rev: New York, NY, USA, 2021. [Google Scholar]
- Shavanov, M.V.; Shigapov, I.I.; Niaz, A. Biological methods for pests and diseases control in agricultural plants. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2022; p. 30081. [Google Scholar]
- Yang, S.; Wei, J.; Yang, S.; Kuang, R. Current Status and Future Trends of Augmentative Release of Aphidius gifuensis for Control of Myzus persicae in Chinaâ€TM s Yunnan Province. J. Entomol. Res. Soc. 2011, 13, 87–99. [Google Scholar]
- Gavkare, O.; Kumar, S.; Japoshvili, G. Effectiveness of native parasitoids of Myzus persicae in greenhouse environments in India. Phytoparasitica 2014, 42, 141–144. [Google Scholar] [CrossRef]
- Rodriguez-saona, C.; Vorsa, N.; Singh, A.P.; Johnson-cicalese, J.; Szendrei, Z.; Mescher, M.C.; Frost, C.J. Tracing the history of plant traits under domestication in cranberries: Potential consequences on anti-herbivore defences. J. Exp. Bot. 2011, 62, 2633–2644. [Google Scholar] [CrossRef]
- Gepts, P. A comparison between crop domestication, classical plant breeding, and genetic engineering. Crop Sci. 2002, 42, 1780–1790. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.; Lomate, P.R.; Joshi, R.S.; Punekar, S.A.; Gupta, V.S.; Giri, A.P. Ecological turmoil in evolutionary dynamics of plant–insect interactions: Defense to offence. Planta 2015, 242, 761–771. [Google Scholar] [CrossRef]
- Ali, J.; Sobhy, I.S.; Bruce, T.J.A. Wild potato ancestors as potential sources of resistance to the aphid Myzus persicae. Pest Manag. Sci. 2022, 78, 3931–3938. [Google Scholar] [CrossRef]
- Ali, J.; Wei, D.; Mahamood, M.; Zhou, F.; King, P.J.H.; Zhou, W.; Shamsi, I.H. Exogenous Application of Methyl Salicylate Induces Defence in Brassica against Peach Potato Aphid Myzus persicae. Plants 2023, 12, 1770. [Google Scholar] [CrossRef]
- Erbilgin, N.; Krokene, P.; Christiansen, E.; Zeneli, G.; Gershenzon, J. Exogenous application of methyl jasmonate elicits defenses in Norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus. Oecologia 2006, 148, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, L.; Yan, H.; Kennedy, J.F.; Meng, X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Wang, S.H.; Liu, T.X. Jasmonate- and salicylate-induced defenses in wheat affect host preference and probing behavior but not performance of the grain aphid, Sitobion avenae. Insect Sci. 2014, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Carrillo, J.; Siemann, E.; Ding, J. Herbivore-specific induction of indirect and direct defensive responses in leaves and roots. AoB Plants 2019, 11, plz003. [Google Scholar] [CrossRef]
- Wei, T.; Li, X.; Yashir, N.; Li, H.; Sun, Y.; Hua, L.; Ren, X.; Guo, J. Effect of exogenous silicon and methyl jasmonate on the alleviation of cadmium-induced phytotoxicity in tomato plants. Environ. Sci. Pollut. Res. 2021, 28, 51854–51864. [Google Scholar] [CrossRef]
- Disi, J.O.; Zebelo, S.; Ngumbi, E.; Fadamiro, H.Y. cis-Jasmone primes defense pathways in tomato via emission of volatile organic compounds and regulation of genes with consequences for Spodoptera exigua oviposition. Arthropod. Plant. Interact. 2017, 11, 591–602. [Google Scholar] [CrossRef]
- Oluwafemi, S.; Dewhirst, S.Y.; Veyrat, N.; Powers, S.; Bruce, T.J.A.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. Priming of production in maize of volatile organic defence compounds by the natural plant activator cis-jasmone. PLoS ONE 2013, 8, e62299. [Google Scholar] [CrossRef]
- Matthes, M.; Bruce, T.; Chamberlain, K.; Pickett, J.; Napier, J. Emerging roles in plant defense for cis-jasmone-induced cytochrome P450 CYP81D11. Plant Signal. Behav. 2011, 6, 563–565. [Google Scholar] [CrossRef] [Green Version]
- Tonğa, A.; Çakmak, S.; Şeker, K.; Temiz, M.G.; Bayram, A. cis-Jasmone treatments affect multiple sucking insect pests and associated predators in cotton. Entomol. Gen. 2020, 40, 49–61. [Google Scholar] [CrossRef]
- Ben Issa, R.; Gautier, H.; Gomez, L. Influence of neighbouring companion plants on the performance of aphid populations on sweet pepper plants under greenhouse conditions. Agric. For. Entomol. 2017, 19, 181–191. [Google Scholar] [CrossRef]
- Ameline, A.; Dorland, J.; Werrie, P.-Y.; Couty, A.; Fauconnier, M.-L.; Lateur, M.; Doury, G. Geranium macrorrhizum, a potential novel companion plant affecting preference and performance of Myzus persicae on sweet pepper. J. Pest Sci. 2022, 96, 671–682. [Google Scholar] [CrossRef]
- Lai, R.; You, M.; Lotz, L.A.P.; Vasseur, L. Response of green peach aphids and other arthropods to garlic intercropped with tobacco. Agron. J. 2011, 103, 856–863. [Google Scholar] [CrossRef]
- Ali, M.Y.; Naseem, T.; Arshad, M.; Ashraf, I.; Rizwan, M.; Tahir, M.; Rizwan, M.; Sayed, S.; Ullah, M.I.; Khan, R.R. Host-plant Variations affect the biotic potential, survival, and population projection of Myzus persicae (Hemiptera: Aphididae). Insects 2021, 12, 375. [Google Scholar] [CrossRef]
- Foglar, H.; Malausa, J.C.; Wajnberg, E. The functional response and preference of Macrolophus caliginosus [heteroptera: Miridae] for two of its prey: Myzus persicae and Tetranychus urticae. Entomophaga 1990, 35, 465–474. [Google Scholar] [CrossRef]
- Tiwari, S.; Sharma, S.; Wratten, S.D. Flowering alyssum (Lobularia maritima) promote arthropod diversity and biological control of Myzus persicae. J. Asia. Pac. Entomol. 2020, 23, 634–640. [Google Scholar] [CrossRef]
- Singh, H.; Joshi, N. Management of the aphid, Myzus persicae (Sulzer) and the whitefly, Bemisia tabaci (Gennadius), using biorational on capsicum under protected cultivation in India. Egypt. J. Biol. Pest Control 2020, 30, 67. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, B.J.; Zidack, N.K.; Larson, B.J. The role of Bacillus-based biological control agents in integrated pest management systems: Plant diseases. Phytopathology 2004, 94, 1272–1275. [Google Scholar] [CrossRef] [Green Version]
- Naranjo, S.E. Conservation and evaluation of natural enemies in IPM systems for Bemisia tabaci. Crop Prot. 2001, 20, 835–852. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, J.A. A conceptual framework for integrated pest management. Trends Plant Sci. 2017, 22, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Lewis, W.J.; Van Lenteren, J.C.; Phatak, S.C.; Tumlinson Iii, J.H. A total system approach to sustainable pest management. Proc. Natl. Acad. Sci. USA 1997, 94, 12243–12248. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, K.; Samu, F. Effects of agricultural diversification on the abundance, distribution, and pest control potential of spiders: A review. Entomol. Exp. Appl. 2000, 95, 1–13. [Google Scholar] [CrossRef]
- Whipps, J.M.; Lumsden, R.D. Commercial use of fungi as plant disease biological control agents: Status and prospects. In Fungi as Biocontrol Agents: Progress, Problems and Potential; Wiley: New York, NY, USA, 2001; pp. 9–22. [Google Scholar]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents. Annu Rev Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, A.; Rao, J. Botanical Pesticides in Agriculture; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ekesi, S.; Dimbi, S.; Maniania, N.K. The role of entomopathogenic fungi in the integrated management of fruit flies (Diptera: Tephritidae) with emphasis on species occurring in Africa. Use Entomopathog. Fungi Biol. Pest Manag. 2007, 239–274. [Google Scholar]
- Skinner, M.; Parker, B.L.; Kim, J.S. Role of entomopathogenic fungi in integrated pest management. Integr. Pest Manag. 2014, 169–191. [Google Scholar] [CrossRef]
- Tomilova, O.G.; Kryukova, N.A.; Efimova, M.V.; Kovtun, I.S.; Kolomeichuk, L.V.; Kryukov, V.Y.; Glupov, V.V. Early physiological response of potato plants to entomopathogenic fungi under hydroponic conditions. Horticulturae 2021, 7, 217. [Google Scholar] [CrossRef]
- Rijal, J.P.; Regmi, R.; Ghimire, R.; Puri, K.D.; Gyawaly, S.; Poudel, S. Farmers’ knowledge on pesticide safety and pest management practices: A case study of vegetable growers in Chitwan, Nepal. Agriculture 2018, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Bottrell, D.G.; Schoenly, K.G. Integrated pest management for resource-limited farmers: Challenges for achieving ecological, social and economic sustainability. J. Agric. Sci. 2018, 156, 408–426. [Google Scholar] [CrossRef]
- Haggblade, S.; Diarra, A.; Traoré, A. Regulating agricultural intensification: Lessons from West Africa’s rapidly growing pesticide markets. Dev. Policy Rev. 2022, 40, e12545. [Google Scholar] [CrossRef]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, history, and classification. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–42. [Google Scholar]
- Denkyirah, E.K.; Okoffo, E.D.; Adu, D.T.; Aziz, A.A.; Ofori, A.; Denkyirah, E.K. Modeling Ghanaian cocoa farmers’ decision to use pesticide and frequency of application: The case of Brong Ahafo Region. Springerplus 2016, 5, 1113. [Google Scholar] [CrossRef] [Green Version]
- Birch, A.N.E.; Begg, G.S.; Squire, G.R. How agro-ecological research helps t o address food security issues under new IPM and pesticide reduction policies fo r global crop production systems. J. Exp. Bot. 2011, 62, 3251–3261. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.Z.A.; Manzoor, S.A.; Gul, H.T.; Ali, M.; Bashir, M.A.; Akmal, M.; Haseeb, M.; Imran, M.U.; Taqi, M.; Manzoor, S.A. Drivers of farmers’ intention to adopt integrated pest management: A case study of vegetable farmers in Pakistan. Ecosphere 2021, 12, e03812. [Google Scholar] [CrossRef]
- Singh, B.; Jasrotia, P.; Crespo-Herreraa, L. Breeding for Aphid Resistance in Wheat: Status and Future Prospects. In New Horizons in Wheat and Barley Research; Springer: Berlin/Heidelberg, Germany, 2022; pp. 381–399. [Google Scholar]
- Dutta, P.; Bhattacharyya, A.; Kumari, A. Innovative Integrated Pest Management Paradigm for Sustainable Crop Production with Special Reference to North East India. In Integrated Pest Management in Diverse Cropping Systems; Apple Academic Press: Waretown, NJ, USA, 2023; pp. 61–90. [Google Scholar]
- Gao, Y.; Zhang, X.; Lu, J.; Wu, L.; Yin, S. Adoption behavior of green control techniques by family farms in China: Evidence from 676 family farms in Huang-huai-hai Plain. Crop Prot. 2017, 99, 76–84. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, J.; Bayram, A.; Mukarram, M.; Zhou, F.; Karim, M.F.; Hafez, M.M.A.; Mahamood, M.; Yusuf, A.A.; King, P.J.H.; Adil, M.F.; et al. Peach–Potato Aphid Myzus persicae: Current Management Strategies, Challenges, and Proposed Solutions. Sustainability 2023, 15, 11150. https://doi.org/10.3390/su151411150
Ali J, Bayram A, Mukarram M, Zhou F, Karim MF, Hafez MMA, Mahamood M, Yusuf AA, King PJH, Adil MF, et al. Peach–Potato Aphid Myzus persicae: Current Management Strategies, Challenges, and Proposed Solutions. Sustainability. 2023; 15(14):11150. https://doi.org/10.3390/su151411150
Chicago/Turabian StyleAli, Jamin, Ahmet Bayram, Mohammad Mukarram, Fanrui Zhou, Muhammad Fazal Karim, Mogeda Mohammed Abdel Hafez, Mohammad Mahamood, Abdullahi Ahmed Yusuf, Patricia Jie Hung King, Muhammad Faheem Adil, and et al. 2023. "Peach–Potato Aphid Myzus persicae: Current Management Strategies, Challenges, and Proposed Solutions" Sustainability 15, no. 14: 11150. https://doi.org/10.3390/su151411150
APA StyleAli, J., Bayram, A., Mukarram, M., Zhou, F., Karim, M. F., Hafez, M. M. A., Mahamood, M., Yusuf, A. A., King, P. J. H., Adil, M. F., Ma, Z., & Shamsi, I. H. (2023). Peach–Potato Aphid Myzus persicae: Current Management Strategies, Challenges, and Proposed Solutions. Sustainability, 15(14), 11150. https://doi.org/10.3390/su151411150








