One-Step Electrosynthesis of Bifunctional NiCu Nanosheets on Iron Foam for Remarkably Enhanced Alkaline Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Material Synthesis
2.2.1. Preparation of NiCu/IF and CN/IF Electrodes
2.2.2. Fabrication of IF-Supported Pt/C and RuO2
2.3. Chemical and Structural Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
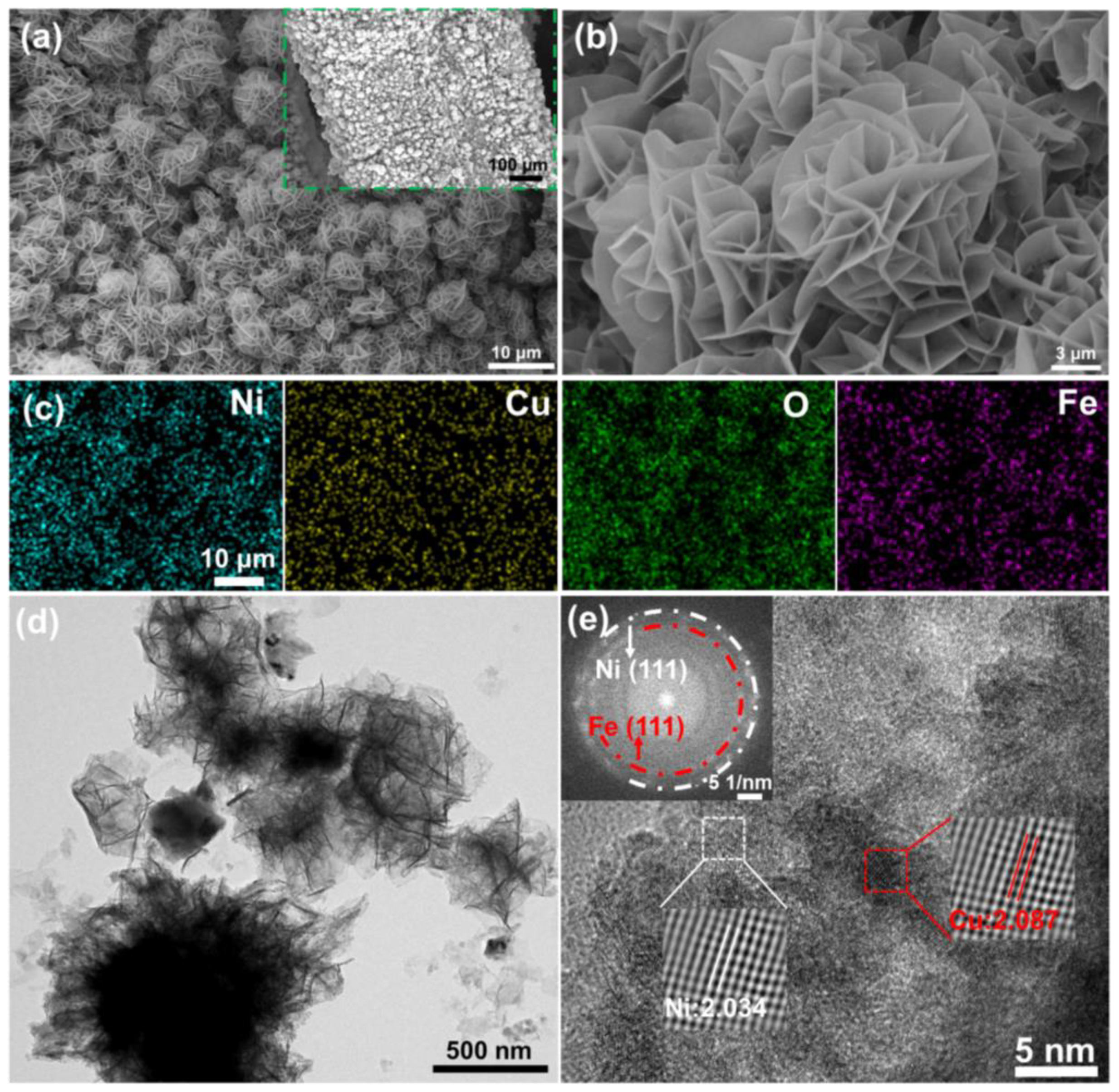
3.1. Structural Characterization
3.2. Electrochemical Performance
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodhankar, P.M.; Sarawade, P.B.; Singh, G.; Vinu, A.; Dhawale, D.S. Recent advances in highly active nanostructured NiFe LDH catalyst for electrochemical water splitting. J. Mater. Chem. A 2021, 9, 3180–3208. [Google Scholar] [CrossRef]
- Du, J.; Li, F.; Sun, L. Metal-organic frameworks and their derivatives as electrocatalysts for the oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 2663–2695. [Google Scholar] [CrossRef]
- Fan, W.; Wang, A.; Wang, L.; Jiang, X.; Xue, Z.; Li, J.; Wang, G. Hollow Carbon Nanopillar Arrays Encapsulated with Pd–Cu Alloy Nanoparticles for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2023, 15, 13600–13608. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Adv. Mater. 2022, 34, e2108133. [Google Scholar] [CrossRef]
- Dong, J.; Wang, Y.; Jiang, Q.; Nan, Z.-A.; Fan, F.R.; Tian, Z.-Q. Charged droplet-driven fast formation of nickel–iron (oxy)hydroxides with rich oxygen defects for boosting overall water splitting. J. Mater. Chem. A 2021, 9, 20058–20067. [Google Scholar] [CrossRef]
- Shah, K.; Dai, R.; Mateen, M.; Hassan, Z.; Zhuang, Z.; Liu, C.; Israr, M.; Cheong, W.C.; Hu, B.; Tu, R.; et al. Cobalt Single Atom Incorporated in Ruthenium Oxide Sphere: A Robust Bifunctional Electrocatalyst for HER and OER. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114951. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, X.; Chen, S.; Xie, H.; Gao, L.; Liu, A.; Ma, T.; Li, Y. Interface engineering of the MoS2/NiS2/CoS2 nanotube as a highly efficient bifunctional electrocatalyst for overall water splitting. Mater. Today Nano 2021, 17, 100156. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Wu, H.; Gao, Y.; Chen, Z.; Jin, W.; Wang, J.; Ma, T.; Wang, L. Corrosion Engineering on Iron Foam toward Efficiently Electrocatalytic Overall Water Splitting Powered by Sustainable Energy. Adv. Funct. Mater. 2021, 31, 2010437. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Li, Y.; Wen, Z. NiFeP-MoO2 hybrid nanorods on nickel foam as high-activity and high-stability electrode for overall water splitting. Chem. Eng. J. 2021, 409, 128161. [Google Scholar] [CrossRef]
- Shaikh, N.; Mukhopadhyay, I.; Ray, A. Heterointerfaces of nickel sulphides and selenides on Ni-foam as efficient bifunctional electrocatalysts in acidic environments. J. Mater. Chem. A 2022, 10, 12733–12746. [Google Scholar] [CrossRef]
- Singh, T.I.; Rajeshkhanna, G.; Pan, U.N.; Kshetri, T.; Lin, H.; Kim, N.H.; Lee, J.H. Alkaline Water Splitting Enhancement by MOF-Derived Fe-Co-Oxide/Co@NC-mNS Heterostructure: Boosting OER and HER through Defect Engineering and In Situ Oxidation. Small 2021, 17, 2101312. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Shi, H.; Luo, J.; Wang, L.; Liang, J.; Li, S.; Yang, L.-M.; Wang, T.; Huang, Y.; et al. Breaking the scaling relations of oxygen evolution reaction on amorphous NiFeP nanostructures with enhanced activity for overall seawater splitting. Appl. Catal. B Environ. 2022, 302, 120862. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, W.; Chen, L.; Meng, A.; Li, G.; Wang, L.; Huang, J.; Song, A.; Zhang, Z.; Li, Z. Surface reconstruction, doping and vacancy engineering to improve the overall water splitting of CoP nanoarrays. Nano Res. 2022, 16, 228–238. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, Y.; Yan, F.; Zhu, C.; Gao, P.; Zhang, X.; Chen, Y. Self-supported NiMo-based nanowire arrays as bifunctional electrocatalysts for full water splitting. J. Mater. Chem. A 2018, 6, 8479–8487. [Google Scholar] [CrossRef]
- Rani, B.J.; Sivanantham, A.; Shridharan, T.S.; Runfa, T.; Cho, I.S. Faceted and defect-rich CuMn2O4 nanoparticles for efficient electrochemical water splitting. J. Mater. Chem. A 2022, 10, 17710–17720. [Google Scholar] [CrossRef]
- Zhao, G.; Yao, Y.; Lu, W.; Liu, G.; Guo, X.; Tricoli, A.; Zhu, Y. Direct Observation of Oxygen Evolution and Surface Restructuring on Mn2O3 Nanocatalysts Using In Situ and Ex Situ Transmission Electron Microscopy. Nano Lett. 2021, 21, 7012–7020. [Google Scholar] [CrossRef]
- Liu, C.; Han, Y.; Yao, L.; Liang, L.; He, J.; Hao, Q.; Zhang, J.; Li, Y.; Liu, H. Engineering Bimetallic NiFe-Based Hydroxides/Selenides Heterostructure Nanosheet Arrays for Highly-Efficient Oxygen Evolution Reaction. Small 2021, 17, e2007334. [Google Scholar] [CrossRef]
- Su, H.; Jiang, J.; Li, N.; Gao, Y.; Ge, L. NiCu alloys anchored defect-rich NiFe layered double-hydroxides as efficient electrocatalysts for overall water splitting. Chem. Eng. J. 2022, 446, 137226. [Google Scholar] [CrossRef]
- Roy, S.; Bagchi, D.; Dheer, L.; Sarma, S.C.; Rajaji, V.; Narayana, C.; Waghmare, U.V.; Peter, S.C. Mechanistic insights into the promotional effect of Ni substitution in non-noble metal carbides for highly enhanced water splitting. Appl. Catal. B Environ. 2021, 298, 120560. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Q.; Yao, H.; Wang, M.; Wu, P.; Wang, H.; Zhang, L.; Guo, L. Rapid Synthesis of C60-MoC Nanocomposites by Molten Salt Electrolysis for Hydrogen Evolution. J. Electrochem. Soc. 2023, 170, acb853. [Google Scholar] [CrossRef]
- Ganesan, P.; Sivanantham, A.; Shanmugam, S. Nanostructured Nickel-Cobalt-Titanium Alloy Grown on Titanium Substrate as Efficient Electrocatalyst for Alkaline Water Electrolysis. ACS Appl. Mater. Interfaces 2017, 9, 12416–12426. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.B.; Zeng, J.R.; Li, X.T.; Hua, Y.X.; Xu, C.Y.; Dong, P. Facile electrochemical preparation of self-supported porous Ni–Mo alloy microsphere films as efficient bifunctional electrocatalysts for water splitting. J. Mater. Chem. A 2017, 5, 5797–5805. [Google Scholar] [CrossRef]
- Gebreslase, G.A.; Martínez-Huerta, M.V.; Lázaro, M.J. Recent progress on bimetallic NiCo and CoFe based electrocatalysts for alkaline oxygen evolution reaction: A review. J. Energy Chem. 2022, 67, 101–137. [Google Scholar] [CrossRef]
- Wang, J.; Xin, S.; Xiao, Y.; Zhang, Z.; Li, Z.; Zhang, W.; Li, C.; Bao, R.; Peng, J.; Yi, J.; et al. Manipulating the Water Dissociation Electrocatalytic Sites of Bimetallic Nickel-Based Alloys for Highly Efficient Alkaline Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202518. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Dai, L.; Chen, Z.N.; Li, L.; Yin, P.; Liu, Z.; Zhang, H. Ultrathin Ni(0)-Embedded Ni(OH)2 Heterostructured Nanosheets with Enhanced Electrochemical Overall Water Splitting. Adv. Mater. 2020, 32, e1906915. [Google Scholar] [CrossRef]
- Molla, C.F.; Gonfa, B.A.; Sabir, F.K.; Gicha, B.B.; Nwaji, N.; Tufa, L.T.; Lee, J. Ni-based ultrathin nanostructures for overall electrochemical water splitting. Mater. Chem. Front. 2023, 7, 194–215. [Google Scholar] [CrossRef]
- Lu, X.; Xie, S.; Yang, H.; Tong, Y.; Ji, H. Photoelectrochemical hydrogen production from biomass derivatives and water. Chem. Soc. Rev. 2014, 43, 7581–7593. [Google Scholar] [CrossRef]
- Zhu, W.; Yue, Z.; Zhang, W.; Hu, N.; Luo, Z.; Ren, M.; Xu, Z.; Wei, Z.; Suo, Y.; Wang, J. Wet-chemistry topotactic synthesis of bimetallic iron–nickel sulfide nanoarrays: An advanced and versatile catalyst for energy efficient overall water and urea electrolysis. J. Mater. Chem. A 2018, 6, 4346–4353. [Google Scholar] [CrossRef]
- Adhikari, S.; Kwon, Y.; Kim, D.-H. Three-dimensional core–shell structured NiCo2O4@CoS/Ni-Foam electrocatalyst for oxygen evolution reaction and electrocatalytic oxidation of urea. Chem. Eng. J. 2020, 402, 126192. [Google Scholar] [CrossRef]
- Fan, L.; Ji, Y.; Wang, G.; Chen, J.; Chen, K.; Liu, X.; Wen, Z. High Entropy Alloy Electrocatalytic Electrode toward Alkaline Glycerol Valorization Coupling with Acidic Hydrogen Production. J. Am. Chem. Soc. 2022, 144, 7224–7235. [Google Scholar] [CrossRef]
- Li, S.; Ma, P.; Gao, C.; Liu, L.; Wang, X.; Shakouri, M.; Chernikov, R.; Wang, K.; Liu, D.; Ma, R.; et al. Reconstruction-induced NiCu-based catalysts towards paired electrochemical refining. Energy Environ. Sci. 2022, 15, 3004–3014. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Wu, P.; Li, B.; Zhang, L.; Shi, J. CoNiFe-LDHs decorated Ta3N5 nanotube array photoanode for remarkably enhanced photoelectrochemical glycerol conversion coupled with hydrogen generation. Nano Energy 2021, 89, 106326. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Z.; Teng, X.; Niu, Y.; Gong, S.; Chen, W.; Meyer, T.J.; Chen, Z. Paired formate and H2 productions via efficient bifunctional Ni-Mo nitride nanowire electrocatalysts. J. Energy Chem. 2022, 72, 432–441. [Google Scholar] [CrossRef]
- Morales, D.M.; Jambrec, D.; Kazakova, M.A.; Braun, M.; Sikdar, N.; Koul, A.; Brix, A.C.; Seisel, S.; Andronescu, C.; Schuhmann, W. Electrocatalytic Conversion of Glycerol to Oxalate on Ni Oxide Nanoparticles-Modified Oxidized Multiwalled Carbon Nanotubes. ACS Catal. 2022, 12, 982–992. [Google Scholar] [CrossRef]
- Pei, Y.; Pi, Z.; Zhong, H.; Cheng, J.; Jin, F. Glycerol oxidation-assisted electrochemical CO2 reduction for the dual production of formate. J. Mater. Chem. A 2022, 10, 1309–1319. [Google Scholar] [CrossRef]
- Pu, H.; Dong, K.; Zhang, T.; Dai, H.; Wang, Y.; Deng, Y. Regulation of the shell thickness and shell components in PtCu/PdCu core–shell tripods for ethylene glycol and glycerol oxidation reactions. J. Mater. Chem. A 2022, 10, 10614–10624. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Li, K.; Huang, J.; Huang, Y.; Liu, Y.; Hu, X.; Zhao, B.; Yi, L.; Jones, T.W.; et al. Cost-effective and durable electrocatalysts for Co-electrolysis of CO2 conversion and glycerol upgrading. Nano Energy 2022, 92, 106751. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Shi, K.; Yu, H.; Deng, K.; Wang, X.; Wang, Z.; Wang, L.; Wang, H. Ru-doping induced lattice strain in hetero-phase Ni2P–Ni12P5 arrays enables simultaneous efficient energy-saving hydrogen generation and formate electrosynthesis. J. Mater. Chem. A 2022, 10, 20365–20374. [Google Scholar] [CrossRef]
- Yu, H.; Wang, W.; Mao, Q.; Deng, K.; Wang, Z.; Xu, Y.; Li, X.; Wang, H.; Wang, L. Pt single atom captured by oxygen vacancy-rich NiCo layered double hydroxides for coupling hydrogen evolution with selective oxidation of glycerol to formate. Appl. Catal. B Environ. 2023, 330, 122617. [Google Scholar] [CrossRef]
- Hao, J.; Zhuang, Z.; Cao, K.; Gao, G.; Wang, C.; Lai, F.; Lu, S.; Ma, P.; Dong, W.; Liu, T.; et al. Unraveling the electronegativity-dominated intermediate adsorption on high-entropy alloy electrocatalysts. Nat. Commun. 2022, 13, 2662. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Zhang, C. Enhanced anodic dissolution of cupronickel alloy scraps by electro-generated reactive oxygen species in acid media. J. Alloys Compd. 2019, 806, 106–112. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, L.; Xie, H.; Zhang, W.; Huang, S.; Yang, Z.; Li, Z.; Mi, X. Enhancing the erosion–corrosion resistance of cupronickel alloy through grain boundary engineering. Corros. Sci. 2023, 219, 111228. [Google Scholar] [CrossRef]
- Arab, N.; Fotouhi, L.; Salis, A. Electrosynthesised CdS@ZnS quantum dots decorated multi walled carbon nanotubes for analysis of propranolol in biological fluids and pharmaceutical samples. Microchem. J. 2021, 168, 106453. [Google Scholar] [CrossRef]
- Ballarin, B.; Berrettoni, M.; Carpani, I.; Scavetta, E.; Tonelli, D. Electrodes modified with an electrosynthesised Ni/Al hydrotalcite as amperometric sensors in flow systems. Anal. Chim. Acta 2005, 538, 219–224. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; McElhenny, B.; Xing, X.; Luo, D.; Zhang, F.; Bao, J.; Chen, S.; Ren, Z. Rational design of core-shell-structured CoP @FeOOH for efficient seawater electrolysis. Appl. Catal. B Environ. 2021, 294, 120256. [Google Scholar] [CrossRef]
- Serre, C.; Yaakoubi, N.; Martínez, S.; Pérez-Rodríguez, A.; Morante, J.R.; Esteve, J.; Montserrat, J. Electrochemical deposition of Cu and Ni/Cu multilayers in Si Microsystem Technologies. Sens. Actuators A Phys. 2005, 123–124, 633–639. [Google Scholar] [CrossRef]
- Yao, H.; Xie, L.; Cheng, Y.; Duan, J.; Chen, Y.; Lyu, S.; Sun, Y.; Liu, J. Tuning the coercivity of Cu/Ni multilayer nanowire arrays by tailoring multiple parameters. Mater. Des. 2017, 123, 165–173. [Google Scholar] [CrossRef]
- Deshpande, M.P.; Patel, K.N.; Gujarati, V.P.; Patel, K.; Chaki, S.H. Structural, Thermal and Optical Properties of Nickel Oxide (NiO) Nanoparticles Synthesized by Chemical Precipitation Method. Adv. Mater. Res. 2016, 1141, 65–71. [Google Scholar] [CrossRef]
- Song, M.-T.; Zhang, Y.; Huang, W.-J.; Hou, H.-Y.; Chen, X.-B. Enhancement of two-magnon scattering in annealed nickel oxide studied by Raman spectroscopy. Acta Phys. Sin. 2021, 70, 167201. [Google Scholar] [CrossRef]
- López, M.C.; Ortiz, G.F.; Lavela, P.; Alcántara, R.; Tirado, J.L. Improved Energy Storage Solution Based on Hybrid Oxide Materials. ACS Sustain. Chem. Eng. 2012, 1, 46–56. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Wu, Y.J.; Yang, J.; Tu, T.X.; Li, W.Q.; Zhang, P.F.; Zhou, Y.; Li, J.F.; Li, J.T.; Sun, S.G. Evolution of Cationic Vacancy Defects: A Motif for Surface Restructuration of OER Precatalyst. Angew. Chem. Int. Ed. Engl. 2021, 60, 26829–26836. [Google Scholar] [CrossRef]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. Ni/NiO nanosheets for alkaline hydrogen evolution reaction: In situ electrochemical-Raman study. Electrochim. Acta 2020, 361, 137040. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, Y.; Xu, P.; Lv, Z.; Shi, X.; Zheng, H. Biomass upgrading coupled with H2 production via a nonprecious and versatile Cu-doped nickel nanotube electrocatalyst. J. Mater. Chem. A 2022, 10, 10181–10191. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.-J.; Zou, Y.; Jiang, L.-W.; Liu, X.-L.; Jiang, W.-J.; Liu, H.; Hu, J.-S. Selective Se doping of NiFe2O4 on an active NiOOH scaffold for efficient and robust water oxidation. Chin. J. Catal. 2021, 42, 1395–1403. [Google Scholar] [CrossRef]
- Wang, H.; Guan, A.; Zhang, J.; Mi, Y.; Li, S.; Yuan, T.; Jing, C.; Zhang, L.; Zheng, G. Copper-doped nickel oxyhydroxide for efficient electrocatalytic ethanol oxidation. Chin. J. Catal. 2022, 43, 1478–1484. [Google Scholar] [CrossRef]
- Deng, Y.; Cao, Y.; Xia, Y.; Xi, X.; Wang, Y.; Jiang, W.; Yang, D.; Dong, A.; Li, T. Self-Templated Synthesis of CoFeP@C Cage-In-Cage Superlattices for Enhanced Electrocatalytic Water Splitting. Adv. Energy Mater. 2022, 12, 43. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, X.; Lu, Q.; Li, Y.; Xiao, F.; Tang, B.; Wang, S.; Yu, D.Y.W.; Rogach, A.L. Electrocatalytic enhancement mechanism of cobalt single atoms anchored on different MXene substrates in oxygen and hydrogen evolution reactions. EcoMat 2022, 5, 12293. [Google Scholar] [CrossRef]
- Gautam, J.; Liu, Y.; Gu, J.; Ma, Z.; Zha, J.; Dahal, B.; Zhang, L.N.; Chishti, A.N.; Ni, L.; Diao, G.; et al. Fabrication of Polyoxometalate Anchored Zinc Cobalt Sulfide Nanowires as a Remarkable Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Funct. Mater. 2021, 31, 46. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Henzie, J.; Jiang, B.; Xia, W.; Chen, T.; Bando, Y.; Kang, Y.M.; Hossain, M.S.A.; Sugahara, Y.; et al. Mesoporous Iron-doped MoS2/CoMo2S4 Heterostructures through Organic-Metal Cooperative Interactions on Spherical Micelles for Electrochemical Water Splitting. ACS Nano 2020, 14, 4141–4152. [Google Scholar]
- Huang, L.; Chen, D.; Luo, G.; Lu, Y.R.; Chen, C.; Zou, Y.; Dong, C.L.; Li, Y.; Wang, S. Zirconium-Regulation-Induced Bifunctionality in 3D Cobalt-Iron Oxide Nanosheets for Overall Water Splitting. Adv. Mater. 2019, 31, e1901439. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Teng, X.; Meyer, T.J.; Chen, Z. CoP Nanoframes as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. ACS Catal. 2019, 10, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Li, R.-Q.; Wan, X.-Y.; Chen, B.-L.; Cao, R.-Y.; Ji, Q.-H.; Deng, J.; Qu, K.-G.; Wang, X.-B.; Zhu, Y.-C. Hierarchical Ni3N/Ni0.2Mo0.8N heterostructure nanorods arrays as efficient electrocatalysts for overall water and urea electrolysis. Chem. Eng. J. 2021, 409, 128240. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, K.; Liu, S.; Cao, X.; Wu, K.; Cheong, W.C.; Chen, Z.; Wang, Y.; Li, Y.; Liu, Y.; et al. Core-Shell ZIF-8@ZIF-67-Derived CoP Nanoparticle-Embedded N-Doped Carbon Nanotube Hollow Polyhedron for Efficient Overall Water Splitting. J. Am. Chem. Soc. 2018, 140, 2610–2618. [Google Scholar] [CrossRef]
- Quan, L.; Li, S.; Zhao, Z.; Liu, J.; Ran, Y.; Cui, J.; Lin, W.; Yu, X.; Wang, L.; Zhang, Y.; et al. Hierarchically Assembling CoFe Prussian Blue Analogue Nanocubes on CoP Nanosheets as Highly Efficient Electrocatalysts for Overall Water Splitting. Small Methods 2021, 5, e2100125. [Google Scholar] [CrossRef]
- Yang, L.; Liu, R.; Jiao, L. Electronic Redistribution: Construction and Modulation of Interface Engineering on CoP for Enhancing Overall Water Splitting. Adv. Funct. Mater. 2020, 30, 1909618. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, Q.; Kong, Q.; Tong, X.; Wu, S.; Zong, N.; Xu, R.; Yang, L. One-Step Electrosynthesis of Bifunctional NiCu Nanosheets on Iron Foam for Remarkably Enhanced Alkaline Water Splitting. Sustainability 2023, 15, 12240. https://doi.org/10.3390/su151612240
Liu Z, Wang Q, Kong Q, Tong X, Wu S, Zong N, Xu R, Yang L. One-Step Electrosynthesis of Bifunctional NiCu Nanosheets on Iron Foam for Remarkably Enhanced Alkaline Water Splitting. Sustainability. 2023; 15(16):12240. https://doi.org/10.3390/su151612240
Chicago/Turabian StyleLiu, Zhenwei, Qiang Wang, Qingxiang Kong, Xiaoning Tong, Song Wu, Naixuan Zong, Ruidong Xu, and Linjing Yang. 2023. "One-Step Electrosynthesis of Bifunctional NiCu Nanosheets on Iron Foam for Remarkably Enhanced Alkaline Water Splitting" Sustainability 15, no. 16: 12240. https://doi.org/10.3390/su151612240
APA StyleLiu, Z., Wang, Q., Kong, Q., Tong, X., Wu, S., Zong, N., Xu, R., & Yang, L. (2023). One-Step Electrosynthesis of Bifunctional NiCu Nanosheets on Iron Foam for Remarkably Enhanced Alkaline Water Splitting. Sustainability, 15(16), 12240. https://doi.org/10.3390/su151612240





