Diversity of Agro-Biological Traits and Development of Diseases in Alfalfa Cultivars during the Contrasting Vegetation Seasons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material and Research Site
2.2. Treatments and Experimental Design
2.3. Evaluation of Agro-biological Traits in Experimental Fields of Alfalfa
2.4. Statistical Analysis
2.5. Meteorological Conditions during Experimental Year
3. Results
3.1. Wintering and the Height of Alfalfa Cultivars at the Spring Regrowth and before Flowering
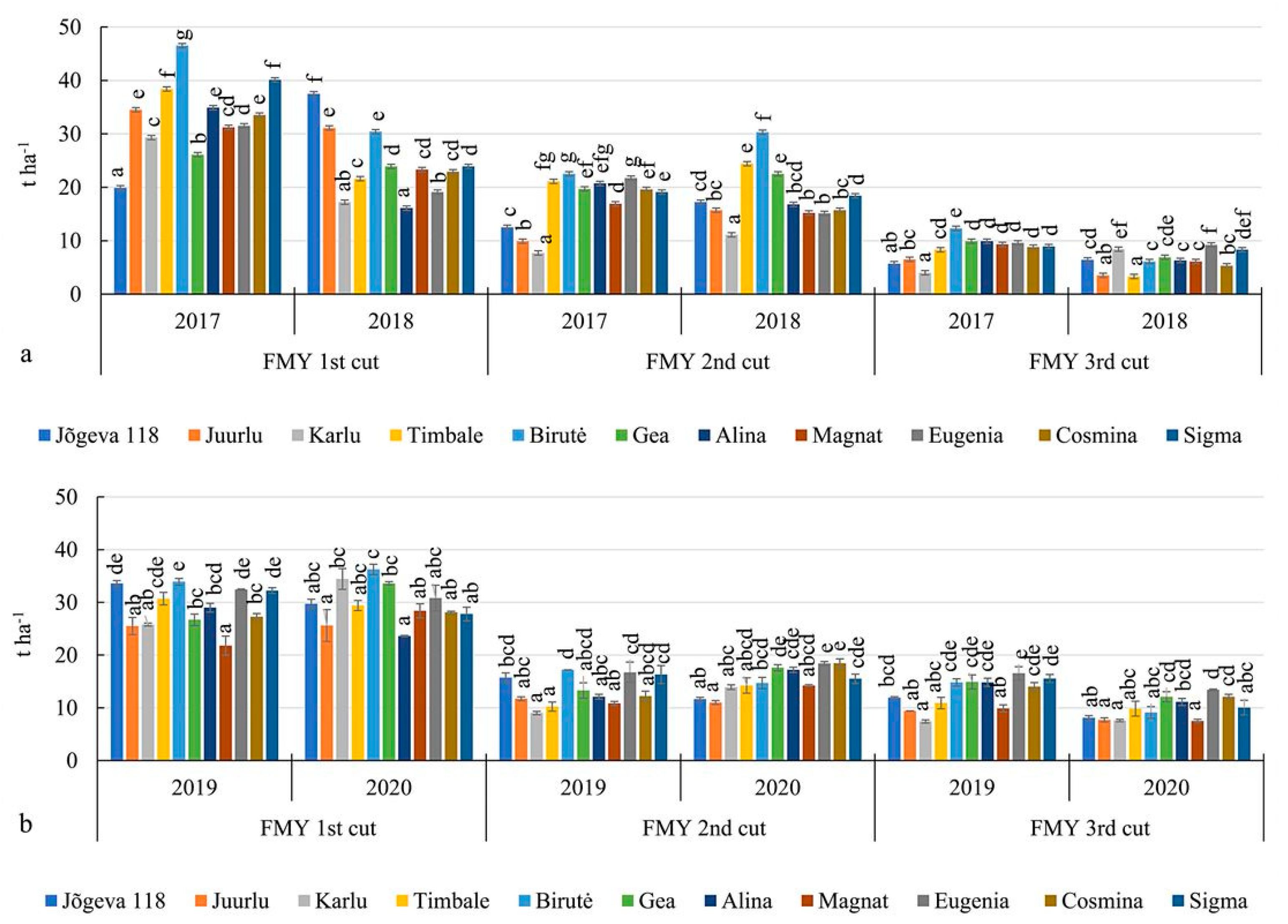
3.2. The Fresh and Dry Matter Yields of Alfalfa Cultivars
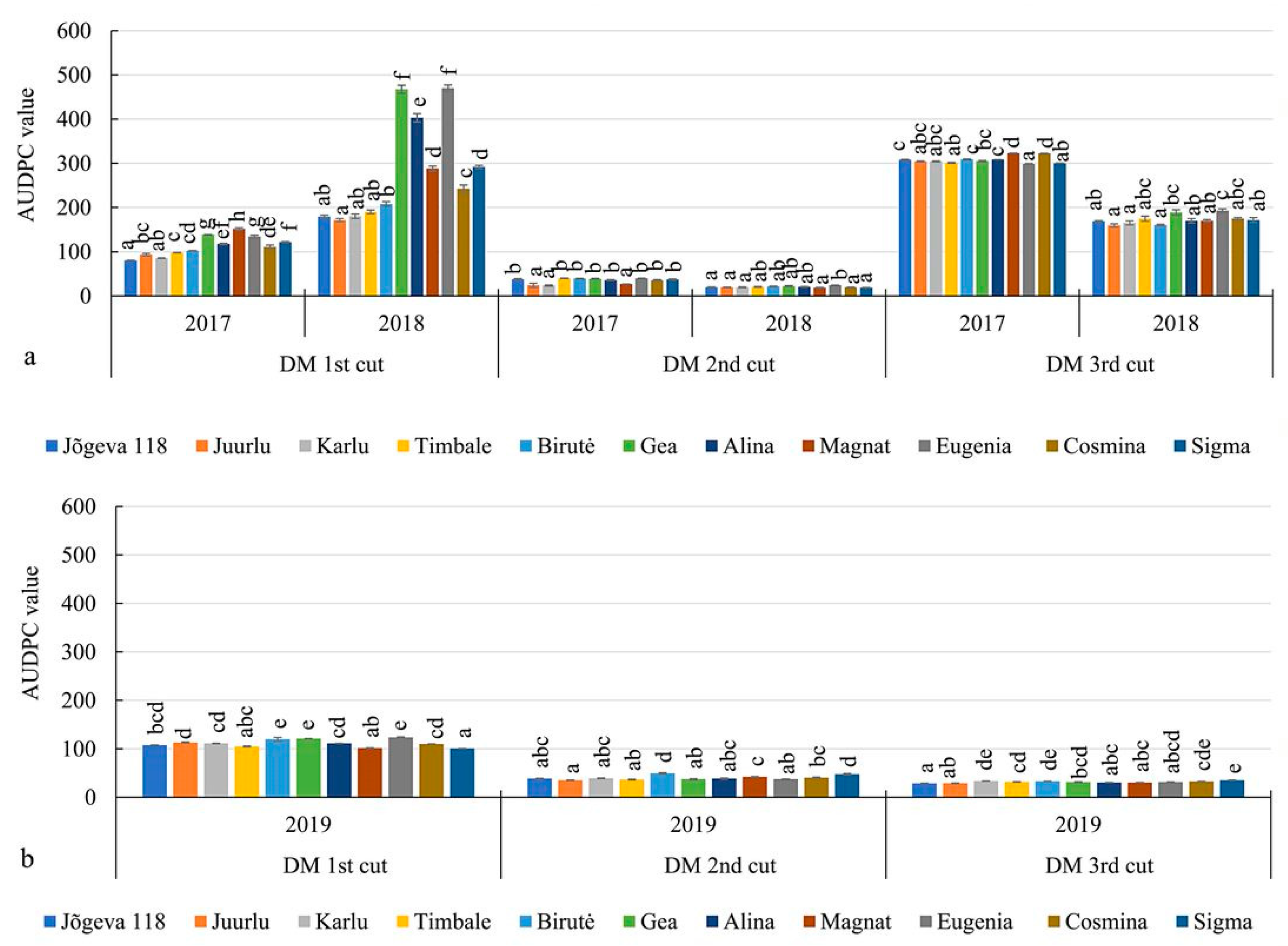
3.3. The Development of Spring Black Stem Leaf Spot and Downy Mildew in Alfalfa Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakić, Ž.; Antic, M.; Djurdjic, I.; Popovic, V. Morphological characteristics of alfalfa genotypes tolerant to low pH. Genetika 2019, 51, 907–922. [Google Scholar] [CrossRef] [Green Version]
- Tucak, M.; Čupić, T.; Horvat, D.; Popović, S.; Krizmanić, G.; Ravlić, M. Variation of Phytoestrogen Content and Major Agronomic Traits in Alfalfa (Medicago sativa L.) Populations. Agronomy 2020, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.; Fageria, N.K. Liming influence on soil chemical properties, nutritional status and yield of alfalfa grown in acid soil. Rev. Bras. Cienc. Solo 2010, 34, 1231–1239. [Google Scholar] [CrossRef]
- Bouton, J.H. Breeding lucerne for persistence. Crops Pasture Sci. 2012, 63, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Sabanci, C.O.; Ertus, M.M.; Zorer, C.S. Collection, conservation, and evaluation for forage yield of alfalfa landraces grown in East Anatolia. Turk. J. Field Crops 2013, 18, 46–51. [Google Scholar]
- Reich, J. Alfalfa’s role in feeding a hungry world. In Proceedings of the 42nd California Alfalfa and Forage Symposium, Sacramento, CA, USA, 10–12 December 2012; Putnam, D., Ed.; Department of Agronomy and Range Science Extension, University of California: Davis, CA, USA, 2012. [Google Scholar]
- Otero, A.; Castro, M. Variability of Alfalfa (Medicago sativa L.) Seasonal Forage Production in the Southwest of Uruguay. Agrocienc. Urug. 2019, 23, e65. [Google Scholar] [CrossRef]
- Bélanger, G.; Rochette, P.; Castonguay, Y.; Bootsma, A.; Mongrain, D.; Ryan, D.A.J. Climate Change and Winter Survival of Perennial Forage Crops in Eastern Canada. Agron. J. 2002, 94, 1120–1130. [Google Scholar] [CrossRef]
- Bélanger, G.; Castonguay, Y.; Bertrand, A.; Dhont, C.; Rochette, P.; Couture, L.; Drapeau, R.; Mongrain, D.; Chalifour, F.P.; Michaud, R. Winter damaged to perennial forage crops in eastern Canada: Causes, mitigation, and prediction. Can. J. Plant. Sci. 2006, 86, 33–47. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Tong, Z.; He, F.; Li, X. Metabolomic analyses reveal substances that contribute to the increased freezing tolerance of alfalfa (Medicago sativa L.) after continuous water deficit. BMC Plant Biol. 2020, 20, 15. [Google Scholar] [CrossRef]
- Xu, H.; Tong, Z.; He, F.; Li, X. Response of Alfalfa (Medicago sativa L.) to Abrupt Chilling as Reflected by Changes in Freezing Tolerance and Soluble Sugars. Agronomy 2020, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-J.; Zhong, T.-X.; Xu, L.-X.; Han, L.-B.; Zhang, X. Water Deficit Irrigation Impacts on Antioxidant Metabolism Associated with Freezing Tolerance in Creeping Bentgrass. J. Am. Soc. Hortic. Sci. 2015, 140, 323–332. [Google Scholar] [CrossRef]
- Castonguay, Y.; Laberge, S.; Brummer, E.C.; Volenec, J.J. Alfalfa Winter Hardiness: A Research Retrospective and Integrated Perspective. Adv. Agron. 2006, 90, 203–265. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wan, L.; Nie, Z.; Li, X. Fractal and Topological Analyses and Antioxidant Defense Systems of Alfalfa (Medicago sativa L.) Root System under Drought and Rehydration Regimes. Agronomy 2020, 10, 805. [Google Scholar] [CrossRef]
- Kavut, Y.T.; Avcioglu, R. Yield and quality performances of various alfalfa (Medicago sativa L.) cultivars in different soil textures in a mediterranean environment. Turk. J. Field Crops 2015, 20, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Morsy, K.M.; Abdel-Moniam, M.F.; Mazen, M.M. Use of abiotic and biotic inducers in controlling fungal diseases and im-proving growth in alfalfa. Aust. J. Basic. Appl. Sci. 2011, 5, 816–824. [Google Scholar]
- Barbetti, M.J.; Riley, I.T.; You, M.; Li, H.; Sivasithamparam, K. The association of necrotrophic fungal pathogens and plant parasitic nematodes with the loss of productivity of annual medic-based pastures in Australia and options for their management. Australas. Plant Pathol. 2006, 35, 691–706. [Google Scholar] [CrossRef]
- Fan, Q.; Creamer, R.; Li, Y. Time-course metabolic profiling in alfalfa leaves under Phoma medicaginis infection. PLoS ONE 2018, 13, e0206641. [Google Scholar] [CrossRef] [PubMed]
- Djebali, N. Aggressiveness and host range of Phoma medicaginis isolated from Medicago species growing in Tunisia. Phytopathol. Mediterr. 2013, 52, 3–15. [Google Scholar]
- Jie, L.C.; Biao, N.Z.; Wen, W.Y.; Wang, Y.R. Evaluation for alfalfa germplasm resistance to downy mildew under Alpine Grassland conditions. Acta. Prat. Sin. 2000, 9, 44–51. [Google Scholar]
- Mario, C.M.; Lira, Z.C. Losses caused by downy mildew (Peronospora trifoliorum) in alfalfa (Medicago sativa L.) growing on the Northern Altiplano, Bolivia. J. Agri. Sci. Food. Res. 2018, 9, 241. [Google Scholar]
- Nan, Z.B. Alfalfa disease and its comprehensive control system in China. Anim. Sci. Vet. Med. 2001, 18, 81–84. [Google Scholar]
- Tucak, M.; Popović, S.; Čupić, T.; Krizmanić, G.; Španić, V.; Šimić, B.; Megalič, V. Agro-Morphological and Forage Quality Traits of Selected Alfalfa Populations and Their Application in Breeding. Turk. J. Field Crops 2014, 19, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Strbanovic, R.; Stanisavljević, R.; Ðukanović, L.; Postić, D.; Marković, J.; Gavrilović, V.; Dolovać, N. Variability and correlation of yield and forage quality in Alfalfa varieties of different origin. J. Agr. Sci. 2017, 23, 128–137. [Google Scholar]
- Çınar, S.; Hatipoglu, R. Forage Yield and Botanical Composition of Mixtures of Some Perennial Warm Season Grasses with Alfalfa (Medicago sativa L.) Under Mediterranean Condi. Turk. J. Field Crops 2014, 19, 13–18. [Google Scholar] [CrossRef] [Green Version]
- WRB. World Reference Base for Soil Resources 2014; World Soil Resources Reports; FAO: Rome, Italy, 2014; pp. 187–189. [Google Scholar]
- Simko, I.; Piepho, H.-P. The Area Under the Disease Progress Stairs: Calculation, Advantage, and Application. Phytopathology 2012, 102, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ma, B.L.; Yan, X.; Han, J.; Guo, Y.; Wang, Y.; Li, P. Yields of Alfalfa Varieties with Different Fall-Dormancy Levels in a Temperate Environment. Agron. J. 2009, 101, 1146–1152. [Google Scholar] [CrossRef]
- Djaman, K.; O’Neill, M.; Lauriault, L.; Marsalis, M.; Koudahe, K.; Darapuneni, M.K. The Dynamics of Forage Yield of Different Fall Dormancy Rating Alfalfa Cultivars in a Semiarid Climate. Agric. Res. 2021, 10, 378–389. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, H.; Gai, Q.; Chen, H.; Zhao, M. Test and comprehensive assessment on the performance of 22 alfalfa varieties. Acta Pratacul. Sin. 2011, 20, 219–229. [Google Scholar]
- Atumo, T.T.; Kauffman, R.; Talore, D.G.; Abera, M.; Tesfaye, T.; Tunkala, B.Z.; Zeleke, M.; Kalsa, G.K. Adaptability, forage yield and nutritional quality of alfalfa (Medicago sativa L.) genotypes. Environ. Sustain. 2021, 1, 1895475. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Diriba, G.; Mekonnen, H.; Ashenafi, M.; Adugna, T. Biomass yield potential and nutritive value of selected Alfalfa (Medicago sativa) cultivars grown under tepid to cool sub-moist agro-ecology of Ethiopia. J. Agric. Res. Develop. 2014, 4, 7–14. [Google Scholar]
- Arab, S.A.; El Shal, M.H.; Hamed, N.M. Evaluation of Some Alfalfa (Medicago sativa L.) Germplasm for Yield and Yield Component Traits. Egypt. J. Agron. 2015, 37, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Djaman, K.; Owen, C.; Koudahe, K.; O’Neill, M. Evaluation of Different Fall Dormancy-Rating Alfalfa Cultivars for Forage Yield in a Semiarid Environment. Agronomy 2020, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Tucak, M.; Popovic, S.; Grljusic, S.; Cupic, T.; Kozumplik, V.; Simic, B. Variability and relationships of important alfalfa germplasm agronomic traits. Period. Biol. 2008, 110, 311–315. [Google Scholar]
- Abdel-Galil, M.M.; Hamed, N.M. Evaluation of yield potential, genetic variances and correlation for nine cultivars of alfalfa under the new valley environment. J. Agric. Sci. Mansoura. Univ. 2008, 33, 4681–4686. [Google Scholar] [CrossRef]
- Seiam, M.A.; Mohamed, E.S. Forage yield, quality characters and genetic variability of some promising egyptian clover populations. Egypt. J. Plant. Breed. 2020, 24, 839–858. [Google Scholar] [CrossRef]
- Veronesi, F.; Brummer, E.C.; Huyghe, C. Alfalfa. In Fodder Crops and Amenity Grasses; Handbook of Plant, Breeding; Boller, B., Posselt, U.K., Veronesi, F., Eds.; Springer: New York, NY, USA, 2010; Volume 5, pp. 395–437. [Google Scholar]
- Acvi, M.A.; Ozkose, A.; Tamkoc, A. Determination of yield and quality characteristics of alfalfa (Medicago sativa L.) varieties grown in different locations. J. Anim. Vet. Adv. 2013, 12, 487–490. [Google Scholar] [CrossRef]
- Luo, K.; Jahufer, M.Z.Z.; Wu, F.; Di, H.; Zhang, D.; Meng, X.; Zhang, J.; Wang, Y. Genotypic Variation in a Breeding Population of Yellow Sweet Clover (Melilotus officinalis). Front. Plant Sci. 2016, 7, 972. [Google Scholar] [CrossRef]
- Testa, G.; Gresta, F.; Cosentino, S.L. Dry matter and qualitative characteristics of alfalfa as affected by harvest times and soil water content. Eur. J. Agron. 2011, 34, 144–152. [Google Scholar] [CrossRef]
- Atis, I.; Celiktas, N.; Can, E.; Yilmaz, S. The effects of cutting intervals and seeding rates on forage yield and quality of alfalfa. Turk. J. Field Crops 2019, 24, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Borreani, G.; Odoardi, M.; Reyneri, A.; Tabacco, E. Effect of cutting height and stage of development on lucerne quality in the Po plain. Ital. J. Agron. 2006, 1, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Geren, H.; Kavut, Y.T.; Unlu, H.B. Effect of different cutting intervals on the forage yield and some silage quality characteristics of giant king grass (Pennisetum hybridum) under mediterranean climatic conditions. Turk. J. Field Crops 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Monirifar, H. Path Analysis of Yield and Quality Traits in Alfalfa. Not. Bot. Horti. Agrobo. 2011, 39, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Takawale, P.S.; Jade, S.S.; Bahulikar, R.A.; Desale, J.S. Diversity in Lucerne (Medicago sativa L.) germplasm for morphology, yield and molecular markers and their correlations. Indian. J. Genet. 2019, 79, 453–459. [Google Scholar] [CrossRef]
- Liatukiene, A.; Skuodiene, R.; Tomchuk, D.; Danyte, V. Evaluation of agro-biological traits of Medicago sativa and M. varia in a Cambisol and Retisol. Zemdirbyste 2020, 107, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Clark, A.J.; Roche, J.R. Climate-change effects and adaptation options for temperate pasture-based dairy farming systems: A review. Grass Forage Sci. 2013, 68, 485–503. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate Impacts on Agriculture: Implications for Crop Production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Fang, D.; Ameen, A.; Li, X.; Guo, K.; Liu, X.; Han, L. Dynamics of Spring Regrowth and Comparative Production Performance of 50 Autumn-Sown Alfalfa Cultivars in the Coastal Saline Soil of North China. Life 2021, 11, 1436. [Google Scholar] [CrossRef]
- Svirskis, A. Improving of Lucerne productivity by means of breeding. Zemdirbyste 2002, 78, 149–157. [Google Scholar]
- Annicchiarico, P. Diversity, genetic structure, distinctness and agronomic value of Italian lucerne (Medicago sativa L.). Euphytica 2006, 148, 269–282. [Google Scholar] [CrossRef]
- Castell-Miller, C.; Zeyen, R.; Samac, D. Infection and development of Phoma medicaginis on moderately resistant and susceptible alfalfa genotypes. Can. J. Plant Pathol. 2007, 29, 290–298. [Google Scholar] [CrossRef]
- Liatukienė, A.; Liatukas, Ž.; Ruzgas, V. Reaction of the Lithuanian alfalfa breeding populations to Phoma medicaginis under cool temperate climate conditions. Pak. J. Bot. 2015, 47, 919–925. [Google Scholar]
- Liatukienė, A.; Liatukas, Ž.; Schitea, M.; Ruzgas, V. Disease reaction of several Lithuanian and Romanian alfalfa cultivars. Rom. Agric.Res. 2013, 30, 99–108. [Google Scholar]
- Ellwood, S.R.; Kamphuis, L.G.; Oliver, R.P. Identification of Sources of Resistance to Phoma medicaginis Isolates in Medicago truncatula SARDI Core Collection Accessions, and Multigene Differentiation of Isolates. Phytopathology 2006, 96, 1330–1336. [Google Scholar] [CrossRef] [Green Version]
- Kamphuis, L.G.; Lichtenzveig, J.; Oliver, R.P.; Ellwood, S.R. Two alternative recessive quantitative trait loci influence resistance to spring black stem and leaf spot in Medicago truncatula. BMC Plant Biol. 2008, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Chang, K.F.; Gossen, B.D.; Turnbull, G.D.; Howard, R.J.; Hwang, S.F. Assessing resistance to spring black stem and leaf spot of alfalfa caused by Phoma spp. Can. J. Plant Sci. 2004, 84, 311–317. [Google Scholar] [CrossRef]
- Barbetti, M.J. Resistance in Annual Medicago spp. to Phoma medicaginis and Leptosphaerulina trifolii and Its Relationship to Induced Production of a Phytoestrogen. Plant Dis. 2007, 91, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Liatukiene, A.; Liatukas, Ž. Downy mildew reaction of alfalfa accessions of different geographical origin under Lithuanian conditions. Int. J. Agric. Biol. 2014, 16, 905–910. [Google Scholar]
- Yaege, J.R.; Stuteville, D.L. Reactions in the Annual Medicago Core Germ Plasm Collection to Two Isolates of Peronospora trifoliorum from Alfalfa. Plant Dis. 2000, 84, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Nagl, N.; Taski-Ajdukovic, K.; Barac, G.; Baburski, A.; Seccareccia, I.; Milic, D.; Katic, S. Estimation of the Genetic Diversity in Tetraploid Alfalfa Populations Based on RAPD Markers for Breeding Purposes. Int. J. Mol. Sci. 2011, 12, 5449–5460. [Google Scholar] [CrossRef] [PubMed]





| Month | 2016 Sowing Year | 2018 Sowing Year | ||||||
|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | |||||
| T, °C | P, mm | T, °C | P, mm | T, °C | P, mm | T, °C | P, mm | |
| January | −3.2 | 14.2 | −1.6 | 55.5 | −4.4 | 48.8 | 2.7 | 52.2 |
| February | −1.6 | 25.1 | −6.1 | 16.2 | 1.2 | 39.2 | 2.3 | 47.3 |
| March | 3.5 | 38.9 | −1.9 | 17.3 | 3.3 | 37.0 | 3.5 | 31.7 |
| April | 5.6 | 48.2 | 9.9 | 52.1 | 8.9 | 0.0 | 6.8 | 9.4 |
| May | 12.8 | 3.4 | 16.9 | 40.2 | 12.9 | 55.4 | 10.6 | 50.1 |
| June | 15.4 | 72.1 | 17.5 | 34.1 | 20.6 | 16.1 | 18.9 | 165.9 |
| July | 16.7 | 153.8 | 20.5 | 83.3 | 17.3 | 66.0 | 17.4 | 65.6 |
| August | 17.3 | 53.2 | 19.5 | 37.0 | 18.2 | 107.0 | 18.5 | 47.7 |
| September | 13.3 | 123.1 | 15.0 | 19.1 | 12.8 | 48.5 | 15.0 | 14.8 |
| October | 7.4 | 89.3 | 8.3 | 32.8 | 9.3 | 34.9 | 10.2 | 49.4 |
| November | 4.2 | 41.0 | 2.9 | 13.1 | 4.9 | 29.5 | 5.3 | 33.3 |
| December | 1.0 | 64.4 | −1.1 | 60.8 | 2.4 | 45.3 | 0.6 | 24.4 |
| Source | 2016 Sowing Year (2017–2018), p-Value | |||||
|---|---|---|---|---|---|---|
| df | W | SR | HF 1st Cut | HF 2nd Cut | HF 3rd Cut | |
| Cultivar | 10 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| Year | 1 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| Cultivar × Year | 10 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| 2018 sowing year (2019–2020), p-value | ||||||
| Cultivar | 10 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| Year | 1 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| Cultivar × Year | 10 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| Trait | 2016 sowing year | 2018 sowing year | ||||
| 2017 | 2018 | 2019 | 2020 | |||
| W, Score | 2.0 a | 2.5 b | 2.6 a | 2.9 b | ||
| SR cm | 19.1 a | 29.1 b | 33.4 b | 20.3 a | ||
| HF 1st cut, cm | 95.5 b | 79.3 a | 98.8 b | 95.1 a | ||
| HF 2nd cut, cm | 75.2 b | 63.4 a | 57.9 a | 68.3 a | ||
| HF 3rd cut, cm | 63.6 b | 50.8 a | 62.0 a | 57.4 a | ||
| Source | 2016 Sowing Year (2017–2018) p-Value | ||||||
|---|---|---|---|---|---|---|---|
| df | FMY 1st Cut | FMY 2nd Cut | FMY 3rd Cut | DMY 1st Cut | DMY 2nd Cut | DMY 3rd Cut | |
| Cultivar | 10 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0003 |
| Year | 1 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.6518 |
| Cultivar × Year | 10 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| 2018 sowing year (2019–2020) p-value | |||||||
| Cultivar | 10 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| Year | 1 | <0.1465 | <0.0000 | <0.0000 | <0.0000 | <0.3667 | <0.0000 |
| Cultivar × Year | 10 | <0.0000 | <0.0000 | <0.0064 | <0.0000 | <0.0000 | <0.0027 |
| Trait | 2016 sowing year | 2018 sowing year | |||||
| 2017 | 2018 | 2019 | 2020 | ||||
| FMY 1st cut, t ha−1 | 33.3 b | 24.3 a | 29.0 a | 29.8 a | |||
| FMY 2nd cut, t ha−1 | 17.4 a | 18.4 a | 13.2 a | 15.2 b | |||
| FMY 3rd cut, t ha−1 | 8.5 b | 6.3 a | 12.7 b | 9.9 a | |||
| DMY 1st cut, t ha−1 | 8.8 b | 7.0 a | 5.6 b | 4.8 a | |||
| DMY 2nd cut, t ha−1 | 2.3 a | 4.3 b | 3.1 a | 3.1 a | |||
| DMY 3rd cut, t ha−1 | 2.2 a | 2.2 a | 2.9 b | 2.4 a | |||
| Source | 2016 Sowing Year (2017–2018) p-Value | ||||||
|---|---|---|---|---|---|---|---|
| df | SBSLS 1st Cut | SBSLS 2nd Cut | SBSLS 3rd Cut | DM 1st Cut | DM 2nd Cut | DM 3rd Cut | |
| Cultivar | 10 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| Year | 1 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| Cultivar × Year | 10 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| 2018 sowing year (2019–2020) p-value | |||||||
| Cultivar | 10 | <0.0081 | <0.0002 | <0.1324 | <0.0000 | <0.0000 | <0.0000 |
| Year | 1 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| Cultivar × Year | 10 | <0.0125 | <0.0023 | <0.0300 | <0.0000 | <0.0000 | <0.0000 |
| Trait | 2016 sowing year | 2018 sowing year | |||||
| 2017 | 2018 | 2019 | 2020 | ||||
| SBSLS at the 1st cut, AUDPC value | 1037.9 b | 353.6 a | 254.8 a | 319.4 b | |||
| SBSLS at the 2nd cut, AUDPC value | 300.4 b | 184.3 a | 127.3 a | 293.5 b | |||
| SBSLS at the 3rd cut, AUDPC value | 476.1 b | 210.8 a | 324.5 a | 438.5 b | |||
| DM at the 1st cut, AUDPC value | 112.2 a | 281.2 b | 111.2 b | 0.0 a | |||
| DM at the 2nd cut, AUDPC value | 34.4 b | 20.5 a | 40.1 b | 0.0 a | |||
| DMat the 3rd cut, AUDPC value | 307.7 b | 172.5 a | 31.4 b | 0.0 a | |||
| Traits | SR | HF1 | HF2 | HF3 | FMY2 | FMY3 | DMY1 | DMY2 | DMY3 | DM1 | DM2 | DM3 | SBSLS2 | SBSLS3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W | 0.376 | 0.26 | 0.419 | 0.547 | 0.243 | 0.75 ** | −0.102 | 0.219 | 0.825 ** | 0.699 * | 0.519 | 0.425 | 0.081 | 0.118 |
| SR | 0.917 ** | 0.846 ** | 0.849 ** | 0.912 ** | 0.631 * | 0.295 | 0.847 ** | 0.42 | 0.545 | 0.822 ** | 0.448 | 0.49 | 0.028 | |
| HF1 | 0.782 ** | 0.747 ** | 0.822 ** | 0.654 * | 0.382 | 0.752 ** | 0.439 | 0.463 | 0.682 * | 0.378 | 0.381 | −0.013 | ||
| HF2 | 0.859 ** | 0.726 * | 0.673 * | 0.149 | 0.552 | 0.48 | 0.668 * | 0.481 | 0.418 | 0.227 | 0.281 | |||
| HF3 | 0.654 * | 0.665 * | −0.028 | 0.541 | 0.53 | 0.795 ** | 0.68 * | 0.435 | 0.218 | 0.167 | ||||
| FMY1 | 0.507 | 0.048 | 0.958 ** | 0.563 | −0.105 | −0.455 | 0.113 | −0.501 | 0.654 * | −0.525 | ||||
| FMY2 | 0.569 | 0.541 | 0.96 ** | 0.335 | 0.283 | 0.778 ** | 0.155 | 0.727 * | −0.237 | |||||
| FMY3 | 0.165 | 0.487 | 0.953 ** | 0.725 * | 0.571 | 0.387 | 0.245 | −0.064 | ||||||
| DMY1 | 0.575 | 0.029 | −0.296 | 0.173 | −0.341 | 0.632 * | −0.392 | |||||||
| DMY2 | 0.286 | 0.131 | 0.821 ** | 0.083 | 0.779 ** | −0.347 | ||||||||
| DMY3 | 0.711 * | 0.447 | 0.409 | 0.068 | −0.007 | |||||||||
| DM1 | 0.466 | 0.642 * | −0.059 | 0.277 | ||||||||||
| DM3 | −0.36 | 0.665 * | ||||||||||||
| SBSLS2 | −0.747 ** |
| Traits | SR | HF1 | HF2 | HF3 | FMY2 | FMY3 | DMY1 | DMY2 | DMY3 | DM3 | SBSLS3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W | 0.564 | 0.646 * | 0.681 * | 0.709 * | 0.363 | 0.496 | 0.355 | 0.427 | 0.559 | -0.18 | 0.372 |
| SR | 0.733 * | 0.859 ** | 0.882 ** | 0.658 * | 0.772 ** | 0.738 ** | 0.703 * | 0.868 ** | 0.213 | −0.266 | |
| HF1 | 0.705 * | 0.684 * | 0.477 | 0.51 | 0.733 * | 0.495 | 0.605 * | 0.409 | −0.145 | ||
| HF2 | 0.945 ** | 0.707 * | 0.865 ** | 0.763 ** | 0.748 ** | 0.934 ** | 0.121 | −0.029 | |||
| HF3 | 0.601 | 0.785 ** | 0.733 ** | 0.668 * | 0.876 ** | 0.152 | −0.068 | ||||
| FMY1 | 0.478 | 0.302 | 0.385 | 0.384 | 0.19 | 0.357 | 0.177 | ||||
| FMY2 | 0.934 ** | 0.778 ** | 0.988 ** | 0.875 ** | 0.312 | 0.214 | |||||
| FMY3 | 0.743 ** | 0.94 ** | 0.978 ** | 0.216 | 0.091 | ||||||
| DMY1 | 0.788 ** | 0.769 ** | 0.467 | −0.06 | |||||||
| DMY2 | 0.895 ** | 0.258 | 0.26 | ||||||||
| DM2 | 0.651 * | −0.103 | |||||||||
| SBSLS2 | −0.647 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liatukienė, A.; Norkevičienė, E.; Danytė, V.; Liatukas, Ž. Diversity of Agro-Biological Traits and Development of Diseases in Alfalfa Cultivars during the Contrasting Vegetation Seasons. Sustainability 2023, 15, 1445. https://doi.org/10.3390/su15021445
Liatukienė A, Norkevičienė E, Danytė V, Liatukas Ž. Diversity of Agro-Biological Traits and Development of Diseases in Alfalfa Cultivars during the Contrasting Vegetation Seasons. Sustainability. 2023; 15(2):1445. https://doi.org/10.3390/su15021445
Chicago/Turabian StyleLiatukienė, Aurelija, Eglė Norkevičienė, Vida Danytė, and Žilvinas Liatukas. 2023. "Diversity of Agro-Biological Traits and Development of Diseases in Alfalfa Cultivars during the Contrasting Vegetation Seasons" Sustainability 15, no. 2: 1445. https://doi.org/10.3390/su15021445





