The Protective Power of Cognitive Reserve: Examining White Matter Integrity and Cognitive Function in the Aging Brain for Sustainable Cognitive Health
Abstract
:1. Introduction
2. Materials and Methods
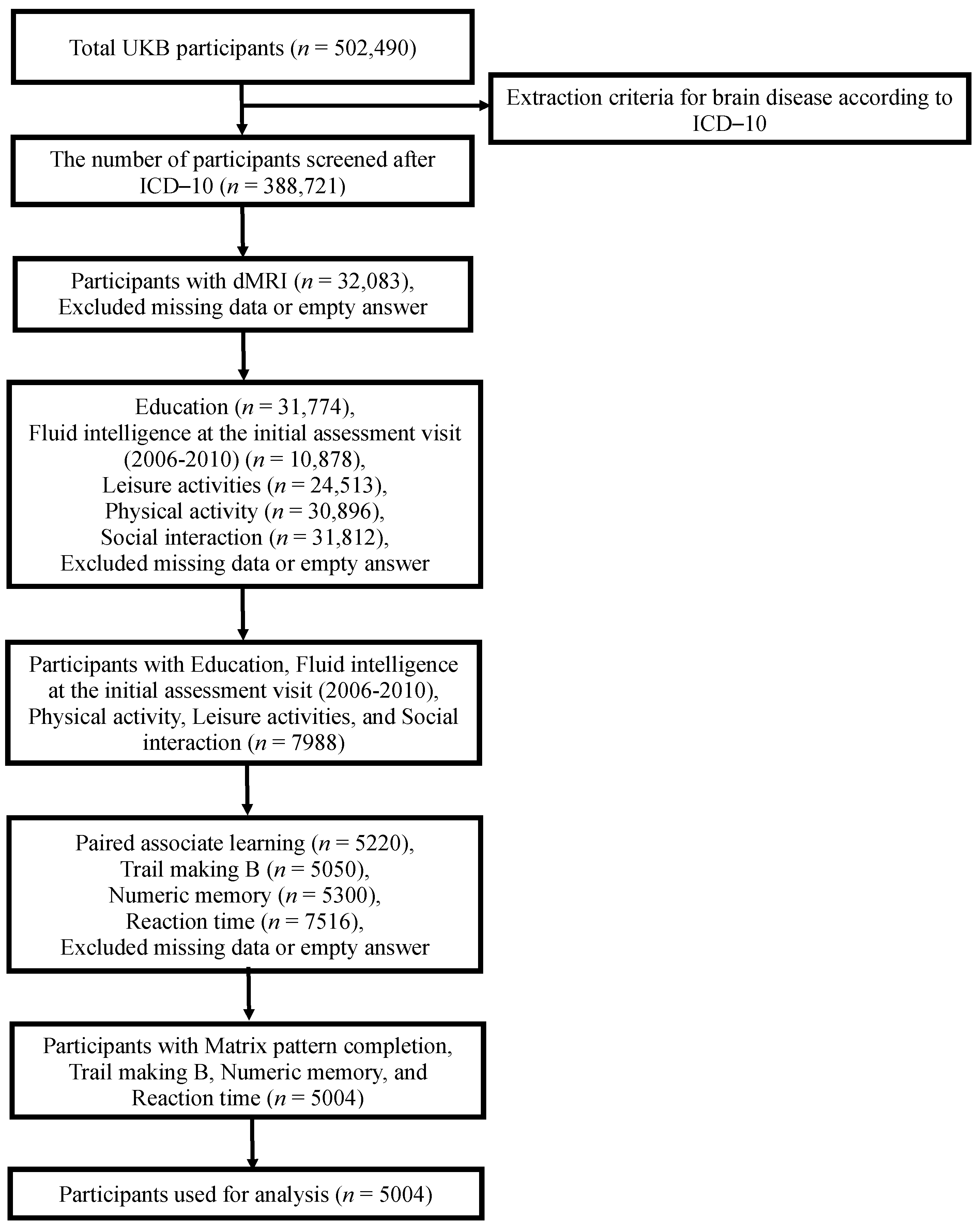
2.1. Participants
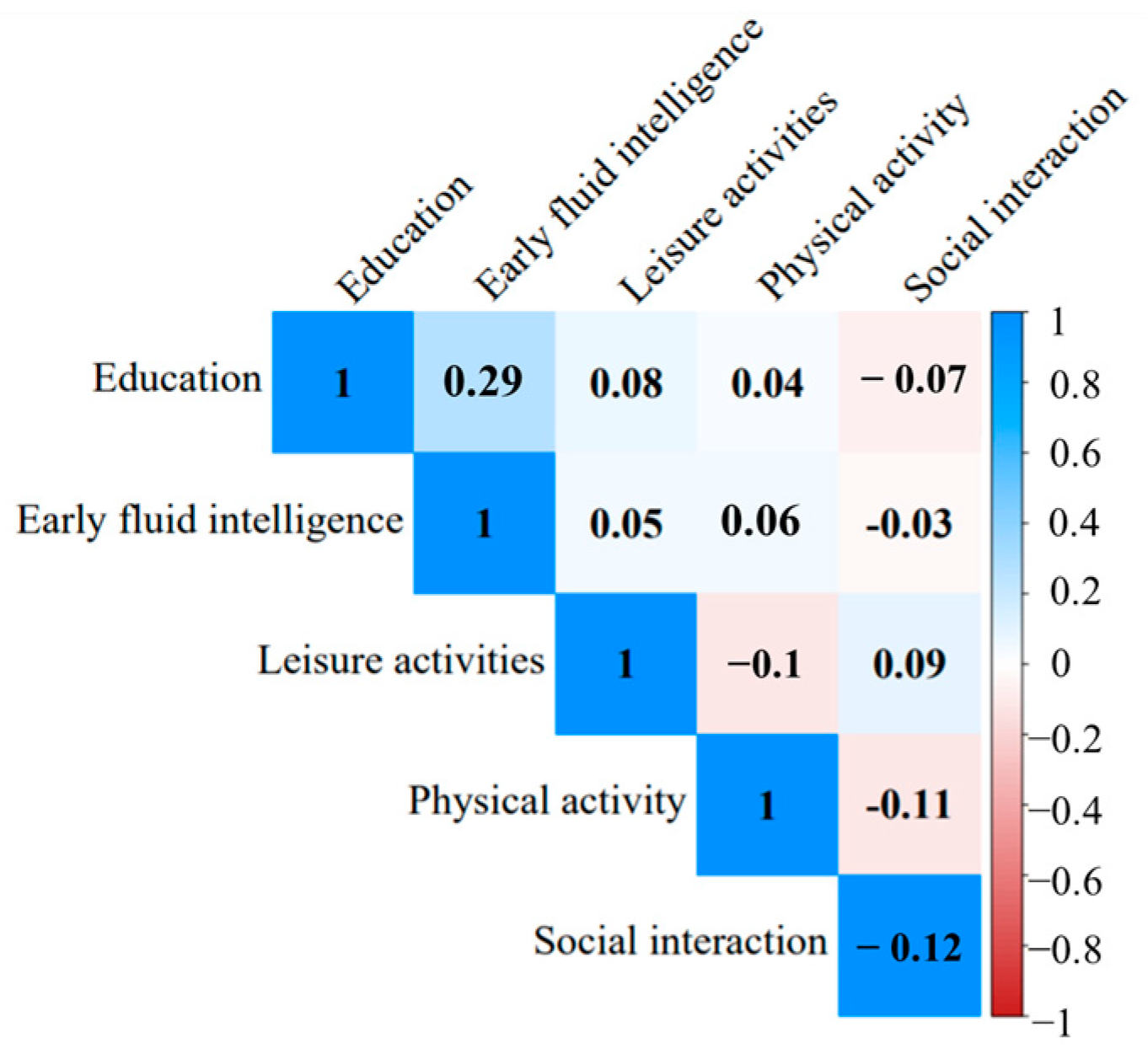
2.2. CR Proxies
2.3. Neuropsychological Exam
2.4. White Matter Integrity
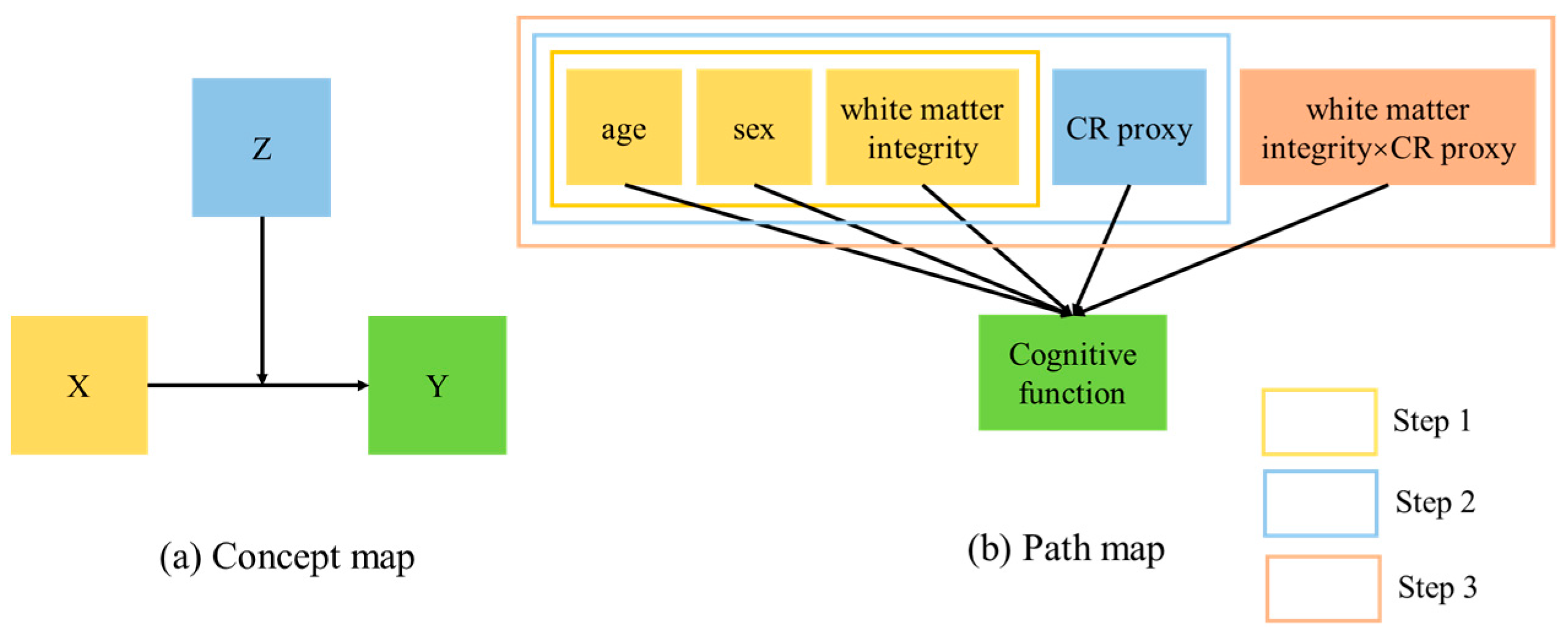
2.5. Analysis
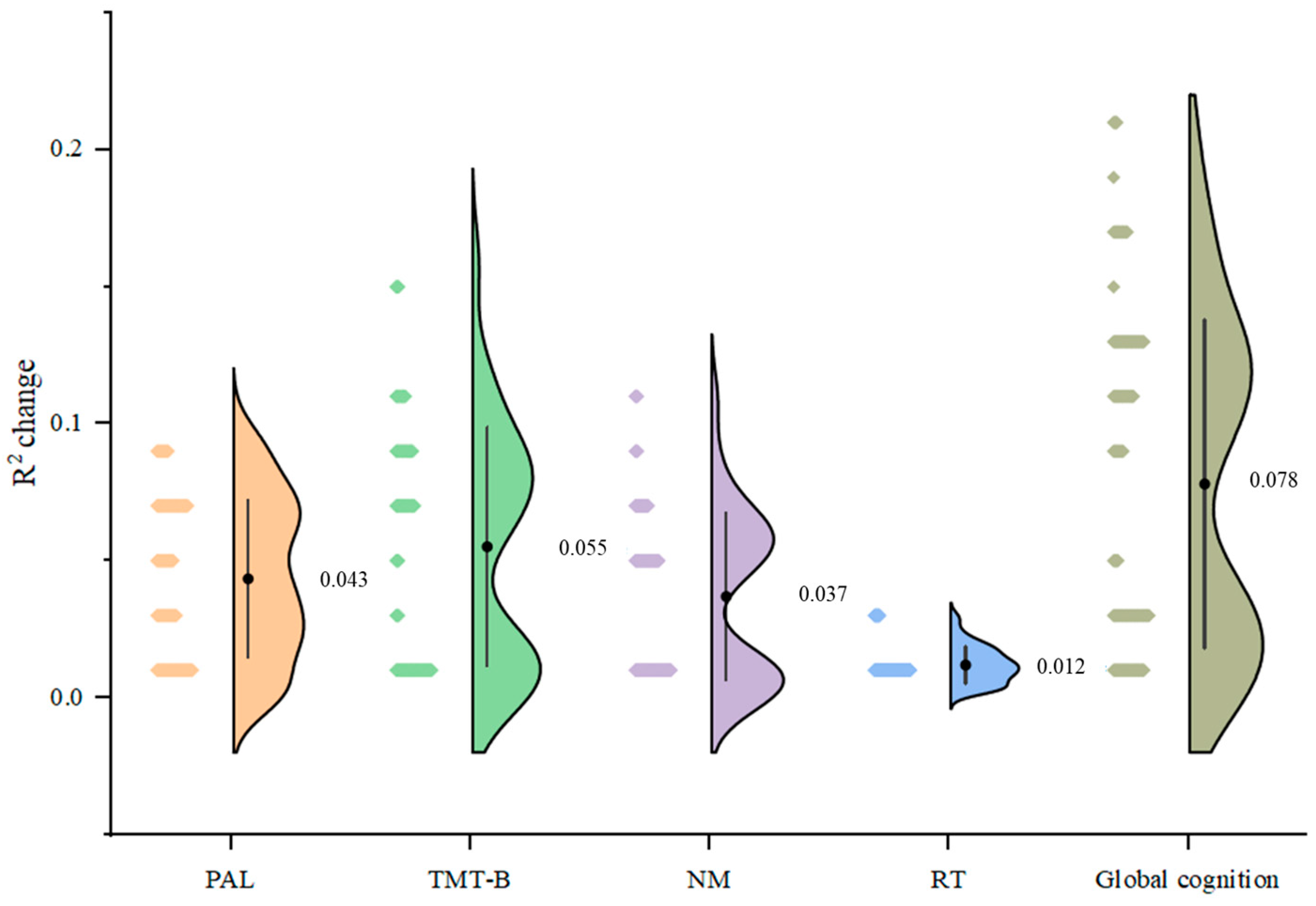
3. Results
3.1. White Matter Integrity–Cognition Relationships
3.2. CR-Cognition Relationships
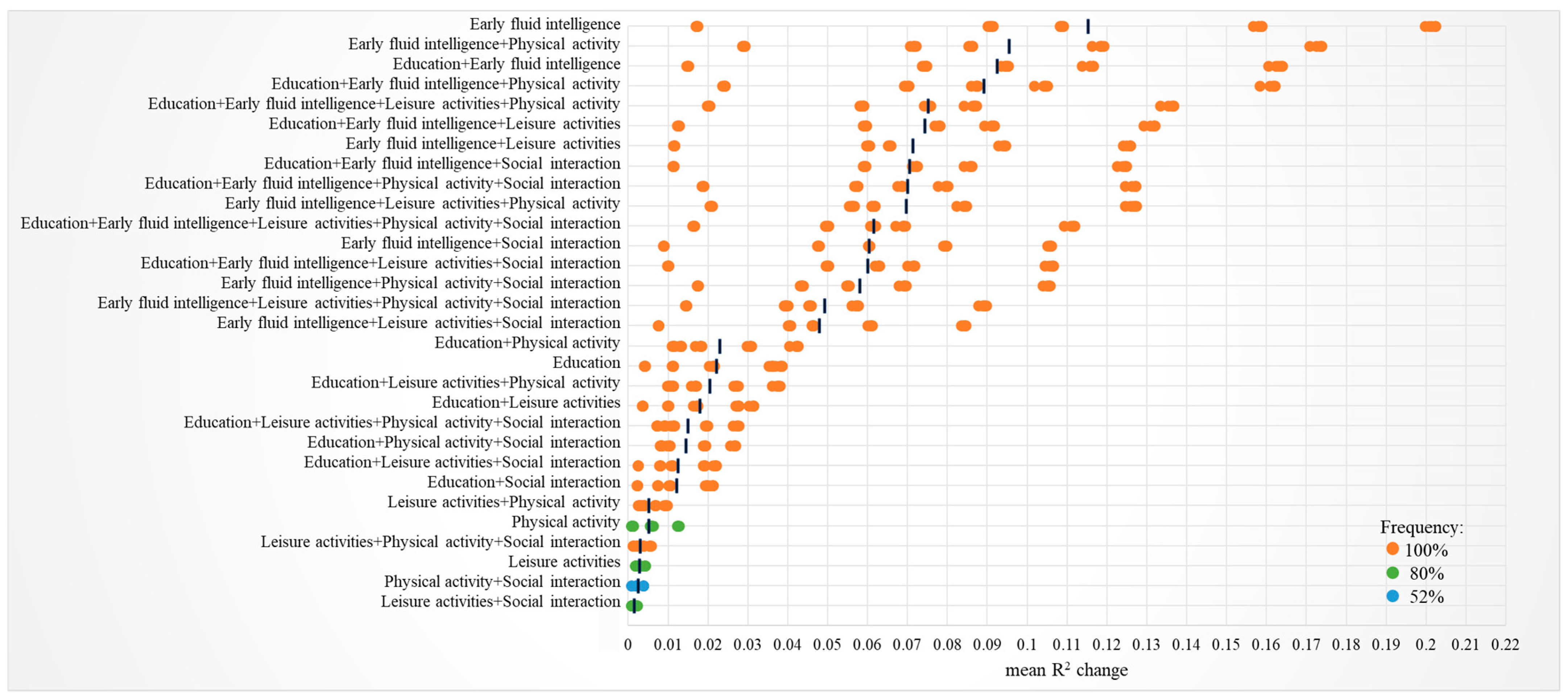
3.3. Moderation Effects
4. Discussion
4.1. White Matter Integrity
4.2. CR Proxy
4.3. CR’s Moderation Effect
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzman, R.; Beard, J.R.; Boerma, T.; Chatterji, S. Health in an ageing world—What do we know? Lancet 2015, 385, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Ihle, A.; Gouveia, É.R.; Gouveia, B.R.; van der Linden, B.W.A.; Sauter, J.; Gabriel, R.; Oris, M.; Fagot, D.; Kliegel, M. The Role of Leisure Activities in Mediating the Relationship between Physical Health and Well-Being: Differential Patterns in Old and Very Old Age. Gerontology 2017, 63, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Tomažič, T.; Čelofiga, A.K. The Role of Different Behavioral and Psychosocial Factors in the Context of Pharmaceutical Cognitive Enhancers’ Misuse. Healthcare 2022, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xiong, M.; Jin, Y.; Kang, W.; Wu, S.; Sun, S.; Fu, Z. Quantifying Brain and Cognitive Maintenance as Key Indicators for Sustainable Cognitive Aging: Insights from the UK Biobank. Sustainability 2023, 15, 9620. [Google Scholar] [CrossRef]
- Ihle, A.; Ghisletta, P.; Gouveia, É.R.; Gouveia, B.R.; Oris, M.; Maurer, J.; Kliegel, M. Lower executive functioning predicts steeper subsequent decline in well-being only in young-old but not old-old age. Int. J. Behav. Dev. 2021, 45, 97–108. [Google Scholar] [CrossRef]
- Enkvist, A.; Ekström, H.; Elmståhl, S. Associations between cognitive abilities and life satisfaction in the oldest-old. Results from the longitudinal population study Good Aging in Skåne. Clin. Interv. Aging 2013, 8, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, R.; Albert, M.; Belleville, S.; Craik, F.I.M.; Duarte, A.; Grady, C.L.; Lindenberger, U.; Nyberg, L.; Park, D.C.; Reuter-Lorenz, P.A.; et al. Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 2018, 19, 701–710. [Google Scholar] [CrossRef]
- Steffener, J.; Stern, Y. Exploring the neural basis of cognitive reserve in aging. Biochim. Biophys. Acta 2012, 1822, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Barulli, D.; Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Robertson, I.H. A noradrenergic theory of cognitive reserve: Implications for Alzheimer’s disease. Neurobiol. Aging 2013, 34, 298–308. [Google Scholar] [CrossRef]
- Mondini, S.; Madella, I.; Zangrossi, A.; Bigolin, A.; Tomasi, C.; Michieletto, M.; Villani, D.; Di Giovanni, G.; Mapelli, D. Cognitive Reserve in Dementia: Implications for Cognitive Training. Front. Aging Neurosci. 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gates, N.J.; Rutjes, A.W.; Di Nisio, M.; Karim, S.; Chong, L.Y.; March, E.; Martínez, G.; Vernooij, R.W. Computerised cognitive training for maintaining cognitive function in cognitively healthy people in midlife. Cochrane Database Syst. Rev. 2019, 3, CD012278. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.R. Towards a network theory of cognition. Neural Netw. 2000, 13, 861–870. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev. Neurosci. 2010, 21, 187–221. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N. Disconnexion syndromes in animals and man. I. Brain 1965, 88, 237–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geschwind, N. Disconnexion syndromes in animals and man. II. Brain 1965, 88, 585–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catani, M.; Ffytche, D.H. The rises and falls of disconnection syndromes. Brain 2005, 128, 2224–2239. [Google Scholar] [CrossRef] [Green Version]
- Fjell, A.M.; Sneve, M.H.; Grydeland, H.; Storsve, A.B.; Walhovd, K.B. The Disconnected Brain and Executive Function Decline in Aging. Cereb. Cortex 2017, 27, 2303–2317. [Google Scholar] [CrossRef] [Green Version]
- Salat, D.H. The declining infrastructure of the aging brain. Brain Connect. 2011, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Filley, C.M. The behavioral neurology of cerebral white matter. Neurology 1998, 50, 1535–1540. [Google Scholar] [CrossRef]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Amato, M.P.; De Stefano, N.; Enzinger, C.; Geurts, J.J.; Penner, I.K.; Rovira, A.; Sumowski, J.F.; Valsasina, P.; Filippi, M. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015, 14, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Devos, H.; Gustafson, K.M.; Liao, K.; Ahmadnezhad, P.; Kuhlmann, E.; Estes, B.J.; Martin, L.E.; Mahnken, J.D.; Brooks, W.M.; Burns, J.M. Effect of Cognitive Reserve on Physiological Measures of Cognitive Workload in Older Adults with Cognitive Impairments. J. Alzheimers Dis. 2023, 92, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.M.; Stern, Y. Cognitive reserve in aging. Curr. Alzheimer Res. 2011, 8, 354–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vemuri, P.; Lesnick, T.G.; Przybelski, S.A.; Machulda, M.; Knopman, D.S.; Mielke, M.M.; Roberts, R.O.; Geda, Y.E.; Rocca, W.A.; Petersen, R.C.; et al. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol. 2014, 71, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, M.S.; Galvin, J.E. Cognitive Resilience in Brain Health and Dementia Research. J. Alzheimers Dis. 2022, 90, 461–473. [Google Scholar] [CrossRef]
- Chapko, D.; McCormack, R.; Black, C.; Staff, R.; Murray, A. Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia—A systematic literature review. Aging Ment. Health 2018, 22, 915–926. [Google Scholar] [CrossRef]
- Lenehan, M.E.; Summers, M.J.; Saunders, N.L.; Summers, J.J.; Vickers, J.C. Relationship between education and age-related cognitive decline: A review of recent research. Psychogeriatrics 2015, 15, 154–162. [Google Scholar] [CrossRef]
- Lee, D.H.; Seo, S.W.; Roh, J.H.; Oh, M.; Oh, J.S.; Oh, S.J.; Kim, J.S.; Jeong, Y. Effects of Cognitive Reserve in Alzheimer’s Disease and Cognitively Unimpaired Individuals. Front. Aging Neurosci. 2022, 13, 784054. [Google Scholar] [CrossRef]
- Li, X.; Song, R.; Qi, X.; Xu, H.; Yang, W.; Kivipelto, M.; Bennett, D.A.; Xu, W. Influence of Cognitive Reserve on Cognitive Trajectories: Role of Brain Pathologies. Neurology 2021, 97, e1695–e1706. [Google Scholar] [CrossRef]
- Meng, X.; D’Arcy, C. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS ONE 2012, 7, e38268. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.T.C.; Lechner, C.M.; Danner, D. New wine in an old bottle? A facet-level perspective on the added value of Grit over BFI-2 Conscientiousness. PLoS ONE 2020, 15, e0228969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Nancekivell, S.; Gelman, S.A.; Shah, P. Perceptions of the malleability of fluid and crystallized intelligence. J. Exp. Psychol. Gen. 2021, 150, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Fu, T. Meta-analysis of fluid intelligence tests of children from the Chinese mainland with learning difficulties. PLoS ONE 2013, 8, e78311. [Google Scholar] [CrossRef] [Green Version]
- Foley, J.M.; Ettenhofer, M.L.; Kim, M.S.; Behdin, N.; Castellon, S.A.; Hinkin, C.H. Cognitive reserve as a protective factor in older HIV-positive patients at risk for cognitive decline. Appl. Neuropsychol. Adult 2012, 19, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Hullinger, R.; Burger, C. Learning impairments identified early in life are predictive of future impairments associated with aging. Behav. Brain Res. 2015, 294, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Salas, N.; Escobar, J.; Huepe, D. Two Sides of the Same Coin: Fluid Intelligence and Crystallized Intelligence as Cognitive Reserve Predictors of Social Cognition and Executive Functions Among Vulnerable Elderly People. Front. Neurol. 2021, 12, 599378. [Google Scholar] [CrossRef]
- Huepe, D.; Roca, M.; Salas, N.; Canales-Johnson, A.; Rivera-Rei, Á.A.; Zamorano, L.; Concepción, A.; Manes, F.; Ibañez, A. Fluid intelligence and psychosocial outcome: From logical problem solving to social adaptation. PLoS ONE 2011, 6, e24858. [Google Scholar] [CrossRef] [Green Version]
- Akbaraly, T.N.; Portet, F.; Fustinoni, S.; Dartigues, J.F.; Artero, S.; Rouaud, O.; Touchon, J.; Ritchie, K.; Berr, C. Leisure activities and the risk of dementia in the elderly: Results from the Three-City Study. Neurology 2009, 73, 854–861. [Google Scholar] [CrossRef]
- Sumowski, J.F.; Wylie, G.R.; Gonnella, A.; Chiaravalloti, N.; Deluca, J. Premorbid cognitive leisure independently contributes to cognitive reserve in multiple sclerosis. Neurology 2010, 75, 1428–1431. [Google Scholar] [CrossRef] [Green Version]
- Kamegaya, T.; Araki, Y.; Kigure, H.; Yamaguchi, H. Twelve-week physical and leisure activity programme improved cognitive function in community-dwelling elderly subjects: A randomized controlled trial. Psychogeriatrics 2014, 14, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Brooks, S.J.; Kullberg, J.; Nordenskjöld, R.; Burgos, J.; Le Grevès, M.; Kilander, L.; Larsson, E.M.; Johansson, L.; Ahlström, H.; et al. Association between physical activity and brain health in older adults. Neurobiol. Aging 2013, 34, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Fischer, F.U.; Riedel, D.; Knaepen, K.; Kollmann, B.; Kocabayoglu, M.; Brüggen, K.; Teipel, S.; Tüscher, O.; Binder, H.; et al. The Impact of Age on the Association Between Physical Activity and White Matter Integrity in Cognitively Healthy Older Adults. Front. Aging Neurosci. 2020, 12, 579470. [Google Scholar] [CrossRef]
- Zhang, Y.; Natale, G.; Clouston, S. The Characteristics of Social Network Structure in Later Life in Relation to Incidence of Mild Cognitive Impairment and Conversion to Probable Dementia. J. Alzheimers Dis. 2021, 81, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, P.; Fang, Y. Social Engagement and Its Change are Associated with Dementia Risk among Chinese Older Adults: A Longitudinal Study. Sci. Rep. 2018, 8, 1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve and brain maintenance. Alzheimers Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Hindle, J.V.; Martyr, A.; Clare, L. Cognitive reserve in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2014, 20, 1–7. [Google Scholar] [CrossRef]
- Mitchell, M.B.; Shaughnessy, L.W.; Shirk, S.D.; Yang, F.M.; Atri, A. Neuropsychological test performance and cognitive reserve in healthy aging and the Alzheimer’s disease spectrum: A theoretically driven factor analysis. J. Int. Neuropsychol. Soc. 2012, 18, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Lin, L.; Xiong, M.; Sun, S.; Wu, S.C. Moderating effects of cognitive reserve on the relationship between brain structure and cognitive abilities in middle-aged and older adults. Neurobiol. Aging 2023, 128, 49–64. [Google Scholar] [CrossRef]
- Christensen, H.; Anstey, K.J.; Parslow, R.A.; Maller, J.; Mackinnon, A.; Sachdev, P. The brain reserve hypothesis, brain atrophy and aging. Gerontology 2007, 53, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Ihle, A.; Oris, M.; Sauter, J.; Rimmele, U.; Kliegel, M. Cognitive Reserve and Social Capital Accrued in Early and Midlife Moderate the Relation of Psychological Stress to Cognitive Performance in Old Age. Dement. Geriatr. Cogn. Disord. 2018, 45, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Vuoksimaa, E.; Panizzon, M.S.; Chen, C.H.; Eyler, L.T.; Fennema-Notestine, C.; Fiecas, M.J.; Fischl, B.; Franz, C.E.; Grant, M.D.; Jak, A.J.; et al. Cognitive reserve moderates the association between hippocampal volume and episodic memory in middle age. Neuropsychologia 2013, 51, 1124–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchman, A.S.; Yu, L.; Wilson, R.S.; Lim, A.; Dawe, R.J.; Gaiteri, C.; Leurgans, S.E.; Schneider, J.A.; Bennett, D.A. Physical activity, common brain pathologies, and cognition in community-dwelling older adults. Neurology 2019, 92, e811–e822. [Google Scholar] [CrossRef]
- Kwak, S.; Shin, M.; Kim, H.; Cho, B.; Ha, J.H.; Han, G.; Kim, H.; Koo, Y.; Kwon, S.; Lee, C.; et al. Moderating effect of cognitive reserve on the association between grey matter atrophy and memory varies with age in older adults. Psychogeriatrics 2020, 20, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Boyle, R.; Knight, S.P.; De Looze, C.; Carey, D.; Scarlett, S.; Stern, Y.; Robertson, I.H.; Kenny, R.A.; Whelan, R. Verbal intelligence is a more robust cross-sectional measure of cognitive reserve than level of education in healthy older adults. Alzheimers Res. Ther. 2021, 13, 128. [Google Scholar] [CrossRef]
- Baker, L.M.; Laidlaw, D.H.; Cabeen, R.; Akbudak, E.; Conturo, T.E.; Correia, S.; Tate, D.F.; Heaps-Woodruff, J.M.; Brier, M.R.; Bolzenius, J.; et al. Cognitive reserve moderates the relationship between neuropsychological performance and white matter fiber bundle length in healthy older adults. Brain Imaging Behav. 2017, 11, 632–639. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [Green Version]
- Amin, V.; Fletcher, J.M.; Sun, Z.; Lu, Q. Higher educational attainment is associated with longer telomeres in midlife: Evidence from sibling comparisons in the UK Biobank. SSM Popul. Health 2021, 17, 101018. [Google Scholar] [CrossRef]
- Fawns-Ritchie, C.; Deary, I.J. Reliability and validity of the UK Biobank cognitive tests. PLoS ONE 2020, 15, e0231627. [Google Scholar] [CrossRef]
- Ihle, A.; Ghisletta, P.; Ballhausen, N.; Fagot, D.; Vallet, F.; Baeriswyl, M.; Sauter, J.; Oris, M.; Maurer, J.; Kliegel, M. The role of cognitive reserve accumulated in midlife for the relation between chronic diseases and cognitive decline in old age: A longitudinal follow-up across six years. Neuropsychologia 2018, 121, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Satz, P.; Cole, M.A.; Hardy, D.J.; Rassovsky, Y. Brain and cognitive reserve: Mediator(s) and construct validity, a critique. J. Clin. Exp. Neuropsychol. 2011, 33, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Naughton, F.; Matthews, F.E. Exposure to secondhand smoke and cognitive impairment in non-smokers: National cross sectional study with cotinine measurement. BMJ 2009, 338, b462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, J.L.; Sotiropoulos, S.N. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. Neuroimage 2015, 122, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basser, P.J.; Mattiello, J.; LeBihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B 1994, 103, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef]
- Daducci, A.; Canales-Rodríguez, E.J.; Zhang, H.; Dyrby, T.B.; Alexander, D.C.; Thiran, J.P. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. Neuroimage 2015, 105, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Lin, F.; Zhou, Y.; Du, Y.; Qin, L.; Zhao, Z.; Xu, J.; Lei, H. Abnormal white matter integrity in adolescents with internet addiction disorder: A tract-based spatial statistics study. PLoS ONE 2012, 7, e30253. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, K.M.; Raz, N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 2009, 47, 916–927. [Google Scholar] [CrossRef] [Green Version]
- Voineskos, A.N.; Rajji, T.K.; Lobaugh, N.J.; Miranda, D.; Shenton, M.E.; Kennedy, J.L.; Pollock, B.G.; Mulsant, B.H. Age-related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiol. Aging 2012, 33, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimny, A.; Szewczyk, P.; Bladowska, J.; Trypka, E.; Wojtynska, R.; Leszek, J.; Sasiadek, M. Quantitative evaluation of changes in the selected white matter tracts using diffusion tensor imaging in patients with Alzheimer’s disease and mild cognitive impairment. Neuroradiol. J. 2012, 25, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, X.; Yuan, Z.; Qiu, J.; Lu, W. A comparison between diffusion tensor imaging and generalized q-sampling imaging in the age prediction of healthy adults via machine learning approaches. J. Neural Eng. 2022, 19, 016013. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, K.M.; McQueeny, T.; Howe, S.R.; Shear, P.; Szaflarski, J. Superior longitudinal fasciculus and language functioning in healthy aging. Brain Res. 2014, 1562, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Molloy, C.J.; Nugent, S.; Bokde, A.L.W. Alterations in Diffusion Measures of White Matter Integrity Associated with Healthy Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 945–954. [Google Scholar] [CrossRef]
- Lebel, C.; Caverhill-Godkewitsch, S.; Beaulieu, C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage 2010, 52, 20–31. [Google Scholar] [CrossRef]
- Chiapponi, C.; Piras, F.; Piras, F.; Fagioli, S.; Caltagirone, C.; Spalletta, G. Cortical grey matter and subcortical white matter brain microstructural changes in schizophrenia are localised and age independent: A case-control diffusion tensor imaging study. PLoS ONE 2013, 8, e75115. [Google Scholar] [CrossRef] [Green Version]
- Kodiweera, C.; Alexander, A.L.; Harezlak, J.; McAllister, T.W.; Wu, Y.C. Age effects and sex differences in human brain white matter of young to middle-aged adults: A DTI, NODDI and q-space study. Neuroimage 2016, 128, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.S.; Owen, J.P.; Pojman, N.J.; Thieu, T.; Bukshpun, P.; Wakahiro, M.L.; Berman, J.I.; Roberts, T.P.; Nagarajan, S.S.; Sherr, E.H.; et al. White Matter Changes of Neurite Density and Fiber Orientation Dispersion during Human Brain Maturation. PLoS ONE 2015, 10, e0123656. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenbulcke, M.; Mädler, B.; Peeters, R.; Dhollander, T.; Zhang, H.; Deprez, S.; Van den Bergh, B.R.; Sunaert, S.; Emsell, L. Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol. Aging 2015, 36, 2107–2121. [Google Scholar] [CrossRef] [Green Version]
- Sumowski, J.F.; Wylie, G.R.; Deluca, J.; Chiaravalloti, N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: Functional magnetic resonance imaging evidence for cognitive reserve. Brain 2010, 133, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.S.; Kaufman, J.C.; Liu, X.; Johnson, C.K. How do educational attainment and gender relate to fluid intelligence, crystallized intelligence and academic skills at ages 22–90 years? Arch. Clin. Neuropsychol. 2009, 24, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, J.T.; Vianen, A.; Flier, H. Score gains on g-loaded tests: No g. Intelligence 2007, 35, 283–300. [Google Scholar] [CrossRef]
- Tikhomirova, T.; Malykh, A.; Malykh, S. Predicting Academic Achievement with Cognitive Abilities: Cross-Sectional Study across School Education. Behav. Sci. 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.D. Appraisal of the pleiotropic effects of intelligence and education on schizophrenia: A univariable and multivariable Mendelian randomization study. Cold Spring Harb. 2019, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.L.; Howe, L.D.; Wade, K.H.; Ben-Shlomo, Y.; Hill, W.D.; Deary, I.J.; Sanderson, E.C.; Zheng, J.; Korologou-Linden, R.; Stergiakouli, E.; et al. Education, intelligence and Alzheimer’s disease: Evidence from a multivariable two-sample Mendelian randomization study. Int. J. Epidemiol. 2020, 49, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Baltes, M.M.; Kühl, K.P.; Sowarka, D. Testing for limits of cognitive reserve capacity: A promising strategy for early diagnosis of dementia? J. Gerontol. 1992, 47, P165–P167. [Google Scholar] [CrossRef]
- Baltes, P.B.; Dittmann-Kohli, F.; Kliegl, R. Reserve capacity of the elderly in aging-sensitive tests of fluid intelligence: Replication and extension. Psychol. Aging 1986, 1, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Schnermann, M.E.; Schulz, C.A.; Ludwig, C.; Alexy, U.; Nöthlings, U. A lifestyle score in childhood and adolescence was positively associated with subsequently measured fluid intelligence in the DONALD cohort study. Eur. J. Nutr. 2022, 61, 3719–3729. [Google Scholar] [CrossRef]
- Ihle, A.; Gouveia, É.R.; Gouveia, B.R.; Marques, A.; Marconcin, P.; de Maio Nascimento, M.; Jurema, J.; Tinôco, M.A.; Kliegel, M. Cognitive Functioning Mediates the Association of Cognitive Reserve with Health-Related Quality of Life. Sustainability 2022, 14, 826. [Google Scholar] [CrossRef]
- Iso-Markku, P.; Kaprio, J.; Lindgrén, N.; Rinne, J.O.; Vuoksimaa, E. Education as a moderator of middle-age cardiovascular risk factor-old-age cognition relationships: Testing cognitive reserve hypothesis in epidemiological study. Age Ageing 2022, 51, afab228. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, A.; Karim, H.T.; Ly, M.J.; Cohen, A.D.; Lopresti, B.J.; Mathis, C.A.; Klunk, W.E.; Aizenstein, H.J.; Snitz, B.E. An Effect of Education on Memory-Encoding Activation in Subjective Cognitive Decline. J. Alzheimers Dis. 2021, 81, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Pinter, D.; Sumowski, J.; DeLuca, J.; Fazekas, F.; Pichler, A.; Khalil, M.; Langkammer, C.; Fuchs, S.; Enzinger, C. Higher education moderates the effect of T2 lesion load and third ventricle width on cognition in multiple sclerosis. PLoS ONE 2014, 9, e87567. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, S.; Chikhi, S.; Maltais, D. The benefits of physical activities on cognitive and mental health in healthy and pathological aging. Geriatr. Psychol. Neuropsychiatr. Vieil. 2018, 16, 197–205. [Google Scholar] [CrossRef]
- Kim, R.E.; Yun, C.H.; Thomas, R.J.; Oh, J.H.; Johnson, H.J.; Kim, S.; Lee, S.; Seo, H.S.; Shin, C. Lifestyle-dependent brain change: A longitudinal cohort MRI study. Neurobiol. Aging 2018, 69, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.J.; Tucker-Drob, E.M.; Cox, S.R.; Dickie, D.A.; Hernández, M.D.C.V.; Corley, J.; Royle, N.A.; Redmond, P.; Muñoz Maniega, S.; Pattie, A.; et al. Risk and protective factors for structural brain ageing in the eighth decade of life. Brain Struct. Funct. 2017, 222, 3477–3490. [Google Scholar] [CrossRef] [Green Version]
- Vemuri, P.; Lesnick, T.G.; Przybelski, S.A.; Knopman, D.S.; Machulda, M.; Lowe, V.J.; Mielke, M.M.; Roberts, R.O.; Gunter, J.L.; Senjem, M.L.; et al. Effect of intellectual enrichment on AD biomarker trajectories: Longitudinal imaging study. Neurology 2016, 86, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Jammula, V.R.; Leeper, H.; Gilbert, M.R.; Cooper, D.; Armstrong, T.S. Effects of Cognitive Reserve on Cognition in Individuals with Central Nervous System Disease. Cogn. Behav. Neurol. 2021, 34, 245–258. [Google Scholar] [CrossRef]
- Fleck, J.I.; Arnold, M.; Dykstra, B.; Casario, K.; Douglas, E.; Morris, O. Distinct Functional Connectivity Patterns Are Associated with Social and Cognitive Lifestyle Factors: Pathways to Cognitive Reserve. Front. Aging Neurosci. 2019, 11, 310. [Google Scholar] [CrossRef]
- Bellani, M.; Ricciardi, C.; Rossetti, M.G.; Zovetti, N.; Perlini, C.; Brambilla, P. Cognitive remediation in schizophrenia: The earlier the better? Epidemiol. Psychiatr. Sci. 2019, 29, e57. [Google Scholar] [CrossRef] [Green Version]
- Fingerhut, H.; Gozdas, E.; Hosseini, S.M.H. Quantitative MRI Evidence for Cognitive Reserve in Healthy Elders and Prodromal Alzheimer’s Disease. J. Alzheimers Dis. 2022, 89, 849–863. [Google Scholar] [CrossRef] [PubMed]
| Testing | UKB ID | Description | Cognitive Domain | Variables | Range |
|---|---|---|---|---|---|
| PAL | 506 | Participants viewed 12-word pairs on a screen and were instructed to memorize them for a later test. In the test, they then selected the correct partner word from four options. | Verbal declarative memory | Total correct score | 0–10 |
| TMT-B | 505 | Participants switched between touching 1–13 numbers in numeric order and the letters A-L in alphabetical order while in a pseudo-random arrangement. | Executive functions | Time to complete (deci-seconds) | 187–2986 |
| NM | 100029 | Participants completed a backward digit span task where they had to enter a two-digit number in reverse order after a short delay. The task progressively added one digit with each correctly recalled sequence. The participant’s task ended once they failed two trials of the same length or successfully recalled a 12-digit number. | Working memory | Maximum digits remembered correctly | 2–11 |
| RT | 100032 | Participants pushed a button-box when two matching symbol cards appeared on screen. Out of 12 trials, the first 5 were practice trials, and the remaining 7 had 4 matching card trials for the score. | Processing speed | Average response time (milliseconds) | 348–1508 |
| Cognition | Model Statistics | White Matter Integrity | Sex | Age | ||
|---|---|---|---|---|---|---|
| R2 | F | Variable | β | β | Β | |
| PAL | 0.059 | 104.477 ** | FA | 0.062 ** | −0.167 ** | −0.134 ** |
| TMT-B | 0.145 | 283.335 ** | FA | 0.094 ** | 0.005 | −0.336 ** |
| NM | 0.017 | 29.392 ** | FA | 0.044 | 0.069 ** | −0.092 ** |
| RT | 0.107 | 199.579 ** | FA | 0.044 | 0.108 ** | −0.297 ** |
| Global cognitive | 0.142 | 276.878 ** | FA | 0.094 ** | 0.006 | −0.333 ** |
| PAL | 0.058 | 102.895 ** | MD | −0.055 * | −0.164 ** | −0.133 ** |
| TMT-B | 0.140 | 270.715 ** | MD | −0.051 * | 0.009 | −0.349 ** |
| NM | 0.016 | 27.524 ** | MD | −0.026 | 0.071 ** | −0.097 ** |
| RT | 0.106 | 197.512 ** | MD | −0.029 | 0.110 ** | −0.300 ** |
| Global cognitive | 0.138 | 266.688 ** | MD | −0.063 ** | 0.010 | −0.340 ** |
| PAL | 0.056 | 99.334 ** | ICVF | 0.024 | −0.166 ** | −0.150 ** |
| TMT-B | 0.141 | 274.217 ** | ICVF | 0.063 ** | 0.006 | −0.354 ** |
| NM | 0.016 | 26.852 ** | ICVF | 0.013 | 0.070 ** | −0.105 ** |
| RT | 0.106 | 196.900 ** | ICVF | 0.020 | 0.109 ** | −0.308 ** |
| Global cognitive | 0.137 | 264.173 ** | ICVF | 0.047 * | 0.007 | −0.355 ** |
| PAL | 0.057 | 101.421 ** | OD | −0.042 | −0.167 ** | −0.148 ** |
| TMT-B | 0.140 | 271.945 ** | OD | −0.053 ** | 0.006 | −0.360 ** |
| NM | 0.018 | 30.897 ** | OD | −0.051 * | 0.070 ** | −0.098 ** |
| RT | 0.107 | 199.924 ** | OD | −0.043 | 0.108 ** | −0.304 ** |
| Global cognitive | 0.140 | 271.168 ** | OD | −0.073 ** | 0.007 | −0.352 ** |
| PAL | 0.057 | 101.054 ** | ISOVF | −0.042 | −0.165 ** | −0.139 ** |
| TMT-B | 0.138 | 266.618 ** | ISOVF | −0.019 | 0.007 | −0.363 ** |
| NM | 0.016 | 26.860 ** | ISOVF | −0.014 | 0.070 ** | −0.102 ** |
| RT | 0.106 | 196.669 ** | ISOVF | −0.017 | 0.109 ** | −0.306 ** |
| Global cognitive | 0.136 | 262.060 ** | ISOVF | −0.036 | 0.008 | −0.352 ** |
| Cognition Function | Brain Structure × CR Proxies | ΔR2 | β |
|---|---|---|---|
| PAL | FA × Education | 0.0017 | 0.041 ** |
| TMT-B | ICVF × Physical activity | 0.0010 | 0.032 * |
| RT | FA × Early fluid intelligence + Physical activity | 0.0009 | −0.031 * |
| PAL | FA × Education + Physical activity | 0.0009 | 0.029 * |
| RT | FA × Early fluid intelligence + Leisure activities + Physical activity | 0.0009 | −0.029 * |
| TMT-B | FA × Early fluid intelligence | 0.0008 | −0.028 * |
| RT | FA × Early fluid intelligence | 0.0007 | −0.026 * |
| Cognition Function | Brain Structure × CR Proxies | ΔR2 | β |
|---|---|---|---|
| TMT-B | OD × Early fluid intelligence + Physical activity | 0.0023 | 0.048 ** |
| RT | ISOVF × Early fluid intelligence + Physical activity | 0.0018 | 0.042 ** |
| RT | ISOVF × Early fluid intelligence + Leisure activities + Physical activity | 0.0017 | 0.041 ** |
| TMT-B | OD × Early fluid intelligence | 0.0016 | 0.040 ** |
| RT | ISOVF × Education + Early fluid intelligence + Leisure activities + Physical activity | 0.0016 | 0.040 ** |
| RT | ISOVF × Education + Early fluid intelligence + Physical activity | 0.0015 | 0.038 ** |
| TMT-B | OD × Education + Early fluid intelligence + Physical activity | 0.0014 | 0.039 ** |
| Global cognition | OD × Early fluid intelligence + Physical activity | 0.0014 | 0.037 ** |
| RT | ISOVF × Education + Early fluid intelligence + Leisure activities | 0.0013 | 0.037 ** |
| Global cognition | OD × Early fluid intelligence + Leisure activities + Physical activity | 0.0013 | 0.036 ** |
| RT | ISOVF × Early fluid intelligence + Leisure activities | 0.0013 | 0.036 ** |
| TMT-B | OD × Education + Early fluid intelligence | 0.0012 | 0.037 ** |
| RT | ISOVF × Education + Early fluid intelligence | 0.0012 | 0.034 ** |
| Global cognition | OD × Education + Early fluid intelligence + Leisure activities | 0.0012 | 0.035 ** |
| RT | ISOVF × Early fluid intelligence | 0.0011 | 0.033 ** |
| Global cognition | OD × Education + Early fluid intelligence + Leisure activities + Physical activity | 0.0011 | 0.034 ** |
| NM | OD × Early fluid intelligence + Leisure activities + Physical activity | 0.0011 | 0.033 ** |
| RT | MD × Early fluid intelligence + Leisure activities | 0.0011 | 0.033 ** |
| Global cognition | OD × Early fluid intelligence + Leisure activities | 0.0010 | 0.032 ** |
| Global cognition | OD × Early fluid intelligence | 0.0010 | 0.032 ** |
| TMT-B | OD × Early fluid intelligence + Physical activity + Social interaction | 0.0010 | 0.031 * |
| RT | MD × Early fluid intelligence + Leisure activities + Physical activity | 0.0010 | 0.031 * |
| TMT-B | ISOVF × Education | 0.0010 | 0.031 * |
| NM | OD × Early fluid intelligence + Leisure activities + Physical activity + Social interaction | 0.0009 | 0.031 * |
| NM | OD × Early fluid intelligence + Leisure activities + Social interaction | 0.0009 | 0.031 * |
| TMT-B | OD × Early fluid intelligence + Leisure activities + Physical activity | 0.0009 | 0.030 * |
| TMT-B | OD × Education + Early fluid intelligence + Leisure activities + Physical activity | 0.0009 | 0.031 * |
| NM | OD × Education + Early fluid intelligence + Leisure activities + Physical activity | 0.0009 | 0.031 * |
| NM | ISOVF × Early fluid intelligence | 0.0009 | −0.030 * |
| NM | ISOVF × Physical activity | 0.0009 | −0.030 * |
| NM | OD × Education + Early fluid intelligence + Leisure activities | 0.0009 | 0.030 * |
| NM | OD × Early fluid intelligence + Leisure activities | 0.0009 | 0.030 * |
| NM | OD × Education + Early fluid intelligence + Leisure activities + Social interaction | 0.0009 | 0.030 * |
| NM | OD × Education + Leisure activities + Social interaction | 0.0009 | 0.030 * |
| Global cognition | OD × Education + Early fluid intelligence | 0.0008 | 0.030 * |
| NM | OD × Education + Leisure activities | 0.0008 | 0.029 * |
| Global cognition | MD × Leisure activities | 0.0008 | 0.029 * |
| TMT-B | ISOVF × Education + Early fluid intelligence + Physical activity | 0.0008 | 0.029 * |
| NM | OD × Education + Early fluid intelligence + Leisure activities + Physical activity + Social interaction | 0.0008 | 0.029 * |
| TMT-B | ISOVF × Early fluid intelligence + Physical activity | 0.0008 | 0.028 * |
| TMT-B | OD × Education + Early fluid intelligence + Physical activity + Social interaction | 0.0008 | 0.029 * |
| Global cognition | OD × Education + Early fluid intelligence + Leisure activities + Social interaction | 0.0008 | 0.029 * |
| RT | ISOVF × Education + Leisure activities + Physical activity | 0.0008 | 0.028 * |
| Global cognition | OD × Education + Early fluid intelligence + Physical activity | 0.0008 | 0.029 * |
| TMT-B | ISOVF × Education + Early fluid intelligence | 0.0008 | 0.027 * |
| RT | MD × Early fluid intelligence + Physical activity | 0.0007 | 0.028 * |
| TMT-B | OD × Early fluid intelligence + Social interaction | 0.0007 | 0.028 * |
| RT | MD × Early fluid intelligence | 0.0007 | 0.027 * |
| TMT-B | ISOVF × Education + Early fluid intelligence + Leisure activities | 0.0007 | 0.026 * |
| Global cognition | OD × Early fluid intelligence + Leisure activities + Social interaction | 0.0007 | 0.026 * |
| Global cognition | OD × Early fluid intelligence + Leisure activities + Physical activity + Social interaction | 0.0007 | 0.026 * |
| TMT-B | OD × Education + Early fluid intelligence + Social interaction | 0.0007 | 0.028 * |
| RT | OD × Education + Early fluid intelligence | 0.0007 | 0.027 * |
| TMT-B | OD × Education + Early fluid intelligence + Leisure activities | 0.0007 | 0.027 * |
| Global cognition | OD × Education + Early fluid intelligence + Leisure activities + Physical activity + Social interaction | 0.0007 | 0.026 * |
| TMT-B | ISOVF × Education + Early fluid intelligence + Leisure activities + Physical activity | 0.0006 | 0.025 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Jin, Y.; Xiong, M.; Wu, S.; Sun, S. The Protective Power of Cognitive Reserve: Examining White Matter Integrity and Cognitive Function in the Aging Brain for Sustainable Cognitive Health. Sustainability 2023, 15, 11336. https://doi.org/10.3390/su151411336
Lin L, Jin Y, Xiong M, Wu S, Sun S. The Protective Power of Cognitive Reserve: Examining White Matter Integrity and Cognitive Function in the Aging Brain for Sustainable Cognitive Health. Sustainability. 2023; 15(14):11336. https://doi.org/10.3390/su151411336
Chicago/Turabian StyleLin, Lan, Yue Jin, Min Xiong, Shuicai Wu, and Shen Sun. 2023. "The Protective Power of Cognitive Reserve: Examining White Matter Integrity and Cognitive Function in the Aging Brain for Sustainable Cognitive Health" Sustainability 15, no. 14: 11336. https://doi.org/10.3390/su151411336
APA StyleLin, L., Jin, Y., Xiong, M., Wu, S., & Sun, S. (2023). The Protective Power of Cognitive Reserve: Examining White Matter Integrity and Cognitive Function in the Aging Brain for Sustainable Cognitive Health. Sustainability, 15(14), 11336. https://doi.org/10.3390/su151411336





