Bacterial Communities: Interaction to Abiotic Conditions under Effect of Anthropogenic Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Soil Samples and Properties
2.2. The Investigation of Soil Microorganisms
2.3. Investigation of Soil Chemical and Physical Properties
2.4. Meteorological Conditions
2.5. Statistical Analysis
3. Results and Discussion
3.1. The Variation of Soil Chemical Properties under Different Anthropogenic Intensity
3.2. The Effect of Intensive Soil Management on Physical Properties
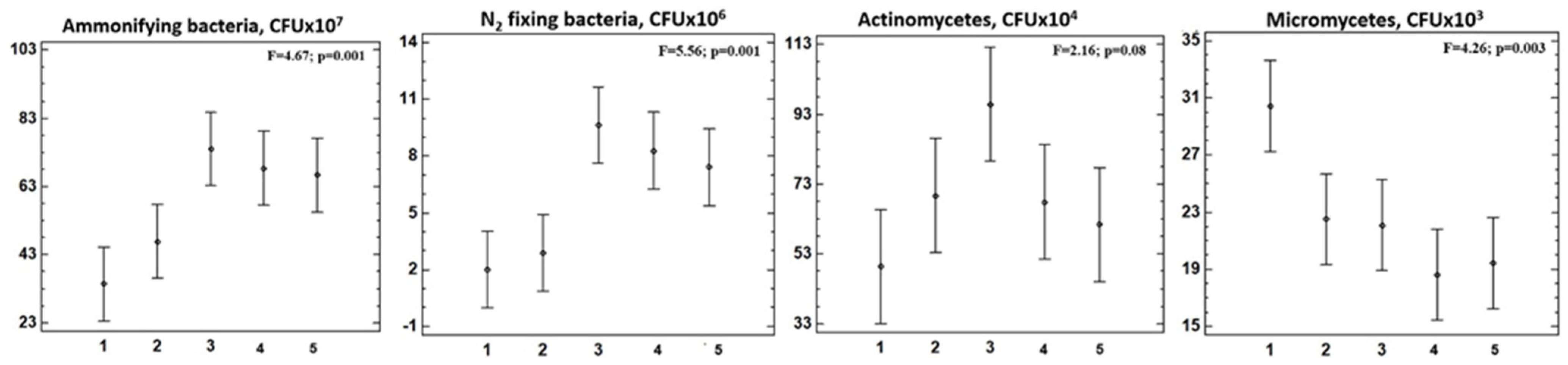
3.3. The Assessment of the Richness of Soil Microorganisms’ Physiological Groups
3.4. The Determination of Soil Microorganisms’ Physiological Group Seasonal Changes
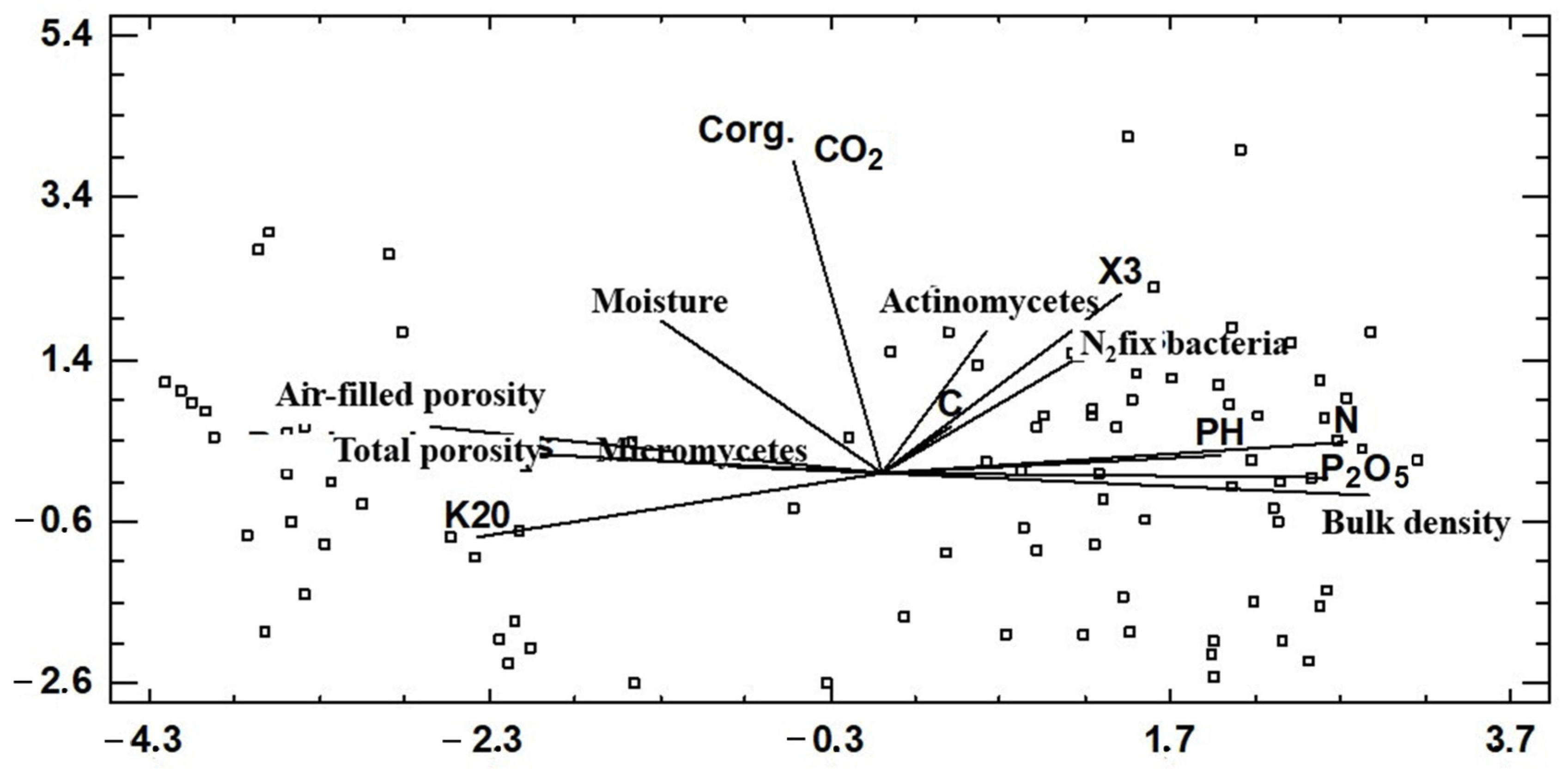
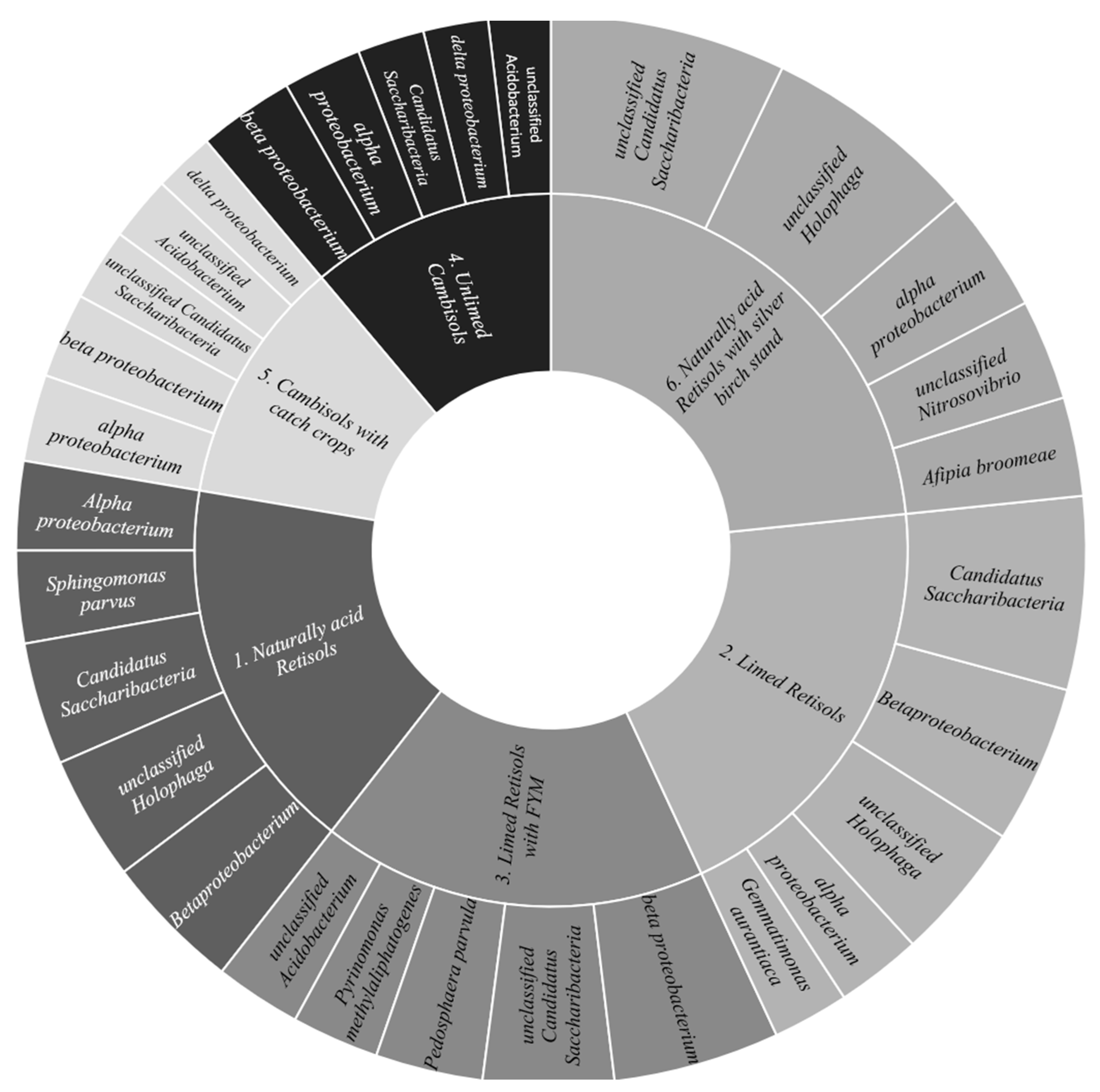
3.5. The Determination of Soil Microorbial Diversity and Abundance under Different Anthropogenic Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Larsbrink, J.; McKee, L. Bacteroidetes bacteria in the soil: Glycan acquisition, enzyme secretion and gliding motility. Appl. Microbiol. 2020, 110, 63–98. [Google Scholar]
- Goss-Souza, D.; Mendes, L.W.; Borges, C.D.; Rodrigues, J.L.M.; Tsai, S.M. Amazon forest-to-agriculture conversion alters rhizosphere microbiome composition while functions are kept. FEMS Microbiol. Ecol. 2019, 95, fiz009. [Google Scholar] [CrossRef] [Green Version]
- Escalas, A.; Hale, L.; Voordeckers, J.W.; Yang, Y.; Firestone, M.K.; Alvarez-Cohen, L.; Zhou, J.Z. Microbial functional diversity: From concepts to applications. Ecol. Evol. 2019, 9, 12000–12016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xu, Z.H. Assessing bacterial diversity in soil. J. Soils Sediment 2008, 8, 379–388. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Bacteria as emerging indicators of soil condition. Appl. Environ. Micro 2017, 83, e02826-16. [Google Scholar] [CrossRef] [Green Version]
- Korneykova, M.V.; Vasenev, V.I.; Nikitin, D.A.; Soshina, A.S.; Dolgikh, A.V.; Sotnikova, Y.L. Urbanization affects soil microbiome profile distribution in the Russian Arctic region. Int. J. Environ. Res. Public Health 2021, 18, 11665. [Google Scholar] [CrossRef]
- Canfora, L.; Bacci, G.; Pinzari, F.; Lo Papa, G.; Dazzi, C.; Benedetti, A. Salinity, and bacterial diversity: To what extent does the concentration of salt affect the bacterial community in a saline soil. PLoS ONE 2014, 9, e106662. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, B.M.; Kim, M.; Singh, D.; Lee-Cruz, L.; Lai-Hoe, A.; Ainuddin, A.N.; Go, R.; Rahim, R.A.; Husni, M.H.A.; Chun, J. Tropical soil bacterial communities in Malaysia: pH dominates in the equatorial tropics too. Micro Ecol. 2012, 64, 474–484. [Google Scholar] [CrossRef]
- Xu, S.; Lu, W.J.; Liu, Y.T.; Ming, Z.Y.; Liu, Y.J.; Meng, R.H.; Wang, H.T. Structure, and diversity of bacterial communities in two large sanitary landfills in China as revealed by high-throughput sequencing (MiSeq). Waste Manag. 2017, 63, 41–48. [Google Scholar] [CrossRef]
- Drobnik, T.; Greiner, L.; Keller, A.; Gret-Regamey, A. Soil quality indicators—From soil functions to ecosystem services. Ecol. Indic. 2018, 94, 151–169. [Google Scholar] [CrossRef]
- Preethi, B.; Poorniammal, R.; Balachandar, D.; Karthikeyan, S.; Chendreyan, K.; Bhattacharyya, P.; Adhya, T.K. Long-term organic nutrient managements foster the biological properties and carbon sequestering capability of a wetland rice soil. Arch. Agron. Soil Sci. 2013, 59, 1607–1624. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Baishya, A.; Gogoi, B.; Hazarika, J.; Hazarika, J.P.; Bora, A.S.; Das, A.K.; Borah, M.; Sutradhar, P. Comparative assessment of organic, inorganic and integrated management practices in rice (Oryza sativa)-based cropping system in acid soil of Assam. Ind. J. Agron. 2017, 62, 118–126. [Google Scholar]
- Saccá, M.L.; Barra Caracciolo, A.; Di Lenola, M.; Grenni, P. Ecosystem Services Provided by Soil Microorganisms. In Soil Biological Communities and Ecosystem Resilience—Sustainability in Plant and Crop Protection; Lukac, M., Grenni, P., Gamboni, M., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Herman, D.J.; Firestone, M.K.; Nuccio, E.; Hodge, A. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol. Ecol. 2012, 80, 236–247. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Krause, S.; Le Roux, X.; Niklaus, P.A.; Van Bodegom, P.M.; Lennon, J.T.; Bertilsson, S.; Grossart, H.P.; Philippot, L.; Bodelier, P.L. Trait based approaches for understanding microbial biodiversity and ecosystem functioning. Front. Microbiol. 2014, 5, 251. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Zhang, J.; Cook, J.; Nearing, J.T.; Zhang, J.; Raudonis, R.; Glick, B.R.; Langille, M.G.I.; Cheng, Z. Harnessing the plant microbiome to promote the growth of agricultural crops. Microbiol. Res. 2021, 245, 126690. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Clemente, J.C.; Shade, A.; Metcalf, J.L.; Song, S.; Prithiviraj, B.; Palmer, B.E.; Knight, R. Our microbial selves: What ecology can teach us. EMBO Rep. 2011, 12, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa-Hueso, R. Global change and the soil microbiome: A human-health perspective. Front. Ecol. Evol. 2017, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, B.M.; Moroenyane, I.; Sherman, C.; Lee, Y.K.; Adams, J.M.; Steinberger, Y. Trends in taxonomic and functional composition of soil microbiome along a precipitation gradient in Israel. Microb. Ecol. 2017, 74, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, V.; Ovreas, L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M.; Gessner, M.O.; Beisner, B.E.; Messier, C.; Paquette, A.; Petermann, J.S.; Soininen, J.; Nock, C.A. Pathways for cross-boundary effects of biodiversity on ecosystem functioning. Trends Ecol. Evol. 2022, 37, 454–467. [Google Scholar] [CrossRef]
- Bailey, K.L.; Lazarovits, G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res. 2003, 72, 169–180. [Google Scholar] [CrossRef]
- Mas-Carrió, E.; Dini-Andreote, F.; Brossi, M.J.d.L.; Salles, J.F.; Olff, H. Organic amendment under increasing agricultural intensification: Effects on soil bacterial communities and plant productivity. Front. Microbiol. 2018, 9, 2612. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Paul, N.C.; Shah, D.H.; Schillinger, W.F.; Bary, A.I.; Sharratt, B.; Paulitz, T.C. Biosolids and tillage practices influence soil bacterial communities in dryland wheat. Microb. Ecol. 2019, 78, 737–752. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; Sharma, K.; Kaku, E.; Karfopoulos, S.; Zelenev, V.V.; Blok, W.J. Soil health indicators and fusarium wilt suppression in organically and conventionally managed greenhouse soils. Appl. Soil Ecol. 2015, 86, 192–201. [Google Scholar] [CrossRef]
- Ye, G.; Banerjee, S.; He, J.-Z.; Fan, J.; Wang, Z.; Wei, X.; Hu, H.-W.; Zheng, Y.; Duan, C.; Wan, S.; et al. Manure application increases microbiome complexity in soil aggregate fractions: Results of an 18-year field experiment. Agric. Ecosyst. Environ. 2021, 307, 107249. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Velickovic, D.; Wietsma, T.W.; Bell, S.L.; Jansson, J.K.; Hofmockel, K.S.; Anderton, C.R. Visualizing microbial community dynamics via a controllable soil environment. Systems 2020, 5, e00645-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaharia, M.; Bolosky, W.J.; Curtis, K.; Fox, A.; Patterson, D.; Shenker, S.; Stoica, I.; Karp, R.M.; Sittler, T. Faster and more accurate sequence alignment with SNAP. arXiv 2011, arXiv:1111.5572. [Google Scholar]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi_recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- Duru, M.; Therond, O.; Martin, G.; Martin-Clouaire, R.; Magne, M.-A.; Justes, E.; Journet, E.P.; Aubertot, J.N.; Savary, S.; Bergez, J.E.; et al. How to implement biodiversity-based agriculture to enhance ecosystem services: A review. Agron. Sustain. Dev. 2015, 35, 1259–1281. [Google Scholar] [CrossRef]
- Liang, F.; Li, J.; Yang, X.; Huang, S.; Cai, Z.; Gao, H.; Ma, J.; Cui, X.; Xu, M. Three-decade long fertilization-induced soil organic carbon sequestration depends on edaphic characteristics in six typical croplands. Sci. Rep. 2016, 6, 30350. [Google Scholar] [CrossRef] [Green Version]
- Almagro, M.; Garcia-Franco, N.; Martínez-Mena, M. The potential of reducing tillage frequency and incorporating plant residues as a strategy for climate change mitigation in semiarid Mediterranean agroecosystems. Agric. Ecosyst. Environ. 2017, 246, 210–220. [Google Scholar] [CrossRef]
- Novara, A.; Minacapilli, M.; Santoro, A.; Rodrigo-Comino, J.; Carrubba, A.; Sarno, M.; Venezia, G.; Gristina, L. Real cover crops contribution to soil organic carbon sequestration in sloping vineyard. Sci. Total Environ. 2019, 652, 300–306. [Google Scholar] [CrossRef]
- Vicente-Vicente, J.L.; Gómez-Muñoz, B.; Hinojosa-Centeno, M.B.; Smith, P.; Garcia-Ruiz, R. Carbon saturation and assessment of soil organic carbon fractions in Mediterranean rainfed olive orchards under plant cover management. Agric. Ecosyst. Environ. 2017, 245, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.A. Sustainability using cover crops in Mediterranean tree crops, olives and vines—Challenges and current knowledge. Hung. Geogr. Bull. 2017, 66, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Meena, B.P.; Fagodiya, R.K.; Prajapat, K.; Dotaniya, M.; Kaledhonkar, M.; Sharma, P. Legume green manuring: An option for soil sustainability. In Legumes for Soil Health and Sustainable Management; Meena, R., Das, A., Yadav, G., Lal, R., Eds.; Springer: Singapore, 2018; pp. 387–408. [Google Scholar]
- Repsiene, R.; Karcauskiene, D. Changes in the chemical properties of acid soil and aggregate stability in the whole profile under long-term management history. Acta Agric. Scand. B-Soil Plant Sci. 2016, 66, 671–676. [Google Scholar] [CrossRef]
- Nazir, A.; Farooq, M.; Farooq, B.; Anjum, S.; Yousuf, S. Soil Microbial Community and Climate Change Drivers. In Climate Change and Microbiome Dynamics—Climate Change Management; Parray, J.A., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- . Romero, F.; Hilfiker, S.; Edlinger, A.; Held, A.; Hartman, K.; Labouyrie, M.; van der Heijden, M.G.A. Soil microbial biodiversity promotes crop productivity and agro-ecosystem functioning in experimental microcosms. Sci. Total Environ. 2023, 885, 163683. [Google Scholar] [CrossRef] [PubMed]
- Karlen, D.L.; Veum, K.S.; Sudduth, K.A.; Obrycki, J.F.; Nunes, M.R. Soil health assessment: Past accomplishments, current activities, and future opportunities. Soil Till Res. 2019, 195, 104365. [Google Scholar] [CrossRef]
- Nunes, M.R.; Karlen, D.L.; Veum, K.S.; Moorman, T.B.; Cambardella, C.A. Biological soil health indicators respond to tillage intensity: AUS meta-analysis. Geoderma 2020, 369, 114335. [Google Scholar] [CrossRef]
- Sun, J.B.; Zou, L.P.; Li, W.B.; Wang, Y.G.; Xia, Q.Y.; Peng, M. Soil microbial and chemical properties influenced by continuous cropping of banana. Sci. Agric. 2018, 75, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Qi, G.F.; Luo, T.; Zhang, H.C.; Jiang, Q.K.; Wang, R.; Zhao, X.Y. Continuous-cropping tobacco caused variance of chemical properties and structure of bacterial network in soils. Land Degrad. Dev. 2018, 29, 4106–4120. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Cui, Y.S.; Li, H.Y.; Kuang, A.X.; Li, X.R.; Wei, Y.L.; Ji, X.L. Diversity and composition of rhizospheric soil and root endogenous bacteria in panax notoginseng during continuous cropping practices. J. Basic Microbiol. 2017, 57, 337–344. [Google Scholar] [CrossRef]
- Kuhwald, M.; Dörnhöfer, K.; Oppelt, N.; Duttmann, R. Spatially explicit soil compaction risk assessment of arable soils at regional scale: The SaSCiA-Model. Sustainability 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Dong, W.; Heinen, M.; Qin, W.; Oenema, O. Soil compaction prevention, amelioration and alleviation measures are effective in mechanized and smallholder agriculture: A meta-analysis. Land 2022, 11, 645. [Google Scholar] [CrossRef]
- Piccoli, I.; Seehusen, T.; Bussell, J.; Vizitu, O.; Calciu, I.; Berti, A.; Börjesson, G.; Kirchmann, H.; Kätterer, T.; Sartori, F.; et al. Opportunities for mitigating soil compaction in Europe—Case studies from the SoilCare project using soil-improving cropping systems. Land 2022, 11, 223. [Google Scholar] [CrossRef]
- Nyéki, A.; Milics, G.; Kovács, A.J.; Neményi, M. Effects of soil compaction on cereal yield. Cereal Res. Comm. 2017, 45, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Feiza, V.; Feizienė, D.; Kadžienė, G. Agro-physical properties of Endocalcari-Epihypogleyic Cambisol arable layer in long-term soil management systems. Zemes Ukio Moksl. 2008, 15, 13–23. [Google Scholar]
- Aislabie, J.; Deslippe, J.R. Soil microbes and their contribution to soil services. In Ecosystem Services in New Zealand—Conditions and Trends; Dymond, J.R., Ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 143–161. Available online: https://www.landcareresearch.co.nz/assets/Publications/Ecosystem-services-in-New-Zealand/1_12_Aislabie.pdf (accessed on 23 April 2023).
- Pereira, P.; Bogunovic, I.; Muñoz-Rojas, M.; Brevik, E.C. Soil ecosystem services, sustainability, valuation, and management. Curr. Opin. Environ. Sci. Health 2018, 5, 7–13. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil ph as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [Green Version]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Delibas, M.; Tezer, A.; Kuzniecow Bacchin, T. Towards embedding soil ecosystem services in spatial planning. Cities 2021, 113, 103150. [Google Scholar] [CrossRef]
- Herdina; Silsbury, J.H. Growth, nitrogen accumulation and partitioning, and N2 fixation in faba bean (Vicia faba cv. Fiord) and pea (Pisum sativum cv. Early Dun). Field Crops Res. 1990, 24, 3–4. [Google Scholar] [CrossRef]
- Volkogon, V.V.; Dimova, S.B.; Volkogon, K.I.; Sidorenko, V.P.; Volkogon, M.V. Biological nitrogen fixation and denitrification in rhizosphere of potato plants in response to the fertilization and inoculation. Front. Sustain. Food Syst. 2021, 5, 606379. [Google Scholar] [CrossRef]
- Wysokinski, A.; Lozak, I. The dynamic of nitrogen uptake from different sources by pea (Pisum sativum L.). Agriculture 2021, 11, 81. [Google Scholar] [CrossRef]
- Klimasmith, I.M.; Kent, A.D. Micromanaging the nitrogen cycle in agroecosystems. Trends Microbiol. 2022, 30, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dou, Y.; Wang, B.; Xue, Z.; Wang, Y.; An, S.; Chang, S.X. Deciphering factors driving soil microbial life-history strategies in restored grasslands. iMeta 2022, 2, 66. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
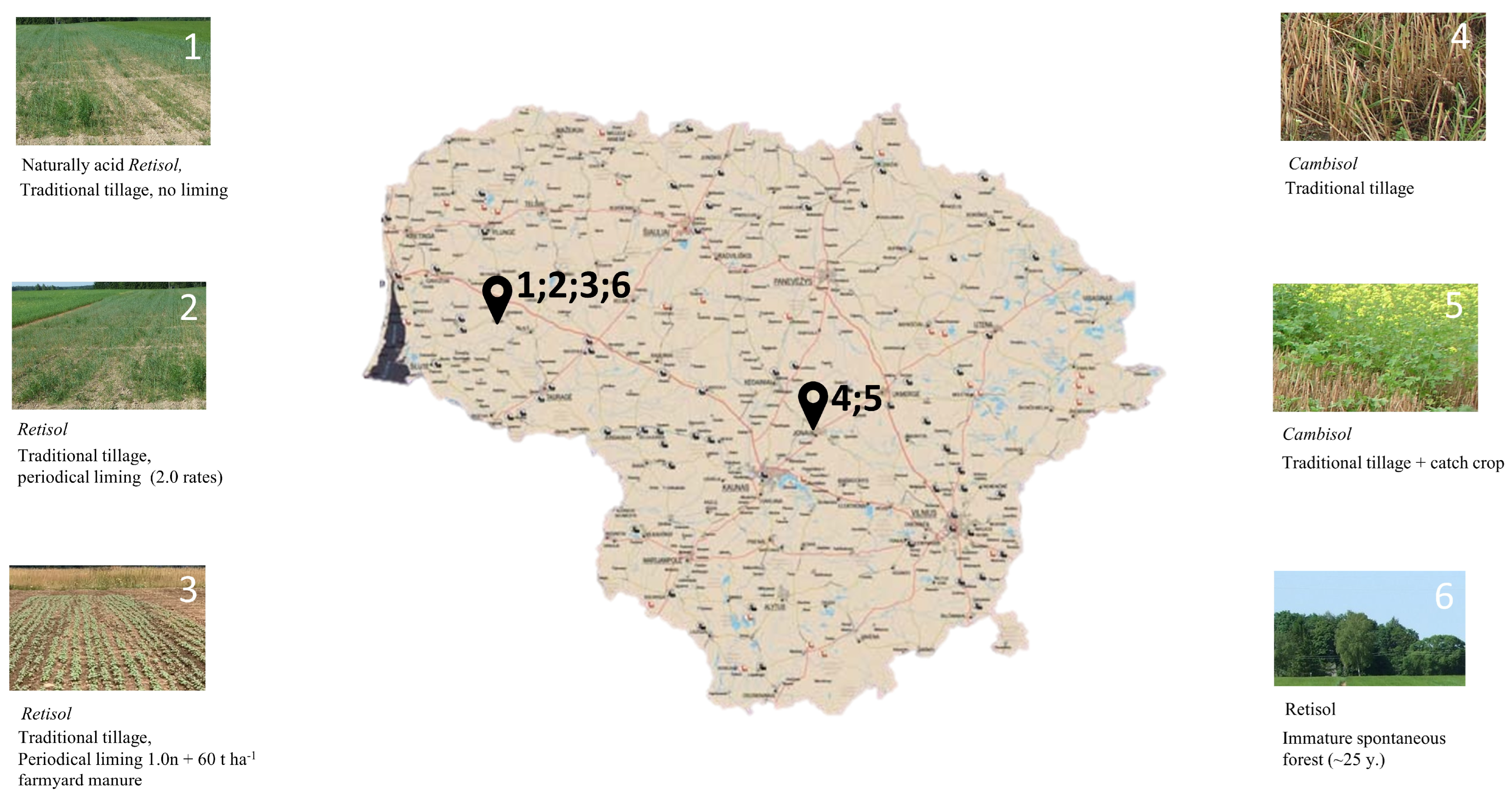




| Sampling Place No. | Coordinates | Type of Soil | pHKCl | Corg | Nsum | Main Applicable Agri-Technics | Intensity of Anthropogenic Activities |
|---|---|---|---|---|---|---|---|
| 1. | N 55°41′, E 21°29′ (Vėžaičiai, Klaipėda dist.) | Bathygleyic Dystric Glossic Retisol | 3.97 ± 0.1 | 1.32 ± 0.04 | 0.11 ± 0.003 | traditional tillage, no liming, every year NPK | 1 |
| 2. | N 55°41′, E 21°29′ (Vėžaičiai, Klaipėda dist.) | Bathygleyic Dystric Glossic Retisol | 6.75 ± 0.2 | 1.30 ± 0.06 | 0.12 ± 0.01 | periodical liming ×2.0 rate every 3–4 years, conventional tillage, every year NPK | 2 |
| 3. | N 55°43′, E 21°27′ (Vėžaičiai, Klaipėda dist.) | Bathygleyic Dystric Glossic Retisol | 5.71 ± 0.1 | 1.55 ± 0.15 | 0.15 ± 0.01 | periodical liming using 1.0 of the liming rate and 60 t ha−1 FYM (farmyard manure) every 5 years, traditional tillage, every year NPK | 3 |
| 4. | N 55°38′, E 23°86′ (Dotnuva, Kėdainiai dist.) | Endocalcari-Epihypogleyic Cambisols | 6.93 ± 0.1 | 1.38 ± 0.18 | 0.16 ± 0.01 | conventional tillage, no liming, every year NPK | 1 |
| 5. | N 55°38′, E 23°86′ (Dotnuva, Kėdainiai dist.) | Endocalcari-Epihypogleyic Cambisols | 7.1 ± 0.2 | 1.19 ± 0.13 | 0.15 ± 0.01 | conventional tillage, no liming, every year NPK + cover crop management | 3 |
| 6. | N 55°41’, E 21°30′ (Vėžaičiai, Klaipėda dist.) | Bathygleyic Distric Glossic Retisol | 4.35 ± 0.2 | 1.08 ± 0.13 | 0.08. ± 0.01 | immature spontaneous forest (~25 years birch) | 0 |
| Month | Total Monthly Rainfall mm | Average Monthly Air Temperature °C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Long-Term Mean 1981–2010 | Year | Long-Term Mean 1981–2010 | |||||||||||||
| 2018 | 2019 | 2020 | 2018 | 2019 | 2020 | |||||||||||
| Vėž | Dot | Vėž | Dot | Vėž | Dot | Vėž | Dot | Vėž | Dot | Vėž | Dot | Vėž | Dot | Vėž | Dot | |
| March | 25.5 | 17.3 | 85.9 | 37.8 | 54.5 | 31.7 | 57.3 | 34.8 | −1.8 | −1.9 | 2.7 | 3.3 | 3.2 | 3.5 | 0.6 | 1.9 |
| April | 47.9 | 52.1 | 1.4 | 0.0 | 6.9 | 9.4 | 37.4 | 31.1 | 8.8 | 9.9 | 8.9 | 8.9 | 6.3 | 6.8 | 6.3 | 8.2 |
| May | 38.8 | 40.2 | 78.0 | 55.4 | 26.9 | 50.1 | 47.7 | 48.8 | 16.5 | 16.9 | 11.5 | 12.9 | 10.0 | 10.6 | 11.7 | 13.8 |
| June | 35.5 | 34.1 | 37.9 | 16.1 | 90.6 | 165.9 | 71.1 | 63.9 | 16.3 | 17.5 | 19.1 | 20.6 | 17.8 | 18.9 | 14.7 | 17.0 |
| July | 61.2 | 83.3 | 99.4 | 66.0 | 80.9 | 65.6 | 84.3 | 75.9 | 20.1 | 20.5 | 16.5 | 17.3 | 16.4 | 17.4 | 17.3 | 18.0 |
| August | 67.7 | 37.0 | 29.8 | 107.0 | 74.4 | 47.7 | 97.0 | 62.6 | 18.7 | 19.5 | 17.5 | 18.2 | 18.2 | 18.5 | 16.8 | 18.0 |
| September | 92.2 | 19.1 | 104.0 | 48.5 | 48.6 | 14.8 | 98.7 | 44.6 | 14.4 | 15.0 | 12.9 | 12.8 | 14.7 | 15.0 | 12.2 | 14.2 |
| Overall | 368.8 | 283.1 | 436.4 | 330.8 | 382.8 | 385.2 | 493.5 | 361.7 | 13.3 | 13.9 | 12.7 | 13.4 | 12.4 | 12.9 | 11.4 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilkiene, M.; Mockeviciene, I.; Kadziene, G.; Karcauskiene, D.; Repsiene, R.; Auskalniene, O. Bacterial Communities: Interaction to Abiotic Conditions under Effect of Anthropogenic Pressure. Sustainability 2023, 15, 11366. https://doi.org/10.3390/su151411366
Vilkiene M, Mockeviciene I, Kadziene G, Karcauskiene D, Repsiene R, Auskalniene O. Bacterial Communities: Interaction to Abiotic Conditions under Effect of Anthropogenic Pressure. Sustainability. 2023; 15(14):11366. https://doi.org/10.3390/su151411366
Chicago/Turabian StyleVilkiene, Monika, Ieva Mockeviciene, Grazina Kadziene, Danute Karcauskiene, Regina Repsiene, and Ona Auskalniene. 2023. "Bacterial Communities: Interaction to Abiotic Conditions under Effect of Anthropogenic Pressure" Sustainability 15, no. 14: 11366. https://doi.org/10.3390/su151411366






