Biosorption Potential of Desmodesmus sp. for the Sequestration of Cadmium and Lead from Contaminated Water
Abstract
1. Introduction
2. Materials and Methods
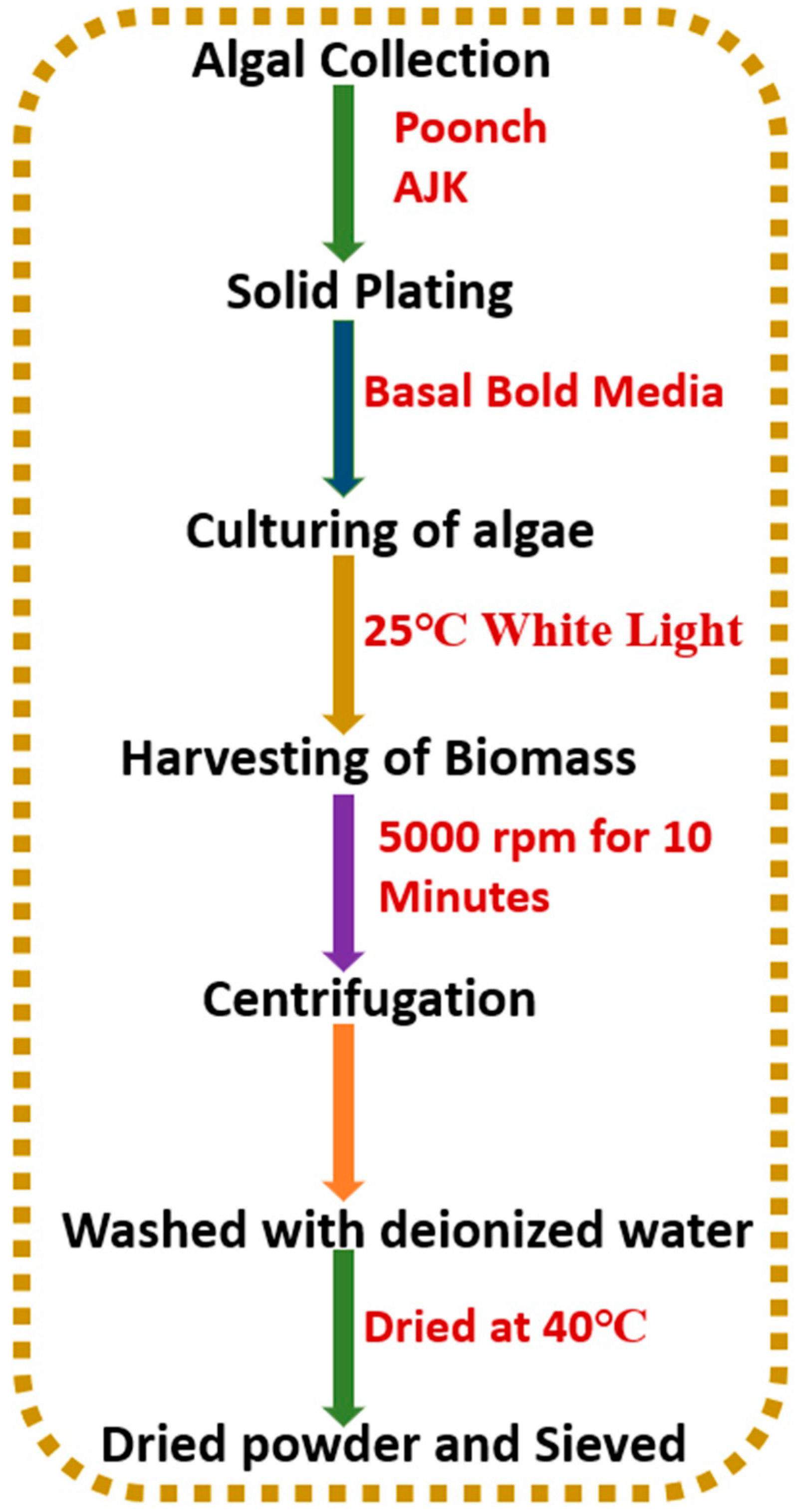
2.1. Preparation of Sorbent
2.2. Reagents Preparation
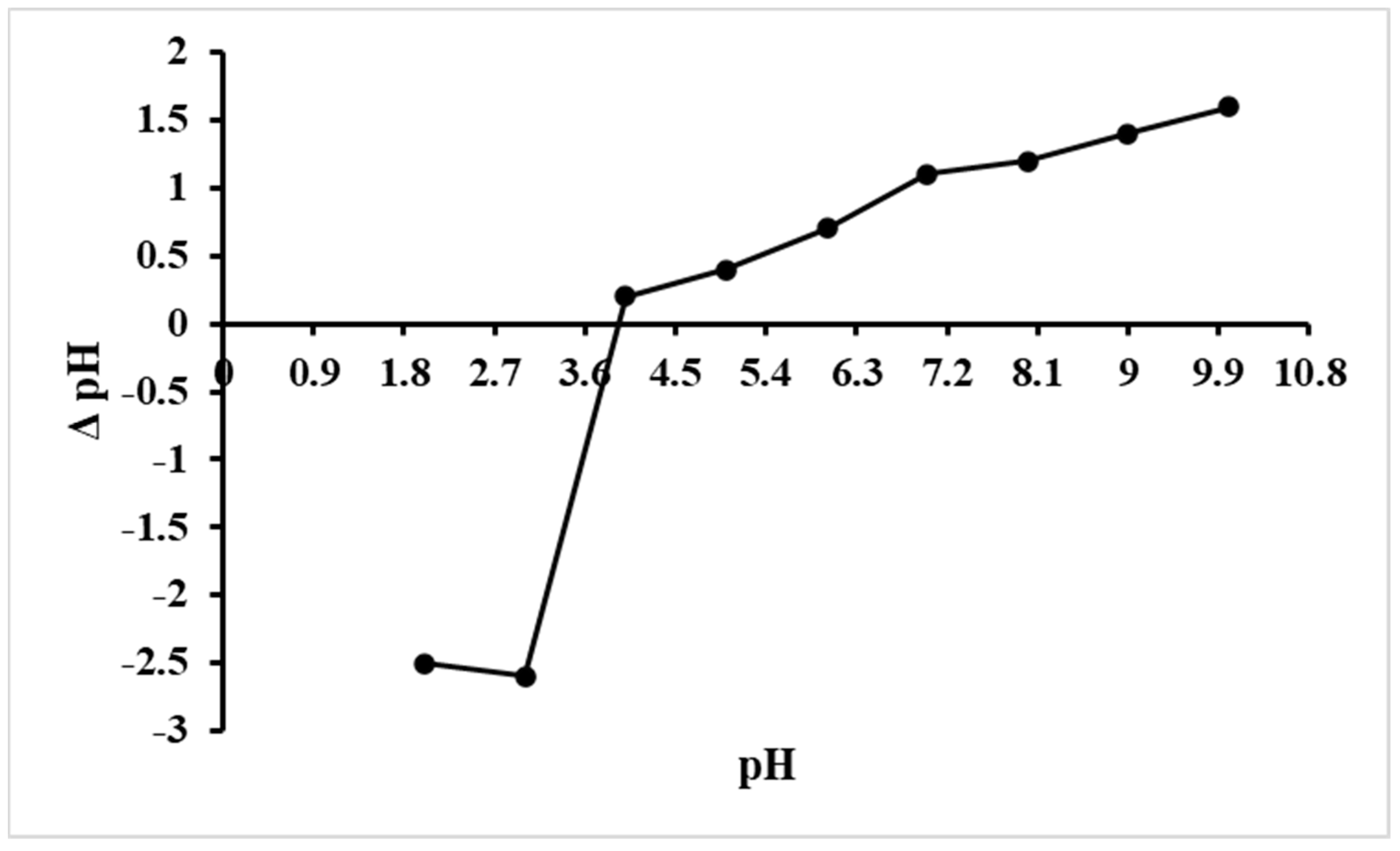
2.3. Point of Zero Charge
2.4. Biosorption Experiments
2.5. Analysis of Adsorption Kinetics
2.6. Adsorption Isotherms
2.7. Desorption of Cd and Pb Ions from Metal Loaded Adsorbent
2.8. Characterization
3. Results
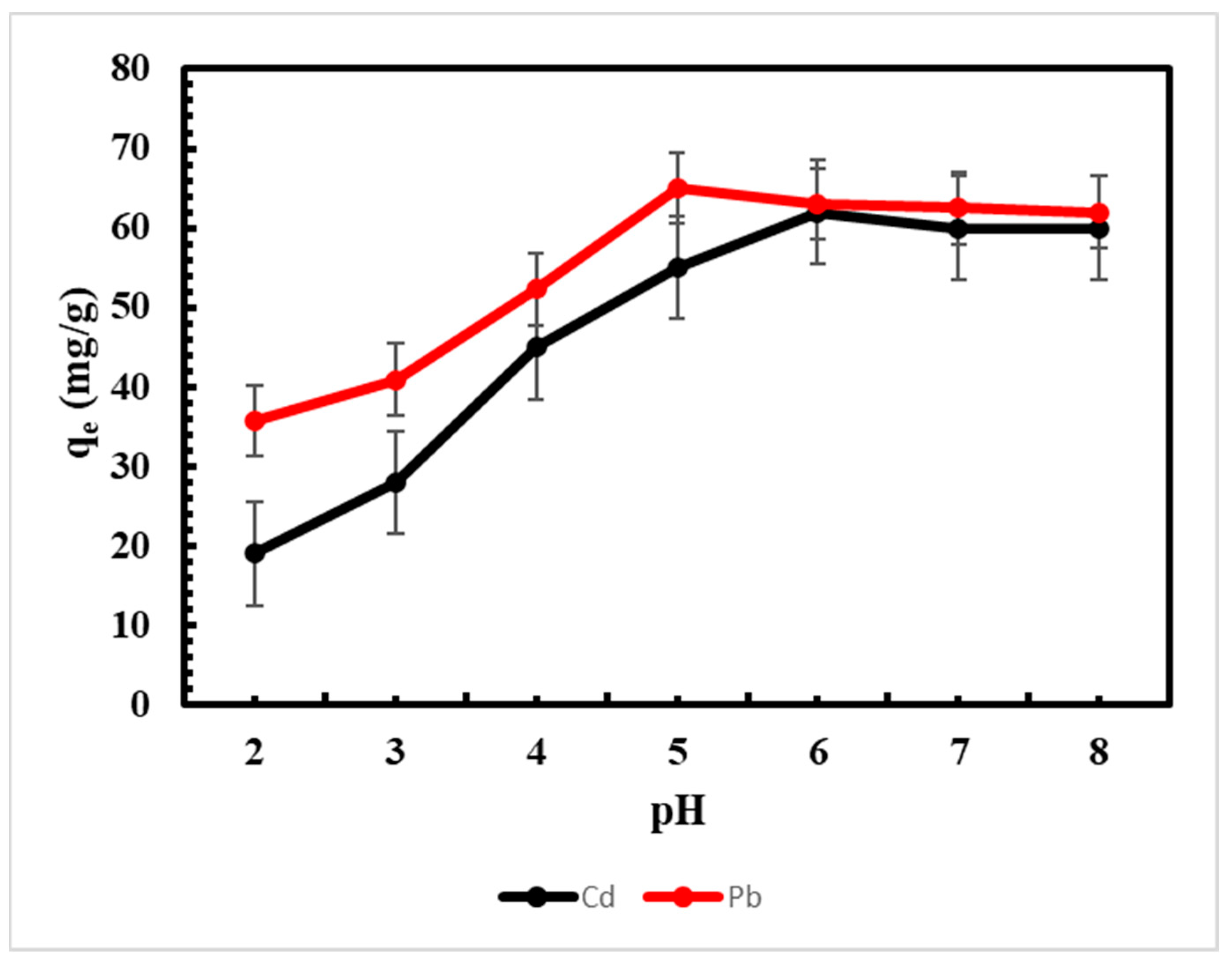
3.1. Effect of pH on Metal Adsorption
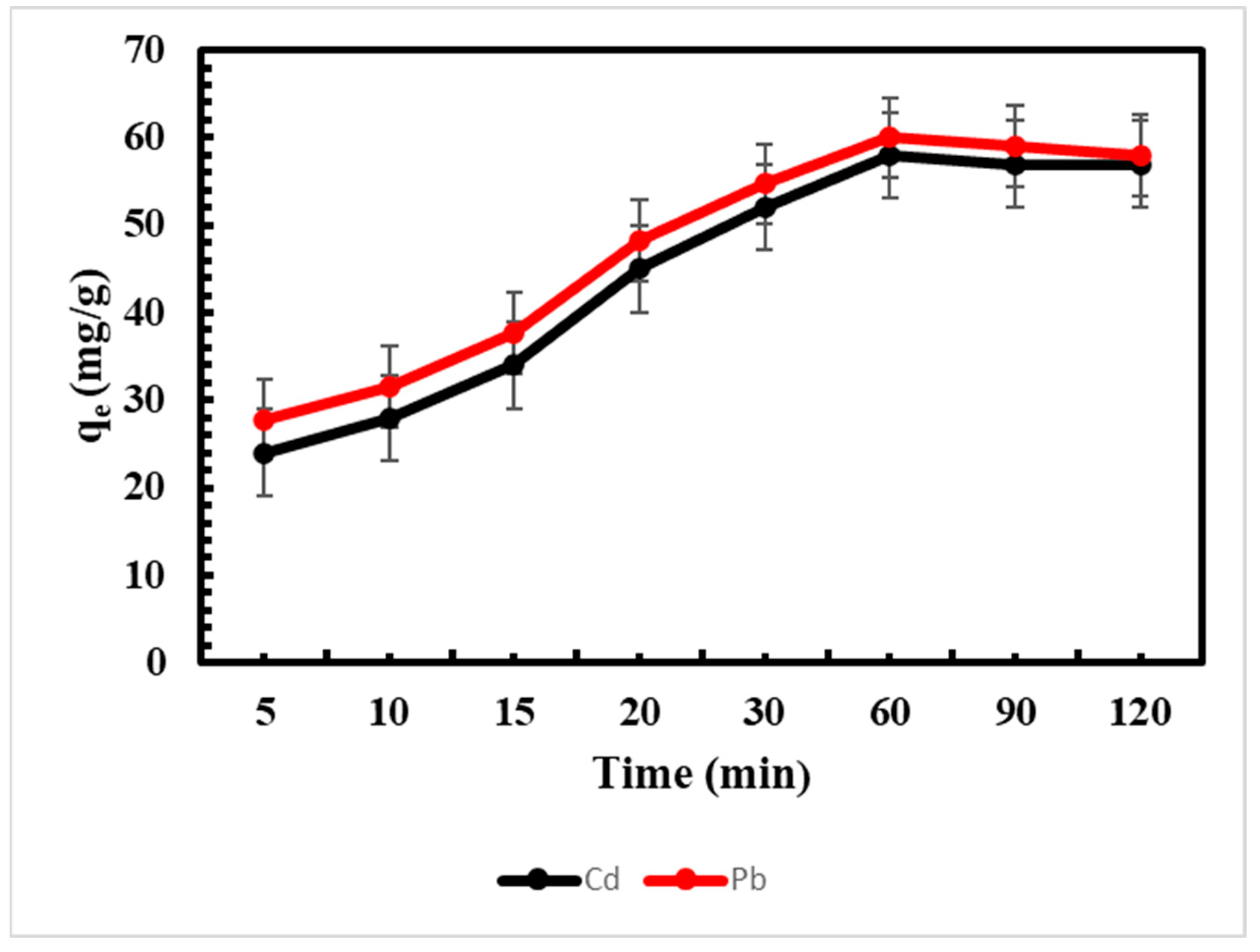
3.2. Effect of Contact Time on Biosorption of Metals Ions
3.3. Effect of Initial Concentration of Cd and Pb on Metal Adsorption
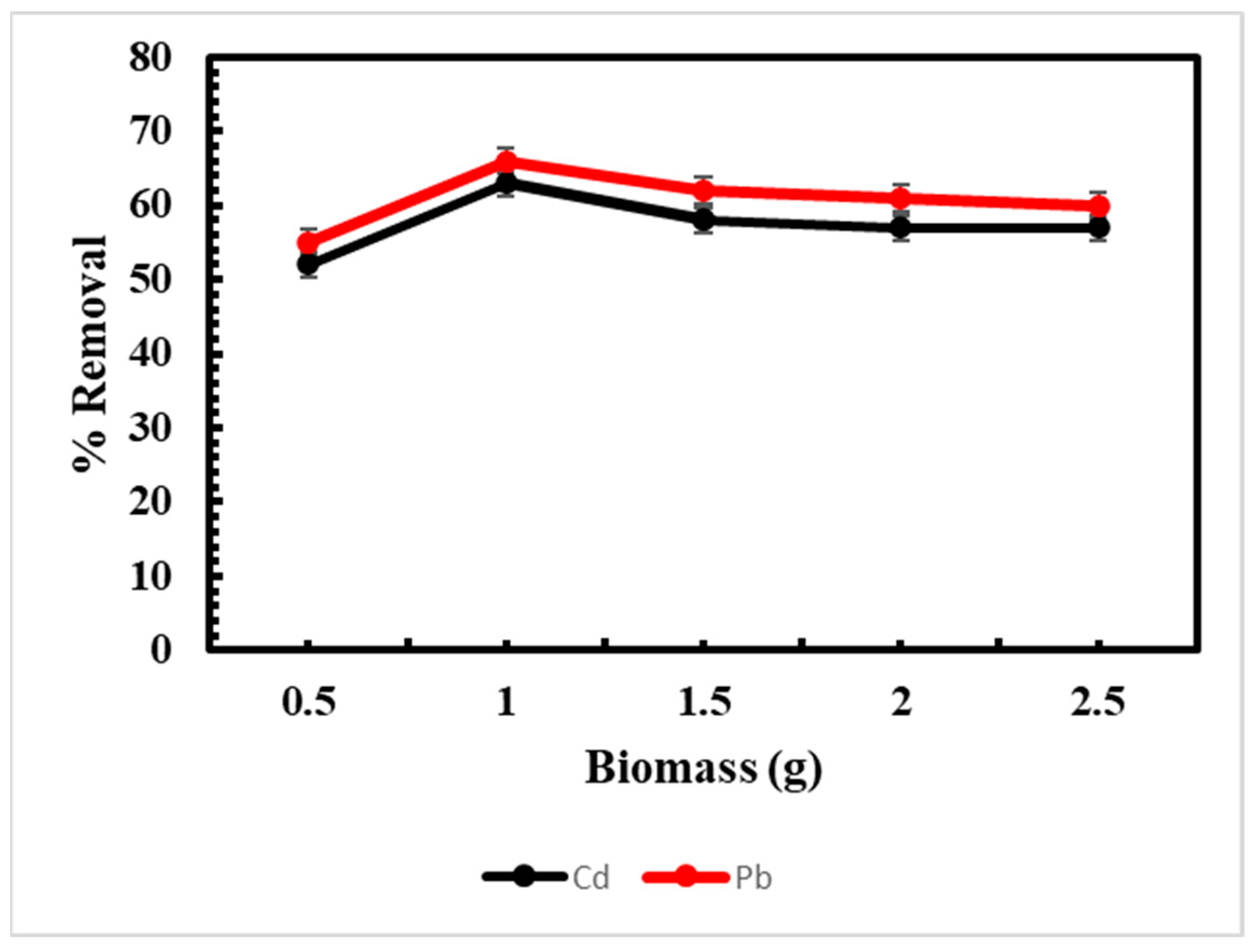
3.4. Effect of Biomass on Adsorption
3.5. Adsorption Kinetics
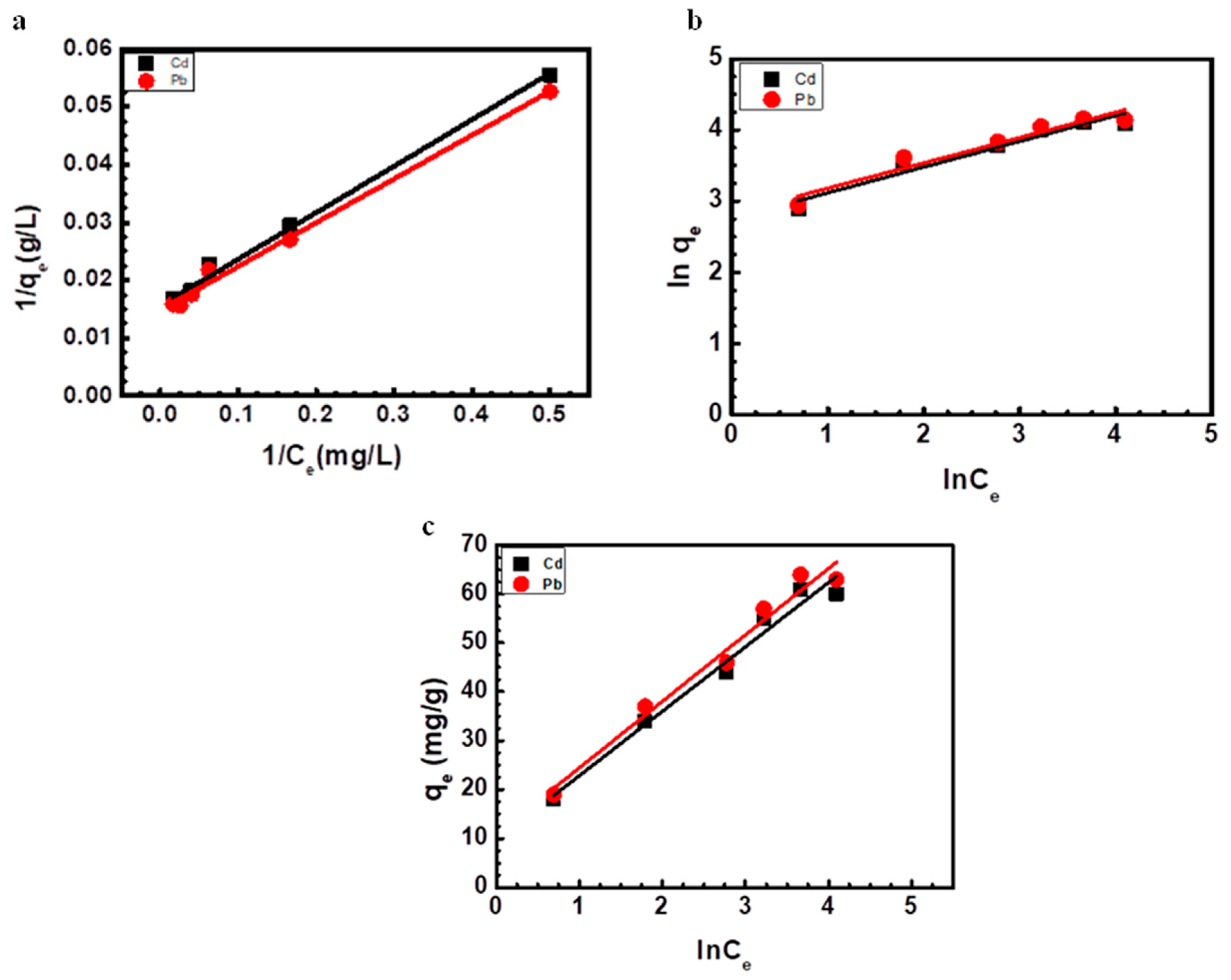
3.6. Adsorption Isotherms
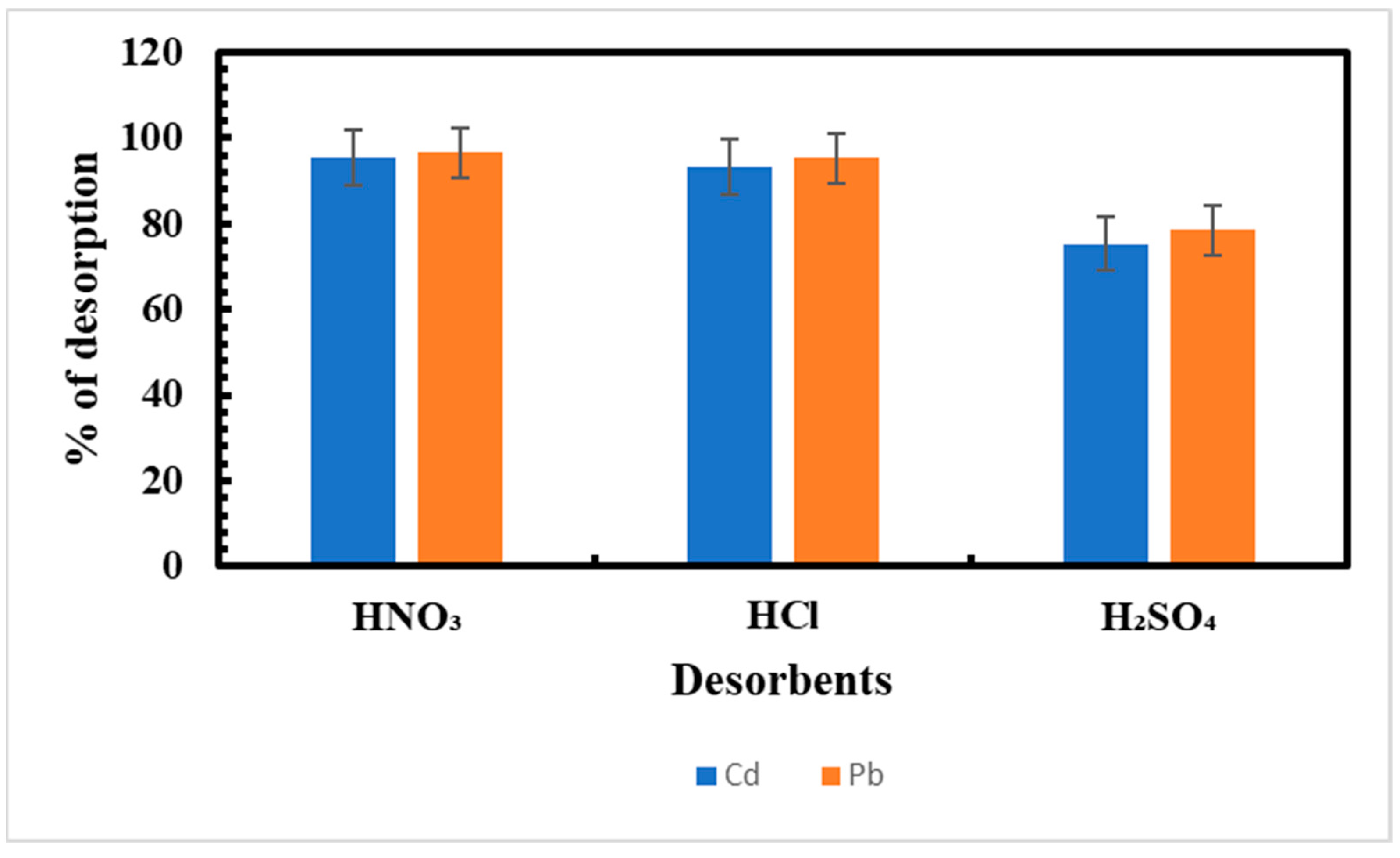
3.7. Desorption Studies
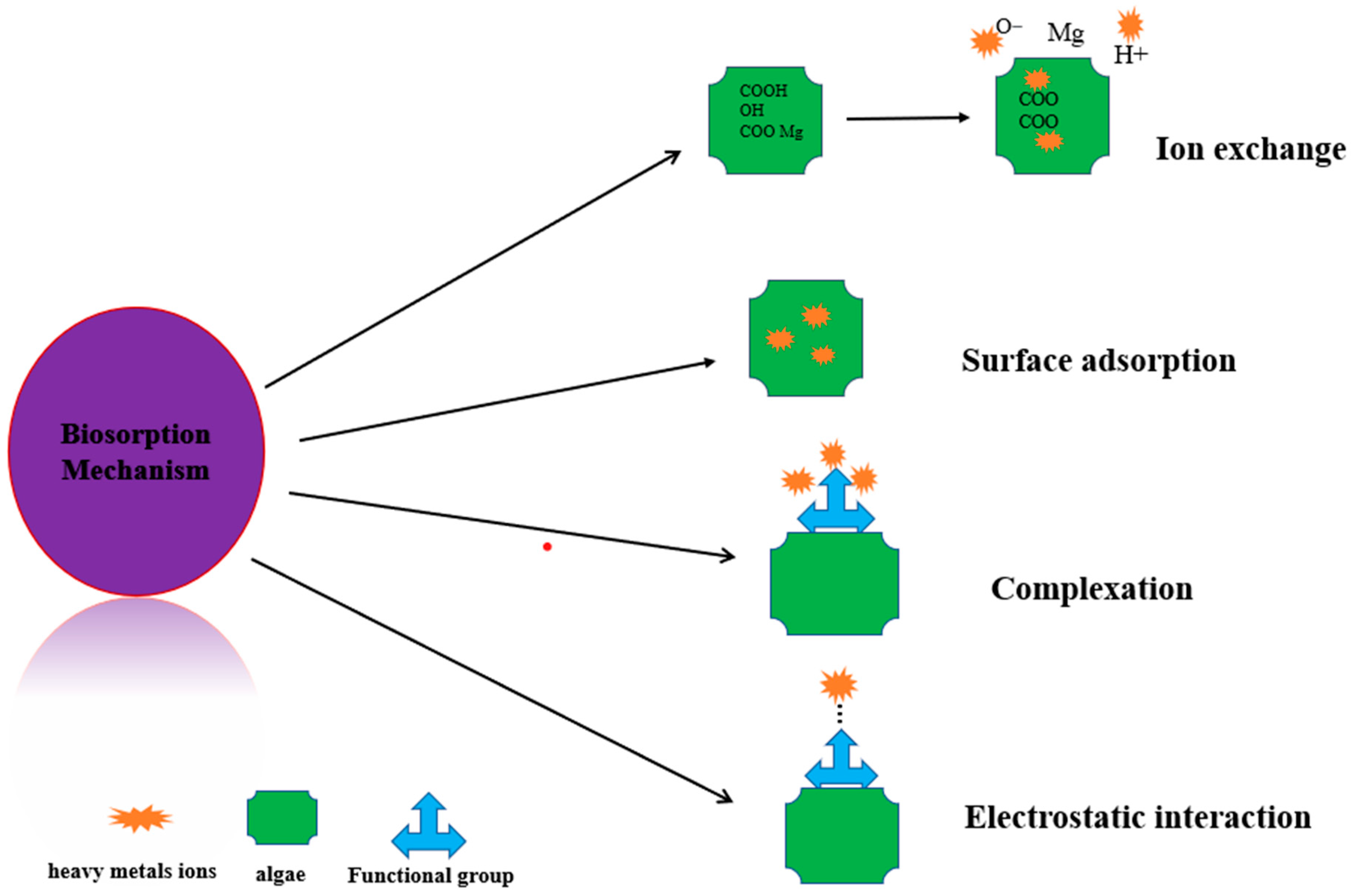
3.8. Adsorption Mechanism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, Y.; Wang, Y.; Lin, N.; Wang, R.; Wang, M.; Zhang, X. Iron-based materials for simultaneous removal of heavy metal (loid) s and emerging organic contaminants from the aquatic environment: Recent advances and perspectives. Environ. Pollut. 2022, 299, 118871. [Google Scholar] [CrossRef]
- Sharma, R.; Agrawal, P.R.; Kumar, R.; Gupta, G. Current scenario of heavy metal contamination in water. Contam. Water 2021, 49–64. [Google Scholar]
- Waheed, A.; Baig, N.; Ullah, N.; Falath, W. Removal of hazardous dyes, toxic metal ions and organic pollutants from wastewater by using porous hyper-cross-linked polymeric materials: A review of recent advances. J. Environ. Manag. 2021, 287, 112360. [Google Scholar] [CrossRef]
- Nateras-Ramírez, O.; Martinez-Macias, M.; Sánchez-Machado, D.; López-Cervantes, J.; Aguilar-Ruiz, R. An overview of microalgae for Cd2+ and Pb2+ biosorption from wastewater. Bioresour. Technol. Rep. 2021, 17, 100932. [Google Scholar] [CrossRef]
- Luo, H.; Gu, R.; Ouyang, H.; Wang, L.; Shi, S.; Ji, Y.; Bao, B.; Liao, G.; Xu, B. Cadmium exposure induces osteoporosis through cellular senescence, associated with activation of NF-κB pathway and mitochondrial dysfunction. Environ. Pollut. 2021, 290, 118043. [Google Scholar] [CrossRef]
- Njati, S.Y.; Maguta, M.M. Lead-based paints and children’s PVC toys are potential sources of domestic lead poisoning–A review. Environ. Pollut. 2019, 249, 1091–1105. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Al-Abbad, A.F.; Al-Hazzani, A.A.; Al-Ghanayem, A.A.; Alabdullatif, J.A. Lead removal by Spirulina platensis biomass. Int. J. Phytoremediation 2016, 18, 184–189. [Google Scholar] [CrossRef]
- Singh, K.; Singh, A.; Hasan, S. Low cost bio-sorbent ‘wheat bran’ for the removal of cadmium from wastewater: Kinetic and equilibrium studies. Bioresour. Technol. 2006, 97, 994–1001. [Google Scholar] [CrossRef]
- Cheng, J.; Yin, W.; Chang, Z.; Lundholm, N.; Jiang, Z. Biosorption capacity and kinetics of cadmium (II) on live and dead Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 211–221. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, X.; Liu, M.; Wu, Z.; Yang, L.; Xia, S.; Zhao, J. Adsorption of Pb (II) from aqueous solution to Ni-doped bamboo charcoal. J. Ind. Eng. Chem. 2013, 19, 353–359. [Google Scholar] [CrossRef]
- Chen, J.M.; Hao, O.J. Microbial chromium (VI) reduction. Crit. Rev. Environ. Sci. Technol. 1998, 28, 219–251. [Google Scholar] [CrossRef]
- Farooqi, H.M.U.; Khalid, M.A.U.; Kim, K.H.; Lee, S.R.; Choi, K.H. Real-time physiological sensor-based liver-on-chip device for monitoring drug toxicity. J. Micromechanics Microeng. 2020, 30, 115013. [Google Scholar] [CrossRef]
- Farooqi, H.M.U.; Kim, K.-H.; Kausar, F.; Muhammad, J.; Bukhari, H.; Choi, K.-H. Frequency and molecular characterization of Staphylococcus aureus from placenta of mothers with term and preterm deliveries. Life 2022, 12, 257. [Google Scholar] [CrossRef]
- Farooqi, H.M.U.; Sammantasinghar, A.; Kausar, F.; Farooqi, M.A.; Chethikkattuveli Salih, A.R.; Hyun, K.; Lim, J.-H.; Khalil, A.A.K.; Mumtaz, A.S.; Choi, K.H. Study of the Anticancer Potential of Plant Extracts Using Liver Tumor Microphysiological System. Life 2022, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, N.; Najafpour, G.D.; Khajouei, M. Macro and micro algae in pollution control and biofuel production–a review. ChemBioEng Rev. 2020, 7, 18–33. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Awwad, A.; Amer, M.; Al-aqarbeh, M. TiO2-kaolinite nanocomposite prepared from the Jordanian Kaolin clay: Adsorption and thermodynamics of Pb (II) and Cd (II) ions in aqueous solution. Chem. Int. 2020, 6, 168–178. [Google Scholar]
- Ahmadzadeh, S.; Yoosefian, M.; Rezayi, M. Comprehensive experimental and theoretical investigations on chromium (III) trace detection in biological and environmental samples using polymeric membrane sensor. Int. J. Environ. Anal. Chem. 2021, 101, 1461–1476. [Google Scholar] [CrossRef]
- Chong, A.; Wong, Y.; Tam, N. Performance of different microalgal species in removing nickel and zinc from industrial wastewater. Chemosphere 2000, 41, 251–257. [Google Scholar] [CrossRef]
- Benabdallah, N.; Harrache, D.; Mir, A.; De La Guardia, M.; Benhachem, F. Bioaccumulation of trace metals by red alga Corallina elongata in the coast of Beni Saf, west coast, Algeria. Chem. Int. 2017, 3, 220–231. [Google Scholar]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Manikandan, A.; Kusuma, H.S.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renew. Sustain. Energy Rev. 2021, 137, 110603. [Google Scholar] [CrossRef]
- Madrid, Y.; Cámara, C. Biological substrates for metal preconcentration and speciation. TrAC Trends Anal. Chem. 1997, 16, 36–44. [Google Scholar] [CrossRef]
- Priyadharshini, S.D.; Babu, P.S.; Manikandan, S.; Subbaiya, R.; Govarthanan, M.; Karmegam, N. Phycoremediation of wastewater for pollutant removal: A green approach to environmental protection and long-term remediation. Environ. Pollut. 2021, 290, 117989. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Chojnacki, A.; Gorecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sirohi, R.; Tong, Y.W.; Kim, S.H.; Pandey, A. Metal and metal (loids) removal efficiency using genetically engineered microbes: Applications and challenges. J. Hazard. Mater. 2021, 416, 125855. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef]
- Taniguchi, J.; Hemmi, H.; Tanahashi, K.; Amano, N.; Nakayama, T.; Nishino, T. Zinc biosorption by a zinc-resistant bacterium, Brevibacterium sp. strain HZM-1. Appl. Microbiol. Biotechnol. 2000, 54, 581–588. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Kausar, F.; Farooqi, M.-A.; Farooqi, H.-M.-U.; Salih, A.-R.-C.; Khalil, A.-A.-K.; Kang, C.-W.; Mahmoud, M.H.; Batiha, G.-E.-S.; Choi, K.-H.; Mumtaz, A.-S. Phytochemical investigation, antimicrobial, antioxidant and anticancer activities of acer cappadocicum gled. Life 2021, 11, 656. [Google Scholar] [CrossRef]
- Kausar, F.; Kim, K.-H.; Farooqi, H.M.U.; Farooqi, M.A.; Kaleem, M.; Waqar, R.; Khalil, A.A.K.; Khuda, F.; Abdul Rahim, C.S.; Hyun, K. Evaluation of antimicrobial and anticancer activities of selected medicinal plants of himalayas, Pakistan. Plants 2021, 11, 48. [Google Scholar] [CrossRef]
- Mehta, S.; Gaur, J. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef] [PubMed]
- Eccles, H. Treatment of metal-contaminated wastes: Why select a biological process? Trends Biotechnol. 1999, 17, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M. Biosorption: An interplay between marine algae and potentially toxic elements—A review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef]
- Manzoor, F.; Karbassi, A.; Golzary, A. Removal of heavy metal contaminants from wastewater by using Chlorella vulgaris beijerinck: A review. Curr. Environ. Manag. (Former. Curr. Environ. Eng.) 2019, 6, 174–187. [Google Scholar] [CrossRef]
- Mustafa, S.; Bhatti, H.N.; Maqbool, M.; Iqbal, M. Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: Prospects, challenges and opportunities. J. Water Process Eng. 2021, 41, 102009. [Google Scholar] [CrossRef]
- Rangsayatorn, N.; Upatham, E.; Kruatrachue, M.; Pokethitiyook, P.; Lanza, G. Phytoremediation potential of Spirulina (Arthrospira) platensis: Biosorption and toxicity studies of cadmium. Environ. Pollut. 2002, 119, 45–53. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Żyłkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Ammar, N.S.; Ghafar, H.H.A.; Ali, R.K. Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J. Adv. Res. 2013, 4, 367–374. [Google Scholar] [CrossRef]
- Mirghaffari, N.; Moeini, E.; Farhadian, O. Biosorption of Cd and Pb ions from aqueous solutions by biomass of the green microalga, Scenedesmus quadricauda. J. Appl. Phycol. 2015, 27, 311–320. [Google Scholar] [CrossRef]
- Munim, S.A.; Saddique, M.T.; Raza, Z.A.; Majeed, M.I. Preparation and physico-chemical characterization of β-cyclodextrin incorporated chitosan biosorbent beads with potential environmental applications. Mater. Res. Express 2018, 5, 065503. [Google Scholar] [CrossRef]
- Al-Rub, F.A.; El-Naas, M.; Ashour, I.; Al-Marzouqi, M. Biosorption of copper on Chlorella vulgaris from single, binary and ternary metal aqueous solutions. Process Biochem. 2006, 41, 457–464. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, A.K.; Sikandar, M. Study of sorption and desorption of Cd (II) from aqueous solution using isolated green algae Chlorella vulgaris. Appl. Water Sci. 2018, 8, 225. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga svenska vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber Jr, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Gu, Q.; Song, S.; Peng, C. Co-influence of the pore size of adsorbents and the structure of adsorbates on adsorption of dyes. Desalination Water Treat. 2016, 57, 14686–14695. [Google Scholar] [CrossRef]
- Chinedu, O.J.; Charles, M.; Onyema, A.M. Equilibrium, kinetic, thermodynamic and thermal stability studies on sorption of Ni (II) ions from aqueous solution using dead biomass of fresh water green algae Cosmarium panamense. Der Chem. Sin. 2012, 3, 38–51. [Google Scholar]
- Radi, S.; El Abiad, C.; Moura, N.M.; Faustino, M.A.; Neves, M.G.P. New hybrid adsorbent based on porphyrin functionalized silica for heavy metals removal: Synthesis, characterization, isotherms, kinetics and thermodynamics studies. J. Hazard. Mater. 2019, 370, 80–90. [Google Scholar] [CrossRef]
- Areco, M.M.; dos Santos Afonso, M. Copper, zinc, cadmium and lead biosorption by Gymnogongrus torulosus. Thermodynamics and kinetics studies. Colloids Surf. B Biointerfaces 2010, 81, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Tüzün, I.; Bayramoğlu, G.; Yalçın, E.; Başaran, G.; Celik, G.; Arıca, M.Y. Equilibrium and kinetic studies on biosorption of Hg (II), Cd (II) and Pb (II) ions onto microalgae Chlamydomonas reinhardtii. J. Environ. Manag. 2005, 77, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.-S. Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.E.; Franca, A.S.; Oliveira, L.S.; Rocha, S.D. Untreated coffee husks as biosorbents for the removal of heavy metals from aqueous solutions. J. Hazard. Mater. 2008, 152, 1073–1081. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M. Biosorption of Pb (II) and Cd (II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Fourest, E.; Roux, J.-C. Heavy metal biosorption by fungal mycelial by-products: Mechanisms and influence of pH. Appl. Microbiol. Biotechnol. 1992, 37, 399–403. [Google Scholar] [CrossRef]
- Roy, D.; Greenlaw, P.N.; Shane, B.S. Adsorption of heavy metals by green algae and ground rice hulls. J. Environ. Sci. Health Part A 1993, 28, 37–50. [Google Scholar] [CrossRef]
- Pavasant, P.; Apiratikul, R.; Sungkhum, V.; Suthiparinyanont, P.; Wattanachira, S.; Marhaba, T.F. Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour. Technol. 2006, 97, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Viraraghavan, T. Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res. 2003, 37, 4486–4496. [Google Scholar] [CrossRef]
- Pan, J.-H.; Liu, R.-X.; Tang, H.-X. Surface reaction of Bacillus cereus biomass and its biosorption for lead and copper ions. J. Environ. Sci. 2007, 19, 403–408. [Google Scholar] [CrossRef]
- Mungasavalli, D.P.; Viraraghavan, T.; Jin, Y.-C. Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: Batch and column studies. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 214–223. [Google Scholar] [CrossRef]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. CLEAN–Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, W.; Han, M. Biosorption of nickel and copper onto treated alga (Undaria pinnatifida): Application of isotherm and kinetic models. J. Hazard. Mater. 2008, 155, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ji, Y.; Cai, F.; Li, J. Heavy metal removal through biosorptive pathways. In Advances in Water Treatment and Pollution Prevention; Springer: Berlin/Heidelberg, Germany, 2012; pp. 95–145. [Google Scholar]
- Shen, W.; Li, Z.; Liu, Y. Surface chemical functional groups modification of porous carbon. Recent Pat. Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Bulgariu, D.; Bulgariu, L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour. Technol. 2012, 103, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, M.; Minhas, L.A.; Hashmi, M.Z.; Ali, M.A.; Mahmoud, R.M.; Saqib, S.; Nazish, M.; Zaman, W.; Samad Mumtaz, A. Biosorption of Cadmium and Lead by Dry Biomass of Nostoc sp. MK-11: Kinetic and Isotherm Study. Molecules 2023, 28, 2292. [Google Scholar] [CrossRef]
- Gupta, V.; Rastogi, A. Equilibrium and kinetic modelling of cadmium (II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J. Hazard. Mater. 2008, 153, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Mane, V.S.; Mall, I.D.; Srivastava, V.C. Kinetic and equilibrium isotherm studies for the adsorptive removal of Brilliant Green dye from aqueous solution by rice husk ash. J. Environ. Manag. 2007, 84, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H. Über die adsorption in lösungen. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Khan, T.A.; Mukhlif, A.A.; Khan, E.A.; Sharma, D.K. Isotherm and kinetics modeling of Pb (II) and Cd (II) adsorptive uptake from aqueous solution by chemically modified green algal biomass. Model. Earth Syst. Environ. 2016, 2, 1–13. [Google Scholar] [CrossRef]
- Lokeshwari, N.; Joshi, K. Biosorption of heavy metal (chromium) using biomass. Glob. J. Environ. Res. 2009, 3, 29–35. [Google Scholar]
- Singh, A.; Mehta, S.; Gaur, J. Removal of heavy metals from aqueous solution by common freshwater filamentous algae. World J. Microbiol. Biotechnol. 2007, 23, 1115–1120. [Google Scholar] [CrossRef]
- Waqar, R.; Kaleem, M.; Iqbal, J.; Minhas, L.A.; Haris, M.; Chalgham, W.; Ahmad, A.; Mumtaz, A.S. Kinetic and Equilibrium Studies on the Adsorption of Lead and Cadmium from Aqueous Solution Using Scenedesmus sp. Sustainability 2023, 15, 6024. [Google Scholar] [CrossRef]
- Hong, J.; Xie, J.; Mirshahghassemi, S.; Lead, J. Metal (Cd, Cr, Ni, Pb) removal from environmentally relevant waters using polyvinylpyrrolidone-coated magnetite nanoparticles. RSC Adv. 2020, 10, 3266–3276. [Google Scholar] [CrossRef]
- Katırcıoğlu, H.; Aslım, B.; Türker, A.R.; Atıcı, T.; Beyatlı, Y. Removal of cadmium (II) ion from aqueous system by dry biomass, immobilized live and heat-inactivated Oscillatoria sp. H1 isolated from freshwater (Mogan Lake). Bioresour. Technol. 2008, 99, 4185–4191. [Google Scholar] [CrossRef]
- Mondal, S.; Aikat, K.; Halder, G. Biosorptive uptake of arsenic (V) by steam activated carbon from mung bean husk: Equilibrium, kinetics, thermodynamics and modeling. Appl. Water Sci. 2017, 7, 4479–4495. [Google Scholar] [CrossRef]



| Kinetic Model | Linearized Equation | Graphical Dependence |
|---|---|---|
| Pseudo 1st order | log (qe − qt) vs. time | |
| Pseudo 2nd order | t/qt vs. time | |
| Intra-Particle diffusion | qt vs. time 0.5 |
| Metal | Pseudo 1st Order | Pseudo 2nd Order | Intraparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K1 (min−1) | qe (mg/g) | R2 | K2 (g mg−1 min−1) | qe (mg/g) | R2 | Ki(mg g−1 min−1) | R2 | qe exp (mg/g) | |
| Cd | −0.034545 | 27.510 | 0.8313 | 27,297.3 | 62.5 | 0.9952 | 3.9189 | 0.7834 | 58 |
| Pb | −0.029478 | 22.819 | 0.7135 | 36,090.7 | 62.8 | 0.9956 | 3.6035 | 0.7554 | 60 |
| Metal | Langmuir Constants | Freundlich Constants | Temkin Constants | |||||
|---|---|---|---|---|---|---|---|---|
| 1/b | qmax (mg g−1) | R2 | KF (mg g−1) | 1/n | R2 | B | R2 | |
| Cd | 5.153 | 64.1 | 0.9941 | 572.137 | 0.361 | 0.9475 | 13.203 | 0.9742 |
| Pb | 2.275 | 62.5 | 0.9864 | 1250.55 | 0.286 | 0.9258 | 11.001 | 0.9628 |
| Biosorbent | Biosorption Capacity (mg/g) | ||
|---|---|---|---|
| Cd | Pb | References | |
| U. lactuca | 29.2 | 34.7 | [54] |
| Rhizopus arrhizus | 27.0 | 56.0 | [55] |
| Chlorella minutissima | 11.1 | 9.74 | [56] |
| Caulerpa lentillifera | 4.7 | 28.7 | [57] |
| Mucor rouxii | 8.5 | 35.7 | [58] |
| Desmodesmus sp | 64.1 | 62.5 | Current study |
| Metal | Cycles | % Sorption | % Desorption |
|---|---|---|---|
| Cd | 1 | 61 ± 0.57 | 93.4 ± 0.63 |
| 2 | 61 ± 0.57 | 90.1 ± 0.63 | |
| 3 | 60 ± 0.57 | 91.6 ± 0.57 | |
| Pb | 1 | 64 ± 0.57 | 95.3 ± 0.57 |
| 2 | 63 ± 0.57 | 93.6 ± 0.57 | |
| 3 | 63 ± 0.57 | 92 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waqar, R.; Rahman, S.; Iqbal, J.; Kaleem, M.; Minhas, L.A.; Ullah, N.; Kausar, F.; Chalgham, W.; Al-Misned, F.A.; El-Serehy, H.A.; et al. Biosorption Potential of Desmodesmus sp. for the Sequestration of Cadmium and Lead from Contaminated Water. Sustainability 2023, 15, 11634. https://doi.org/10.3390/su151511634
Waqar R, Rahman S, Iqbal J, Kaleem M, Minhas LA, Ullah N, Kausar F, Chalgham W, Al-Misned FA, El-Serehy HA, et al. Biosorption Potential of Desmodesmus sp. for the Sequestration of Cadmium and Lead from Contaminated Water. Sustainability. 2023; 15(15):11634. https://doi.org/10.3390/su151511634
Chicago/Turabian StyleWaqar, Rooma, Sultana Rahman, Javed Iqbal, Muhammad Kaleem, Lubna Anjum Minhas, Nabi Ullah, Farzana Kausar, Wadie Chalgham, Fahad A. Al-Misned, Hamed A. El-Serehy, and et al. 2023. "Biosorption Potential of Desmodesmus sp. for the Sequestration of Cadmium and Lead from Contaminated Water" Sustainability 15, no. 15: 11634. https://doi.org/10.3390/su151511634
APA StyleWaqar, R., Rahman, S., Iqbal, J., Kaleem, M., Minhas, L. A., Ullah, N., Kausar, F., Chalgham, W., Al-Misned, F. A., El-Serehy, H. A., & Mumtaz, A. S. (2023). Biosorption Potential of Desmodesmus sp. for the Sequestration of Cadmium and Lead from Contaminated Water. Sustainability, 15(15), 11634. https://doi.org/10.3390/su151511634






