Habitat Selection: Autumn and Winter Behavioral Preferences of Water Deer (Hydropotes inermis) in Northeast China
Abstract
1. Introduction
2. Materials and Methods
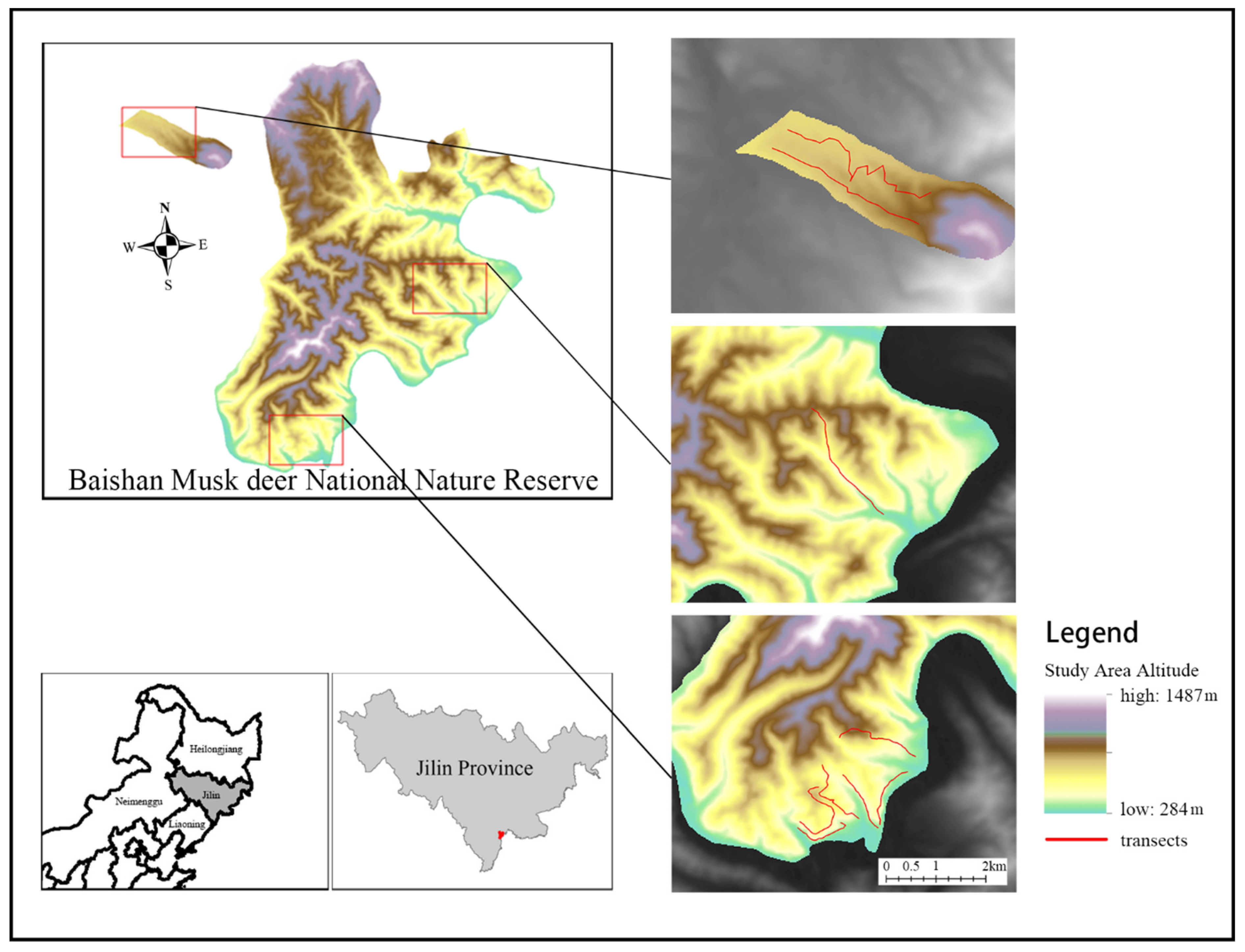
2.1. Study Area
2.2. Climate
2.3. Flora and Fauna
2.4. Data Collection
2.5. Data Analysis
3. Results
3.1. Habitat Selection of Water Deer in Autumn
3.2. Habitat Selection of Water Deer in Winter
4. Discussion
5. Conclusions and Suggestions
6. Limitations and Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, M.L.; Marcot, B.G.; Mannan, W. Wildlife-Habitat Relationship: Concepts and Applications. J. Range Manag. 2007, 57, 980–981. [Google Scholar]
- Grinnell, J. Field Tests of Theories Concerning Distributional Control. Am. Nat. 1917, 51, 115–128. [Google Scholar] [CrossRef]
- Gaillard, J.-M.; Hebblewhite, M.; Loison, A.; Fuller, M.; Powell, R.; Basille, M.; Van Moorter, B. Habitat–performance relationships: Finding the right metric at a given spatial scale. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2255–2265. [Google Scholar] [CrossRef]
- Furstenburg, D.; Scholtz, M. Global Climate Change and Animal Production in Southern Africa: A Short Review. Submitted: Livestock Science. 2009 (Supplement–10th Wrld. Cong. Anim. Prod.). Available online: https://www.researchgate.net/publication/306055750_Global_climate_change_and_animal_production_in_southern_Africa_a_short_review (accessed on 29 June 2023).
- Ehrlén, J.; Morris, W.F. Predicting changes in the distribution and abundance of species under environmental change. Ecol. Lett. 2015, 18, 303–314. [Google Scholar] [CrossRef]
- Singh, B.; Mainali, K.; Jiang, Z.; Thapa, A.; Subedi, N.; Awan, M.N.; Ilyas, O.; Luitel, H.; Zhou, Z.; Hu, H. Projected distribution and climate refugia of endangered Kashmir musk deer Moschus cupreus in greater Himalaya, South Asia. Sci. Rep. 2020, 10, 1511. [Google Scholar] [CrossRef]
- Zhu, G.; Papeş, M.; Armsworth, P.R.; Giam, X. Climate change vulnerability of terrestrial vertebrates in a major refuge and dispersal corridor in North America. Divers. Distrib. 2022, 28, 1227–1241. [Google Scholar] [CrossRef]
- Campos, F.A.; Morris, W.F.; Alberts, S.C.; Altmann, J.; Brockman, D.K.; Cords, M.; Pusey, A.; Stoinski, T.S.; Strier, K.B.; Fedigan, L.M. Does climate variability influence the demography of wild primates? Evidence from long-term life-history data in seven species. Glob. Chang. Biol. 2017, 23, 4907–4921. [Google Scholar] [CrossRef]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.M.; Butchart, S.H.M.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R.; et al. Assessing species vulnerability to climate change. Nat. Clim. Chang. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 1–24. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Kelly, A.E.; Goulden, M.L. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 11823–11826. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Prato, T. Adaptively Managing Wildlife for Climate Change: A Fuzzy Logic Approach. Environ. Manag. 2011, 48, 142–149. [Google Scholar] [CrossRef]
- Leslie, J.D.M.; Bowyer, R.T.; Kie, J.G. Life-History Strategies of Ungulates. J. Mammal. 1999, 80, 1067–1069. [Google Scholar] [CrossRef]
- Underwood, H.B. Deer of the World: Their Evolution, Behaviour, and Ecology. Val. Geist 2000, 75, 196. [Google Scholar]
- Boydston, E.E.; Kapheim, K.M.; Watts, H.E.; Szykman, M.; Holekamp, K.E. Altered behaviour in spotted hyenas associated with increased human activity. Anim. Conserv. 2003, 6, 207–219. [Google Scholar] [CrossRef]
- Inskip, C.; Zimmermann, A. Human-felid conflict: A review of patterns and priorities worldwide. Oryx 2009, 43, 18. [Google Scholar] [CrossRef]
- Shannon, G.; Cordes, L.S.; Hardy, A.R.; Angeloni, L.M.; Crooks, K.R. Behavioral Responses Associated with a Human-Mediated Predator Shelter. PLoS ONE 2014, 9, e94630. [Google Scholar] [CrossRef]
- Moll, R.J.; Cepek, J.D.; Lorch, P.D.; Dennis, P.M.; Robison, T.; Millspaugh, J.J.; Montgomery, R.A. Humans and urban development mediate the sympatry of competing carnivores. Urban Ecosyst. 2018, 21, 765–778. [Google Scholar] [CrossRef]
- Atickem, A.; Loe, L.E.; Stenseth, N.C. Individual Heterogeneity in Use of Human Shields by Mountain Nyala. Ethology 2014, 120, 715–725. [Google Scholar] [CrossRef]
- Won, C.; Smith, K.G. History and current status of mammals of the Korean Peninsula. Mammal Rev. 2002, 29, 3–36. [Google Scholar] [CrossRef]
- Ohtaishi, N. Deer of China: Biology and Management; Elsevier: Amsterdam, The Netherlands, 1993; pp. 4–7. [Google Scholar]
- Li, Y.K.; Shan, J.H.; Li, J.; Yuan, F.K.; Miao, L.J.; Xie, G.Y. Research Advances on the Ecology of Chinese Water Deer (Hydropotes inermis). Chin. J. Wildl. 2013, 34, 270–273. [Google Scholar]
- Harris, R.B.; Duckworth, J.W. Hydropotes inermis. The IUCN Red List of Threatened Species 2015: e.T10329A22163569. Available online: https://www.iucnredlist.org/species/10329/22163569 (accessed on 15 June 2023).
- Zhang, N. Home Range and Habitat Selection of Chinese Water Deer (Hydropotes inermis) after Release in Poyang Lake during the Dry Season. Master’s Thesis, Jiangxi Normal University, Nanchang, China, 2019. [Google Scholar]
- Han, Q.; Liang, T.; Zhang, M.Y.; Liu, M.M.; Wang, Z.Q.; Lu, C.H. Analysis of activity rhythm and habitat selection of water deer based on the infrared-triggered camera technology in autumn and winter in urban forest park. Chin. J. Ecol. 2022, 41, 963. [Google Scholar]
- Li, Z.Z.; Wu, J.P.; Teng, L.W.; Liu, Z.S.; Wang, B.K.; Liu, Y.C.; Xu, T. The Rediscovery of Water Deer (Hydropotes inermis) in Jilin Province. Chin. J. Zool. 2019, 54, 108–112. [Google Scholar]
- Belyaev, D.A.; Jo, Y.-S. Northernmost finding and further information on water deer Hydropotes inermis in Primorskiy Krai, Russia. Mammalia 2021, 85, 71–73. [Google Scholar] [CrossRef]
- Teng, L.W. Population of Chinese Water Deer (Hydropotes inermis) in Yancheng National Nature Reserve and Feasibility of Reintroduction Chinse Water Deer to Shanghai; Research Report of Postdoctoral Fellow; East China Normal University: Shanghai, China, 2007. [Google Scholar]
- Chen, L.C. A brief discussion on the causes of problems in the former musk Deer National Nature Reserve of Baishan in Jilin Province and the results of rectification. Agric. Dev. Equip. 2021, 230–231. Available online: https://kns-cnki-net-443.webvpn.nefu.edu.cn/kcms/detail/detail.aspx?FileName=NJJY202103112&DbName=CJFQ2021 (accessed on 29 June 2023).
- Li, H.J. Investigation and Analysis of Wild Animals in Jilin Baishan Original Musk Deer National Nature Reserve. For. Prospect. Des. 2021, 50, 52–55. [Google Scholar]
- Ding, Y.X. Study on Diets Analysis of Water Deer (Hydropotes inermis) in the Baishan Musk Deer National Nature Reserve in Jilin Province. Master’s Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar]
- Smith, A.T.; Xie, Y. A Guide to the Mammals of China. Changsha, Hunan Province, China; Hunan Education Publishing House: Changsha, China, 2009; pp. 481–482. [Google Scholar]
- Chen, Y.-T.; Chen, M.C. Using chi-square statistics to measure similarities for text categorization. Expert Syst. Appl. 2011, 38, 3085–3090. [Google Scholar] [CrossRef]
- Rosenthal, R. An Application of the Kolmogorov-Smirnov Test for Normality with Estimated Mean and Variance. Psychol. Rep. 1968, 22, 570. [Google Scholar] [CrossRef]
- Lennon, J.J. Resource selection functions: Taking space seriously? Trends Ecol. Evol. 1999, 14, 399–400. [Google Scholar] [CrossRef]
- Murtaugh, P.A. The Statistical Evaluation of Ecological Indicators. Ecol. Appl. 1996, 6, 132–139. [Google Scholar] [CrossRef]
- David, W.H.J.; Stanley, L.; Rodney, X.S. Applied Logistic Regression; John Wiley and Sons: New York, NY, USA, 2013. [Google Scholar]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.E.; Schmiegelow, F.K. Evaluating resource selection functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef]
- Xia, X.; Ren, J.; Li, L.; Wang, H.; Song, Y.; Yang, D.; Jiang, Z. Autumn-winter habitat selection by the re-wild Milu (Elaphurus davidianus) at the early stage after release in Dongting Lake Wetland, China. Biodivers. Sci. 2021, 29, 1087–1096. [Google Scholar] [CrossRef]
- Zheng, X.; Bao, Y.X.; Ge, B.M.; Zheng, R.Q. Seasonal changes in habitat use of black muntjac (Muntiacus crinifrons) in Zhejiang. Acta Theriol. Sin. 2006, 26, 201–205. [Google Scholar]
- Zhang, E.D.; Teng, L.W.; Wu, Y.B. Habitat selection of the Chinese water deer (Hydropotes inermis) in Yancheng Reserve, Jiangsu Province. Acta Theriol. Sin. 2006, 26, 49–53. [Google Scholar]
- Zhang, X.L.; Zhang, E.D. Distribution Pattern of Hydropotes inermis in various habitats in Jiangsu Dafeng Peredavids deer state nature reserve. Sichuan J. Zool. 2002, 21, 19–22. [Google Scholar]
- He, X. Spatial Behavioural Ecology of the Chinese Water Deer. Ph.D. Thesis, East China Normal University, Shanghai, China, 2013. [Google Scholar]
- Teng, L.; Liu, Z.; Song, Y.-L.; Zeng, Z. Forage and bed sites characteristics of Indian muntjac (Muntiacus muntjak) in Hainan Island, China. Ecol. Res. 2004, 19, 675–681. [Google Scholar] [CrossRef]
- Li, Z.Z.; Liu, Z.S.; Mi, S.H.; Wu, J.P.; Teng, L.W. Habitat selection of the Chinese water deer at Baishan Musk Deer Natural Reserve in spring and summer. Acta Ecol. Sin. 2021, 41, 1625–1633. [Google Scholar]
- Huang, Y. Food-Habits of Chinese Water Deer (Hydropotes inermis) in Poyang Lake Area. Master’s Thesis, Jiangxi Normal University, Nanchang, China, 2016. [Google Scholar]
- Kim, B.J.; Lee, N.S.; Lee, S.D. Feeding diets of the Korean water deer (Hydropotes inermis argyropus) based on a 202 bp rbcL sequence analysis. Conserv. Genet. 2011, 12, 851–856. [Google Scholar] [CrossRef]
- Cederlund, G.; Lindström, E. Effects of severe winters and fox predation on roe deer mortality. Acta Theriol. 1983, 28, 129–145. [Google Scholar] [CrossRef]
- Miao, L.J. The Population Viability Analysis, Distribution and Habitat Selection of Chinese Water Deer (Hydropotes inermis) in Poyang Lake. Master’s Thesis, Jiangxi Normal University, Nanchang, China, 2015. [Google Scholar]
- Choi, J.; Lee, S. Application of Habitat Evaluation Procedure with Quantifying the Eco-Corridor in the Process of Environmental Impact Assessment. Int. J. Environ. Res. Public Health 2019, 16, 1437. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E. Winter range arrival and departure of white-tailed deer in northeastern Minnesota. Can. J. Zool. 2011, 73, 1069–1076. [Google Scholar] [CrossRef]
- Godvik, I.M.R.; Loe, L.E.; Vik, J.O.; Veiberg, V.; Langvatn, R.; Mysterud, A. Temporal scales, trade-offs, and functional responses in red deer habitat selection. Ecology 2009, 90, 699–710. [Google Scholar] [CrossRef]
- Putman, R.J.; Moore, N.P. Impact of deer in lowland Britain on agriculture, forestry and conservation habitats. Mammal Rev. 2002, 28, 141–164. [Google Scholar] [CrossRef]
- Gordon, I.J.; Hester, A.J.; Festa-Bianchet, M. REVIEW: The management of wild large herbivores to meet economic, conservation and environmental objectives. J. Appl. Ecol. 2004, 41, 1021–1031. [Google Scholar] [CrossRef]





| Habitat Factors | Abbreviation | Measure Methods |
|---|---|---|
| Vegetation type | VT | Including grassland, coniferous forest, broad-leaved forest, shrub, cropland, and forest edge. |
| Dominant plant | DP | The plant with more than 70%, including Artemisia caruifolia, Amphicarpaea edgeworthii, A. argyi, Betula platyphylla, Quercus mongolica, Senna nomame. |
| Slope positions | SP | Divide the hillside into three equal parts, uphill position: top part, mid-slope position: middle part, downhill position: bottom part. |
| Slope direction | SD | Measured in the counterclockwise direction by the military compass Type 65 with the true north direction at 0°. Sunny slope (157.5°–337.5°), Shady slope (337.5°–157.5°). |
| Herbage coverage (%) | HEC | The average of 5 small plots (1 m × 1 m) sampled at center and 4 corners of the experimental plots or control plots |
| Height of dominant herbage (cm) | HDH | The average of 5 small plots (1 m × 1 m) sampled at center and 4 corners of the experimental plots or control plots |
| Slope degree (°) | S | Measured by the military compass Type 65. |
| Elevation (m) | E | Measured by global positioning instrument. |
| Distance from water (m) | DW | The distance between the center of plots and water resource |
| Distance to human settlements (m) | DHS | The distance between the center of plots and human settlement. |
| Hiding cover (%) | HIC | Erect a 1 m wooden pole at the center of the plot, measure the visibility of the pole at 20 m away from the center in the east, south, west, and north directions, that is, the percentage of the length of the pole in the total length can be seen, and then calculate the average value. |
| Habitat Factors | Regression Coefficient | Wald Chi-Square | p-Value |
|---|---|---|---|
| HDH (height of dominant herbage/cm) | −0.041 | 17.575 | 0.000 *** |
| Constant | 2.864 | 15.610 | 0.000 *** |
| Habitat Factors | Regression Coefficient | Wald Chi-Square | p-Value |
|---|---|---|---|
| DHS (distance to human settlements/m) | −0.013 | 6.804 | 0.009 ** |
| DW (distance from water/m) | 0.009 | 16.007 | 0.000 *** |
| HIC (hiding cover/%) | −0.032 | 7.805 | 0.005 ** |
| Constant | 0.533 | 0.669 | 0.413 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, Z.; Chen, J.; Khattak, R.H.; Liu, Z.; Teng, L. Habitat Selection: Autumn and Winter Behavioral Preferences of Water Deer (Hydropotes inermis) in Northeast China. Sustainability 2023, 15, 12181. https://doi.org/10.3390/su151612181
Sun Y, Li Z, Chen J, Khattak RH, Liu Z, Teng L. Habitat Selection: Autumn and Winter Behavioral Preferences of Water Deer (Hydropotes inermis) in Northeast China. Sustainability. 2023; 15(16):12181. https://doi.org/10.3390/su151612181
Chicago/Turabian StyleSun, Yue, Zongzhi Li, Junda Chen, Romaan Hayat Khattak, Zhensheng Liu, and Liwei Teng. 2023. "Habitat Selection: Autumn and Winter Behavioral Preferences of Water Deer (Hydropotes inermis) in Northeast China" Sustainability 15, no. 16: 12181. https://doi.org/10.3390/su151612181
APA StyleSun, Y., Li, Z., Chen, J., Khattak, R. H., Liu, Z., & Teng, L. (2023). Habitat Selection: Autumn and Winter Behavioral Preferences of Water Deer (Hydropotes inermis) in Northeast China. Sustainability, 15(16), 12181. https://doi.org/10.3390/su151612181






