Climate Change and Anthropogenic Factors Are Influencing the Loss of Habitats and Emerging Human–Elephant Conflict in the Namib Desert
Abstract
:1. Introduction
1.1. The Desert Elephants
1.2. Namibia as One of the Most Vulnerable Countries to Climate Change and HWC
1.3. Namib Desert Ephemeral Rivers and Aims of the Study
2. Methods
2.1. Study Area
2.2. Data Collection
2.2.1. Elephant Observation Movements and Population Structure
2.2.2. Human–Elephant Conflict Events
2.3. Data Analysis
2.3.1. Spatiotemporal Analysis of Elephant Movement Data to Trace Home Range Shift Patterns
2.3.2. Identification of Historic Vegetation Cover Change and Habitat Modification
2.3.3. Identification and Mapping of Migration Corridors
3. Results
3.1. Elephant Movement, Home Range Shift, and Potential Emerging Human–Elephant Conflict Areas
3.2. Habitats, Home Range Shift, and Potential Emerging Human–Elephant Conflict Areas
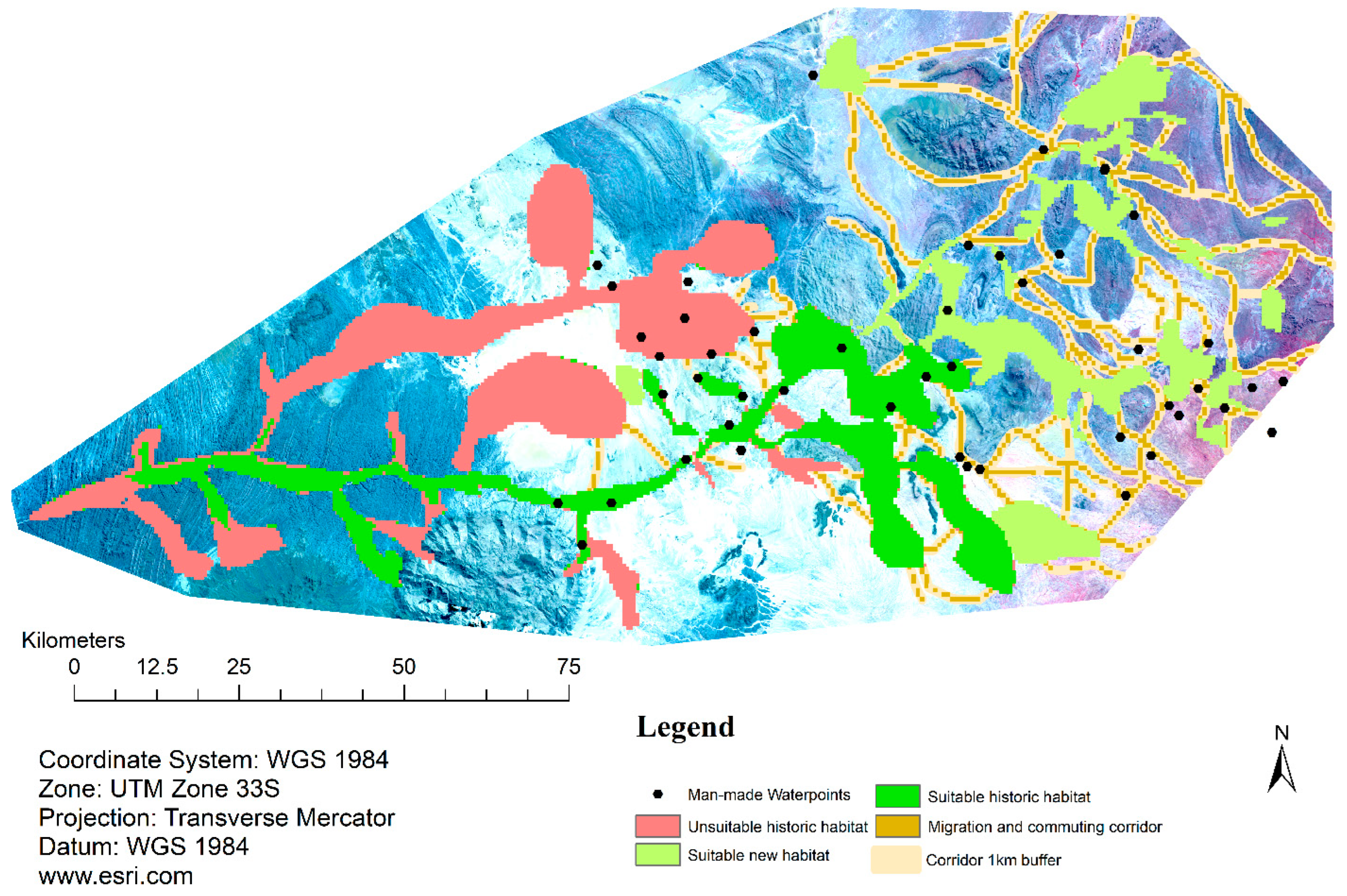
3.3. Habitat Loss, Gain, Connectivity, and Migration Corridors
3.4. Habitat Modification and Food Availability at the Ugab River Lower Catchment
4. Discussion
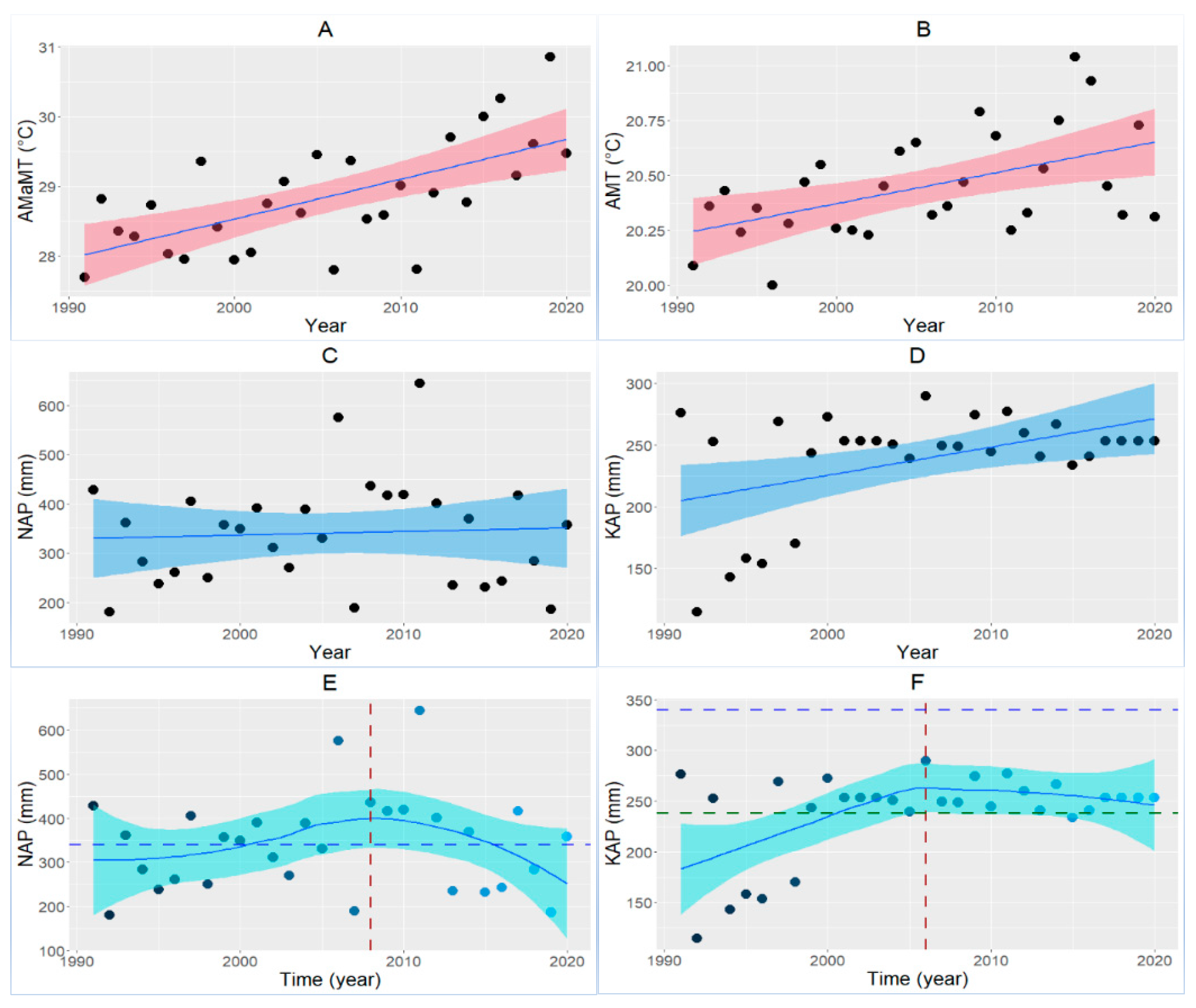
4.1. Vegetation Loss, Climate Change, and Home Range Shift
4.2. Farmers and Human–Elephant Conflict
4.3. Restoring Elephant’s Historic Habitats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poole, J.; Granli, P. Chapter 1. Mind and Movement: Meeting the Interests of Elephants. In An Elephant in the Room: Science and Well Being of Elephants in Captivity; Forthman, L.D., Kane, L.F., Waldau, P., Eds.; Center for Animals and Public Policy: North Grafton, MA, USA, 2008; pp. 1–20. ISBN 9780615229843. [Google Scholar]
- Hauenstein, S.; Kshatriya, M.; Blanc, J.; Dormann, C.F.; Beale, C.M. African Elephant Poaching Rates Correlate with Local Poverty, National Corruption and Global Ivory Price. Nat. Commun. 2019, 10, 2242. [Google Scholar] [CrossRef] [Green Version]
- Schlossberg, S.; Chase, M.J.; Griffin, C.R. Poaching and Human Encroachment Reverse Recovery of African Savannah Elephants in South-East Angola despite 14 Years of Peace. PLoS ONE 2018, 13, e0193469. [Google Scholar] [CrossRef] [Green Version]
- Chase, M.J.; Schlossberg, S.; Griffin, C.R.; Bouché, P.J.C.; Djene, S.W.; Elkan, P.W.; Ferreira, S.; Grossman, F.; Kohi, E.M.; Landen, K.; et al. Continent-Wide Survey Reveals Massive Decline in African Savannah Elephants. PeerJ 2016, 4, e2354. [Google Scholar] [CrossRef] [Green Version]
- Redpath, S.M.; Bhatia, S.; Young, J. Tilting at Wildlife: Reconsidering Human–Wildlife Conflict. Oryx 2015, 49, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Nyhus, P.J. Human–Wildlife Conflict and Coexistence. Annu. Rev. Environ. Resour. 2016, 41, 143–171. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.J.; Muir, R.D.J.; Walpole, M.J.; Balmford, A.; Leader-Williams, N. Governance and the Loss of Biodiversity. Nature 2003, 426, 67–70. [Google Scholar] [CrossRef]
- Laurance, W.F.; Carolina Useche, D.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.A.; Sloan, S.P.; Laurance, S.G.; Campbell, M.; Abernethy, K.; Alvarez, P.; et al. Averting Biodiversity Collapse in Tropical Forest Protected Areas. Nature 2012, 489, 290–294. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future Threats to Biodiversity and Pathways to Their Prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef]
- Hughes, A.C.; Qiao, H.; Orr, M.C. Extinction Targets Are Not SMART (Specific, Measurable, Ambitious, Realistic, and Time Bound). Bioscience 2021, 71, 115–118. [Google Scholar] [CrossRef]
- Hodnebrog, O.; Myhre, G.; Forster, P.M.; Sillmann, J.; Samset, B.H. Local Biomass Burning Is a Dominant Cause of the Observed Precipitation Reduction in Southern Africa. Nat. Commun. 2016, 7, 11236. [Google Scholar] [CrossRef] [Green Version]
- Szczys, P.; Oswald, S.A.; Arnold, J.M. Conservation Implications of Long-Distance Migration Routes: Regional Metapopu-lation Structure, Asymmetrical Dispersal, and Population Declines. Biol. Cons. 2017, 209, 263–272. [Google Scholar] [CrossRef]
- Turpie, J.; Midgley, G.; Brown, C.; Barnes, J.I.; Pallett, J.; Desmet, P.; Tarr, J.; Tarr, P. Climate Change Vulnerability and Adaptation Assessment for Namibia’s Biodiversity and Protected Area System; Climate Systems Analysis Group for the Ministry of Environment and Tourism: Windhoek, Namibia, 2010.
- Bauer, H.; Tehou, A.C.; Gueye, M.; Garba, H.; Doamba, B.; Diouck, D.; Sillero-Zubiri, C. Ignoring Species Hybrids in the IUCN Red List Assessments for African Elephants May Bias Conservation Policy. Nat. Ecol. Evol. 2021, 5, 1050–1051. [Google Scholar] [CrossRef]
- Gobush, K.S.; Edwards, C.T.T.; Balfour, D.; Wittemyer, G.; Maisels, F.; Taylor, R.D. African Savanna Elephant: Loxodonta africana. Available online: https://go.nature.com/3v9Bno6 (accessed on 18 July 2023).
- Hart, J.; Gobush, K.; Maisels, F.; Wasser, S.; Okita-Ouma, B.; Slotow, R. African Forest and Savannah Elephants Treated as Separate Species. Oryx 2021, 55, 170–171. [Google Scholar] [CrossRef]
- Brown, C. Conservation and the Environment in Namibia; Venture Media: Windhoek, Namibia, 2021. [Google Scholar]
- Douglas-Hamilton, l. African Elephants: Population Trends and Their Causes. Oryx 1987, 21, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Chwalibog, A.; Ngcobo, J.N.; Nedambale, T.L.; Nephawe, K.A.; Sawosz, E. The Future Survival of African Elephants: Implications for Conservation. Int. J. Avian Wildl. Biol. 2018, 3, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Pinnock, D.; Bell, C. The Last Elephants; Penguin Random House South Africa: Cape Town, South Africa, 2019; ISBN 9781775846840. [Google Scholar]
- di Minin, E.; Slotow, R.; Fink, C.; Bauer, H.; Packer, C. A Pan-African Spatial Assessment of Human Conflicts with Lions and Elephants. Nat. Commun. 2021, 12, 156. [Google Scholar] [CrossRef]
- Osei-Owusu, Y.; Bakker, L. Human-Wildlife Conflict: Elephant Technical Manual, the Wildlife Management Working Papers Report; FAO: Rome, Italy, 2008. [Google Scholar]
- Ministry of Environment, F. and T. National Elephant Conservation and Management Plan 2020/2021–2029/2030; Ministry of Environment, Forestry and Tourism: Windhoek, Namibia, 2020.
- Leggett, K.; Godfrey, M.; Weiver, E. Annual Report for the International Elephant Foundation; IEF: Azle, TX, USA, 2008. [Google Scholar]
- Brown, L.M.; Ramey, R.R.; Vinjevold, R.; Vinjevold, T.; Jauire, A. 2019 Annual Research Report Status and Distribution of Desert-Dwelling Elephants in the Hoarusib, Hoanib, and Uniab River Drainages, Kunene Region, Namibia. 2020. Available online: https://img1.wsimg.com/blobby/go/bded65a9-b30d-4b15-90f3-4d331465d33e/downloads/2019%20DEC%20Annual%20Research%20Report%20compressed.pdf?ver=1585231842554 (accessed on 27 June 2023).
- Martin, B.R. Transboundary Species Project—Background Study; Environmental Information Service Namibia: Windhoek, Namibia, 2005. [Google Scholar]
- Ishida, Y.; Van Coeverden de Groot, P.J.; Leggett, K.E.A.; Putnam, A.S.; Fox, V.E.; Lai, J.; Boag, P.T.; Georgiadis, N.J.; Roca, A.L. Genetic Connectivity across Marginal Habitats: The Elephants of the Namib Desert. Ecol. Evol. 2016, 6, 6189–6201. [Google Scholar] [CrossRef]
- Viljoen, P.J. Spatial Distribution and Movements of Elephants (Loxodonta africana) in the Northern Namib Desert Region of the Kaokoveld, South West Africa/Namibia. J. Zool. 1989, 219, 1–19. [Google Scholar] [CrossRef]
- Viljoen, P.J.; Bothma, J. du P. The Influence of Desert-Dwelling Elephants on Vegetation in the Northern Namib Desert, South West Africa/Namibia. J. Arid. Environ. 1990, 18, 85–96. [Google Scholar] [CrossRef]
- Leggett, A.E.K. Home Range and Seasonal Movement of Elephants in the Kunene Region, Northwestern Namibia. Afr. Zool. 2006, 41, 17–36. [Google Scholar] [CrossRef]
- Viljoen, P.J. Status and Past and Present Distribution of Elephants in the Kaokoveld, South West Africa/Namibia. S. Afr. J. Zool. 1987, 22, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Environment, Forestry and Tourism. Revised National Strategy on Wildlife Protection and Law Enforcement in the Republic of Namibia; Ministry of Environment, Forestry and Tourism: Windhoek, Namibia, 2021.
- Hitila, M. An Assessment of the Capacity of the Directorate of Disaster Risk Management of Namibia; Namibia University of Science and Technology: Windhoek, Namibia, 2019. [Google Scholar]
- United Nations Framework Convention on Climate Change. Republic of Namibia: Fourth National Communication to the United Nations Framework Convention; on Climate Change; United Nations Framework Convention on Climate Change: Windhoek, Namibia, 2020. [Google Scholar]
- Republic of Namibia Namibia. Namibia’s Updated Nationally Determined Contribution; Ministry of Environment, Forestry and Tourism: Windhoek, Namibia, 2021.
- Fox, J.T.; Vandewalle, M.E.; Alexander, K.A. Land Cover Change in Northern Botswana: The Influence of Climate, Fire, and Elephants on Semi-Arid Savanna Woodlands. Land 2017, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Inman, E.N.; Hobbs, R.J.; Tsvuura, Z. No Safety Net in the Face of Climate Change: The Case of Pastoralists in Kunene Region, Namibia. PLoS ONE 2020, 15, e0238982. [Google Scholar] [CrossRef] [PubMed]
- Dirkx, E.; Claus, H.; Mark, T.; Bethune, S.; Curtis, B. Climate Change Vulnerability & Adaptation Assessment Namibia; Climate Systems Analysis Group for the Ministry of Environment and Tourism: Windhoek, Namibia, 2008. [Google Scholar]
- Thuiller, W.; Midgley, G.F.; Hughes, G.O.; Bomhard, B.; Drew, G.; Rutherford, M.C.; Woodward, F.I. Endemic Species and Ecosystem Sensitivity to Climate Change in Namibia. Glob. Chang. Biol. 2006, 12, 759–776. [Google Scholar] [CrossRef]
- IPCC. IPCC Climate Change 2023: Synthesis Report of the Intergovernmental Panel on Climate Change Sixth Assessment Report (AR6); IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Mannheimer; Curtis, B. Le Roux and Müller’s Field Guide to the Trees & Shrubs of Namibia, 2nd ed.; Mannheimer, Curtis, B., Eds.; Namibia Publishing House: Windhoek, Namibia, 2011; Volume 77, ISBN 978-99916-0-970-6. [Google Scholar]
- Soetaert, F.; Wanke, H.; Dupuy, A.; Lusuekikio, V.; Gaucher, E.C.; Bordmann, V.; Fleury, J.M.; Franceschi, M. Toward the Sustainable Use of Groundwater Springs: A Case Study from Namibia. Sustainability 2022, 14, 3995. [Google Scholar] [CrossRef]
- The World Bank Group, World Bank Climate Change Knowledge Portal (CCKP). For Global Climate Data and Information! Climate Change Knowlwdge Portal: Washington, DC, USA, 2020. [Google Scholar]
- World Bank Group. Climate Risk Profile: Namibia; World Bank Group: Washington, DC, USA, 2021. [Google Scholar]
- Gómez-Rubio, V. Ggplot2—Elegant Graphics for Data Analysis. J. Stat. Softw. 2017, 77, 1–3. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. The R Project for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Enzerink, R.; Liefferink, D. Co-Existing with Wildlife in Namibia’s Conservancies: A Case Study on the Relationship between Human-Wildlife Conflict and Attitudes of Local Communities and the Influence of Community-Based Natural Resource Management on This Relationship; Radboud University: Nijmegen, The Netherlands, 2017. [Google Scholar]
- Naidoo, R.; Weaver, L.C.; de Longcamp, M.; du Plessis, P. Namibia’s Community-Based Natural Resource Management Programme: An Unrecognized Payments for Ecosystem Services Scheme. Environ. Conserv. 2011, 38, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Klintenberg, P.; Seely, M.; Christiansson, C. Local and National Perceptions of Environmental Change in Central Northern Namibia: Do They Correspond? J. Arid. Environ. 2007, 69, 506–525. [Google Scholar] [CrossRef]
- Seely, M.; Moser, P. Connecting Community Action and Science to Combat Desertification: Evaluation of a Process. Environ. Monit. Assess. 2004, 99, 33–55. [Google Scholar] [CrossRef]
- Seely, M.; Wöhl, H. Connecting Research to Combating Desertification. Environ. Monit. Assess. 2004, 99, 23–32. [Google Scholar] [CrossRef] [PubMed]
- MEFT/NACSO. The State of Community Conservation in Namibia (Annual Report 2019); NACSO: Windhoek, Namibia, 2021. [Google Scholar]
- Jones, R.; Weaver, L.C. CBNRM in Namibia: Growth, Trends, Lessons and Constraints. In Evolution and Innovation in Wildlife Conservation; Child, B., Suich, H., Anna, S., Eds.; Routledge: London, UK, 2012; pp. 241–260. ISBN 9781849771283. [Google Scholar]
- Brown, J.; Bird, N. Sustainable Natural Resource Management in Namibia: Successful Community-Based Wildlife Conservation; Overseas Development Institute: London, UK, 2011. [Google Scholar]
- Shaffer, L.J.; Khadka, K.K.; Van Den Hoek, J.; Naithani, K.J. Human–Elephant Conflict: A Review of Current Management Strategies and Future Directions. Front. Ecol. Evol. 2019, 6, 235. [Google Scholar] [CrossRef] [Green Version]
- Trumbull, R.B.; Reid, D.L.; de Beer, C.; van Acken, D.; Romer, R.L. Magmatism and Continental Breakup at the West Margin of Southern Africa: A Geochemical Comparison of Dolerite Dikes from Northwestern Namibia and the Western Cape. S. Afr. J. Geol. 2007, 110, 477–502. [Google Scholar] [CrossRef] [Green Version]
- Shikangalah, R.N.; Mapani, B.S. Ephemeral River Systems and Their Ecosystem Provisions to the Local Populations: A Review of the Huab and Ugab Rivers, Namibia. Int. Sci. Technol. J. Namib. 2021, 14, 46–62. [Google Scholar]
- Curtis, A.B. The Status of Faidherbia Albida Trees in the Hoanib River, Namibia. Namib. J. Environ. 2017, 1, 77–91. [Google Scholar]
- Moss, P. Regeneration and Utilization of Faidherbia Albida and Acacia Erioloba along Ephemeral Rivers of Namibia; Cuvilliier Verlag: Göttingen, Germany, 2006; Volume 42, ISBN 3-86537-948-6. [Google Scholar]
- Jacobson, P.J. The Influence of Elephants on Faidherbia Albida Trees in the Northern Namib Desert: A Reappraisal. In An Ephemeral Perspective of Fluvial Ecosystems: Viewing Ephemeral Rivers in the Context of Current Lotic Ecology; Jacobson, P.J., Richard, W.L.D., Cherry, D.S., Angermeier, P.L., Eds.; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 1997; pp. 111–122. [Google Scholar]
- Douglas, C.M.S. The Distribution and Survival of Riparian Trees along a Dammed Ephemeral River. Ph.D. Thesis, King’s College London, London, UK, 2015. [Google Scholar]
- Douglas, C.M.S.; Mulligan, M.; Harrison, X.A.; Henschel, J.R.; Pettorelli, N.; Cowlishaw, G. Widespread Dieback of Riparian Trees on a Dammed Ephemeral River and Evidence of Local Mitigation by Tributary Flows. PeerJ 2016, 2016, e2622. [Google Scholar] [CrossRef] [Green Version]
- Garstang, M.; Davis, R.E.; Leggett, K.; Frauenfeld, O.W.; Greco, S.; Zipser, E.; Peterson, M. Response of African Elephants (Loxodonta africana) to Seasonal Changes in Rainfall. PLoS ONE 2014, 9, e108736. [Google Scholar] [CrossRef] [Green Version]
- Jasechko, S.; Perrone, D. Global Groundwater Wells at Risk of Running Dry. Science 2021, 372, 418–421. [Google Scholar] [CrossRef]
- Douglas, C.M.S.; Cowlishaw, G.; Harrison, X.A.; Henschel, J.R.; Pettorelli, N.; Mulligan, M. Identifying the Determinants of Tree Distributions along a Large Ephemeral River. Ecosphere 2018, 9, e02223. [Google Scholar] [CrossRef]
- IPCC Summary for Policymakers. Climate Change: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, I.M., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Jacobson, J.P.; Jacobson, M.K.; Seely, K.M. Reviewed Work: Ephemeral Rivers and Their Catchments: Sustaining People and Development in Western Namibia. J. East. Afr. Res. Dev. 1996, 26, 238–241. [Google Scholar]
- Thomson, G. Climate Change in Namibia Part 2: Current and Projected Changes. Conservation and the Environment in Namibia. Windhoek, 29 October 2021. [Google Scholar]
- Viljoen, P.J. Habitat Selection and Preferred Food Plants of a Desert-Dwelling Elephant Population in the Northern Namib Desert, South West Africa/Namibia. Afr. J. Ecol. 1989, 27, 227–240. [Google Scholar] [CrossRef]
- Ramey, E.M.; Ramey, R.R.; Brown, L.M.; Kelley, S.T. Desert-Dwelling African Elephants (Loxodonta africana) in Namibia Dig Wells to Purify Drinking Water. Pachyderm 2013, 53, 66–72. [Google Scholar]
- Ministry of Environment and Tourism. Species Management Plan: Elephants Loxodonta africana; Ministry of Environment and Tourism: Windhoek, Namibia, 2007.
- Viljoen, J.P.; Bothma, P.J. Daily Movements of Desert-Dwelling Elephants in the Northern Namib Desert. S. Afr. J. Wildl. Res. 1990, 20, 69–80. [Google Scholar]
- USGS EarthExplorer: USGS. Available online: https://earthexplorer.usgs.gov/ (accessed on 21 June 2023).
- Office of the Prime. Minister The Annual Report 2019-2020; Office of the Prime Minister: Windhoek, Namibia, 2021. [Google Scholar]
- The Cooperation in International Waters in Africa. Drought Resilience Profiles: Namibia; The Cooperation in International Waters in Africa: Washington, DC, USA, 2021. [Google Scholar]
- Curtis, R. Guide to Animal Tracking: Outdoor Action. Available online: https://outdooraction.princeton.edu/nature/guide-animal-tracking (accessed on 22 November 2021).
- Nchanji, A.C.; Plumptre, A.J. Seasonality in Elephant Dung Decay and Implications for Censusing and Population Moni-toring in South-Western Cameroon. Afr. J. Ecol. 2001, 39, 24–32. [Google Scholar] [CrossRef]
- Barnes, R.F.W.; Asamoah-Boateng, B.; Naada Majam, J.; Agyei-Ohemeng, J. Rainfall and the Population Dynamics of El-ephant Dung-Piles in the Forests of Southern Ghana. Afr. J. Ecol. 1997, 35, 39–52. [Google Scholar] [CrossRef]
- Masunga, G.S.; Andresen, Ø.; Taylor, J.E.; Dhillion, S.S. Elephant Dung Decomposition and Coprophilous Fungi in Two Habitats of Semi-Arid Botswana. Mycol. Res. 2006, 110, 1214–1226. [Google Scholar] [CrossRef]
- Jachmann, H. Estimating Age in African Elephants. Afr. J. Ecol. 1985, 23, 199–202. [Google Scholar] [CrossRef]
- Shrader, A.M.; Ferreira, S.M.; McElveen, M.E.; Lee, P.C.; Moss, C.J.; van Aarde, R.J. Growth and Age Determination of African Savanna Elephants. J. Zool. 2006, 270, 40–48. [Google Scholar] [CrossRef]
- Douglas-Hamilton, I. On the Ecology and Behaviour of the African Elephant. Ph.D. Thesis, University of Oxford, Oxford, UK, 1972. [Google Scholar]
- Elephant Voices How to Age African Elephants. Available online: https://www.elephantvoices.org/features-guide/139-elephantvoices/education/808-how-to-age-african-elephants.html (accessed on 4 August 2022).
- Harris, R.; Winter, C.; Pitot, C.; Shiweda, M. EHRA 2019 Annual Report; Elephant-Human Relations Aid: Swakopmund, Namibia, 2020. [Google Scholar]
- Nelson, T.A.; Boots, B. Detecting Spatial Hot Spots in Landscape Ecology. Ecography 2008, 31, 556–566. [Google Scholar] [CrossRef]
- Zerbe, K.; Polit, C.; McClain, S.; Cook, T. Optimized Hot Spot and Directional Distribution Analyses Characterize the Spa-tiotemporal Variation of Large Wildfires in Washington, USA, 1970−2020. Int. J. Disaster Risk Sci. 2022, 13, 139–150. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Hazen, E.L.; Abrahms, B.; Brodie, S.; Carroll, G.; Welch, H.; Bograd, S.J. Where Did They Not Go? Considerations for Generating Pseudo-Absences for Telemetry-Based Habitat Models. Mov. Ecol. 2021, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.J.; Goossens, B.; Davies, A.B.; Reynolds, G.; Asner, G.P. Natural and Anthropogenic Drivers of Bornean Elephant Movement Strategies. Glob. Ecol. Conserv. 2020, 22, e00906. [Google Scholar] [CrossRef]
- Loarie, S.R.; Van Aarde, R.J.; Pimm, S.L. Fences and Artificial Water Affect African Savannah Elephant Movement Patterns. Biol. Conserv. 2009, 142, 3086–3098. [Google Scholar] [CrossRef]
- Ibrahim, M.; Al-Mashagbah, A. Change Detection of Vegetation Cover Using Remote Sensing Data as a Case Study: Ajloun Area. Civ. Environ. Res. 2016, 8, 1–5. [Google Scholar]
- Ajibade, F. Fidelis.; Adewumi, J.R. Application of GIS and Remote Sensing Technique to Change Detection in Land Use/Land Cover Mapping of Igbokoda, Ondo State, Nigeria. J. Appl. Sci. Process Eng. 2016, 3. [Google Scholar]
- Choate, M.J.; Rengarajan, R.; Storey, J.C.; Beckmann, T. Landsat 8 Thermal Infrared Sensor Scene Select Mechanism Open Loop Operations. Remote Sens. 2021, 13, 617. [Google Scholar] [CrossRef]
- Opedes, H.; Mücher, S.; Baartman, J.E.M.; Nedala, S.; Mugagga, F. Land Cover Change Detection and Subsistence Farming Dynamics in the Fringes of Mount Elgon National Park, Uganda from 1978–2020. Remote Sens. 2022, 14, 2423. [Google Scholar] [CrossRef]
- Al-Kindi, K.M.; Alqurashi, A.F.; Al-Ghafri, A.; Power, D. Assessing the Impact of Land Use and Land Cover Changes on Aflaj Systems over a 36-Year Period. Remote Sens. 2023, 15, 1787. [Google Scholar] [CrossRef]
- Roy, P.S.; Behera, M.D.; Murthy, M.S.R.; Roy, A.; Singh, S.; Kushwaha, S.P.S.; Jha, C.S.; Sudhakar, S.; Joshi, P.K.; Reddy, C.S.; et al. New Vegetation Type Map of India Prepared Using Satellite Remote Sensing: Comparison with Global Vegeta-tion Maps and Utilities. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 142–159. [Google Scholar] [CrossRef]
- Weier, J.; Herring, D. Measuring Vegetation (NDVI & EVI). Available online: https://www.earthobservatory.nasa.gov/features/MeasuringVegetation (accessed on 2 September 2022).
- Islam, K.; Jashimuddin, M.; Nath, B.; Nath, T.K. Land Use Classification and Change Detection by Using Multi-Temporal Remotely Sensed Imagery: The Case of Chunati Wildlife Sanctuary, Bangladesh. Egypt. J. Remote Sens. Space Sci. 2018, 21, 37–47. [Google Scholar] [CrossRef]
- Rudnick, D.; Ryan, S.; Beier, P.; Cushman, S.; Dieffenbach, F.; Epps, C.; Gerber, L.; Hartter, J.; Jenness, J.; Kintsch, J.; et al. The Role of Landscape Connectivity in Planning and Implementing Conservation and Restoration Priorities. Issues Ecology. Ecol. Soc. Am. 2012, 16, 1–23. Available online: https://www.esa.org/esa/wp-content/uploads/2013/03/issuesinecology16.pdf (accessed on 2 September 2022).
- Druce, D.J.; Shannon, G.; Page, B.R.; Grant, R.; Slotow, R. Ecological Thresholds in the Savanna Landscape: Developing a Protocol for Monitoring the Change in Composition and Utilisation of Large Trees. PLoS ONE 2008, 3, e3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beirne, C.; Meier, A.C.; Brumagin, G.; Jasperse-Sjolander, L.; Lewis, M.; Masseloux, J.; Myers, K.; Fay, M.; Okouyi, J.; White, L.J.T.; et al. Climatic and Resource Determinants of Forest Elephant Movements. Front. Ecol. Evol. 2020, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Purdon, A.; Mole, M.A.; Chase, M.J.; van Aarde, R.J. Partial Migration in Savanna Elephant Populations Distributed across Southern Africa. Sci. Rep. 2018, 8, 11331. [Google Scholar] [CrossRef] [Green Version]
- Birkett, P.J.; Vanak, A.T.; Muggeo, V.M.R.; Ferreira, S.M.; Slotow, R. Animal Perception of Seasonal Thresholds: Changes in Elephant Movement in Relation to Rainfall Patterns. PLoS ONE 2012, 7, e38363. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M. Namibia to sell off wild elephants in controversial auction. Mongabay, 29 January 2021. [Google Scholar]
- Thomson, G.C. The Story Behind the Namibian Elephant Auction. Conservation Namibia. Windhoek, 23 February 2021. [Google Scholar]
- Brown, K. Integrating Conservation and Development: A Case of Institutional Misfit. Front. Ecol. Environ. 2003, 1, 479. [Google Scholar] [CrossRef]
- Hobbs, J.S. Community Participation in Biodiversity Monitoring. Ph.D. Thesis, University of York, York, UK, 2012. [Google Scholar]
- Johnson, C.M.; Poulin, M.; Graham, M. Towards an Integrated Approach to the Conservation and Sustainable Use of Bio-diversity: Lessons Learned from the Rideau River Biodiversity Project on JSTOR. Hum. Ecol. Rev. 2003, 10, 40–55. [Google Scholar]
- Boudreaux, K. Community Conservation in Namibia: Devolution as a Tool for the Legal Empowerment of the Poor. Mercatus Policy Paper; Environmental Information Service Namibia: Windhoek, Namibia, 2010; pp. 1–25. [Google Scholar]
- Vaughn, K.J.; Porensky, L.M.; Wilkerson, M.L.; Balachowski, J.; Peffer, E.; Riginos, C.; Young, T.P. Restoration Ecology. Nat. Educ. Knowl. 2010, 3, 66. [Google Scholar]
- Young, T.P. Restoration Ecology and Conservation Biology. Biol. Conserv. 2000, 92, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Favier, C.; Aleman, J.; Bremond, L.; Dubois, M.A.; Freycon, V.; Yangakola, J.M. Abrupt Shifts in African Savanna Tree Cover along a Climatic Gradient. Glob. Ecol. Biogeogr. 2012, 21, 787–797. [Google Scholar] [CrossRef]
- Perring, M.P.; Standish, R.J.; Price, J.N.; Craig, M.D.; Erickson, T.E.; Ruthrof, K.X.; Whiteley, A.S.; Valentine, L.E.; Hobbs, R.J.; Perring, M.P.; et al. Advances in Restoration Ecology: Rising to the Challenges of the Coming Decades. Ecosphere 2015, 6, 1–25. [Google Scholar] [CrossRef] [Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiweda, M.; Shivute, F.; Sales, A.R.; Pereira, M.J. Climate Change and Anthropogenic Factors Are Influencing the Loss of Habitats and Emerging Human–Elephant Conflict in the Namib Desert. Sustainability 2023, 15, 12400. https://doi.org/10.3390/su151612400
Shiweda M, Shivute F, Sales AR, Pereira MJ. Climate Change and Anthropogenic Factors Are Influencing the Loss of Habitats and Emerging Human–Elephant Conflict in the Namib Desert. Sustainability. 2023; 15(16):12400. https://doi.org/10.3390/su151612400
Chicago/Turabian StyleShiweda, Markus, Fillipus Shivute, Ana Raquel Sales, and Mário J. Pereira. 2023. "Climate Change and Anthropogenic Factors Are Influencing the Loss of Habitats and Emerging Human–Elephant Conflict in the Namib Desert" Sustainability 15, no. 16: 12400. https://doi.org/10.3390/su151612400





