Accelerating Electricity Generation and Cr (VI) Removal Using Anatase–Biochar-Modified Cathode Microbial Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals for the Experiment
2.2. Preparation of Maize Biochar and Ana-B
2.3. Characterization of Composites
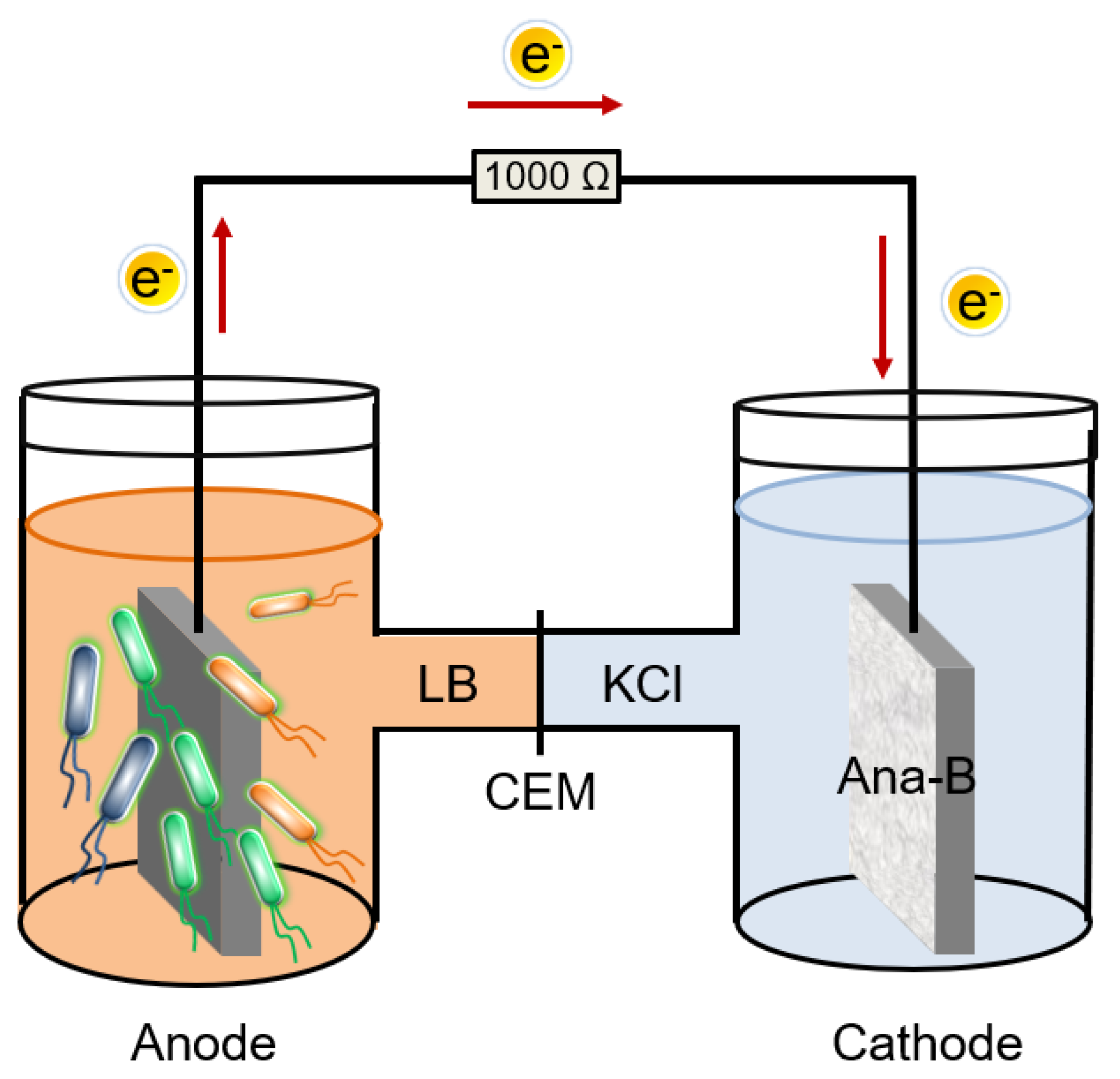
2.4. Construction and Operation of the MFC
2.5. Measurement and Analysis of the Pollutant Degradation Performance
3. Results
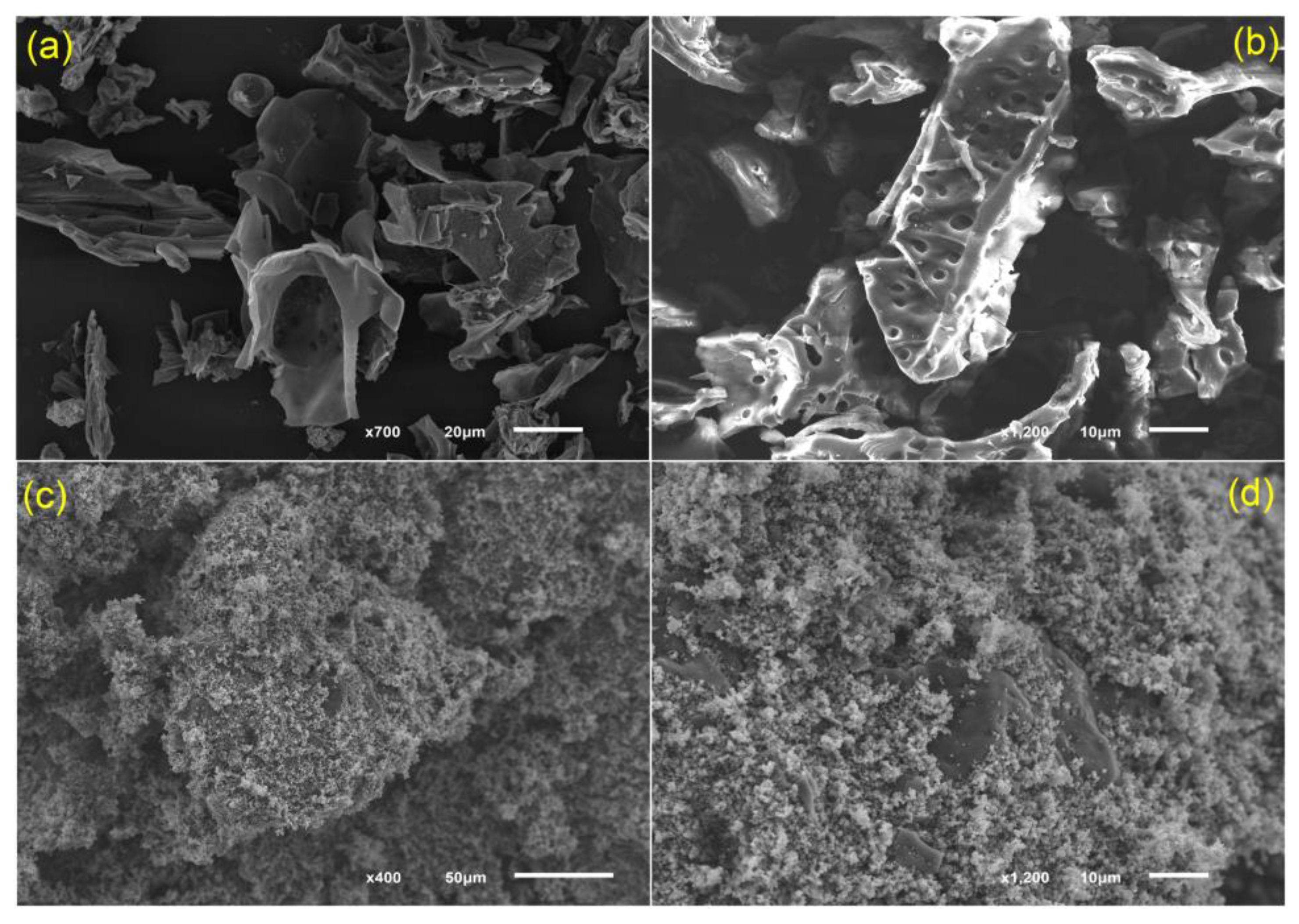
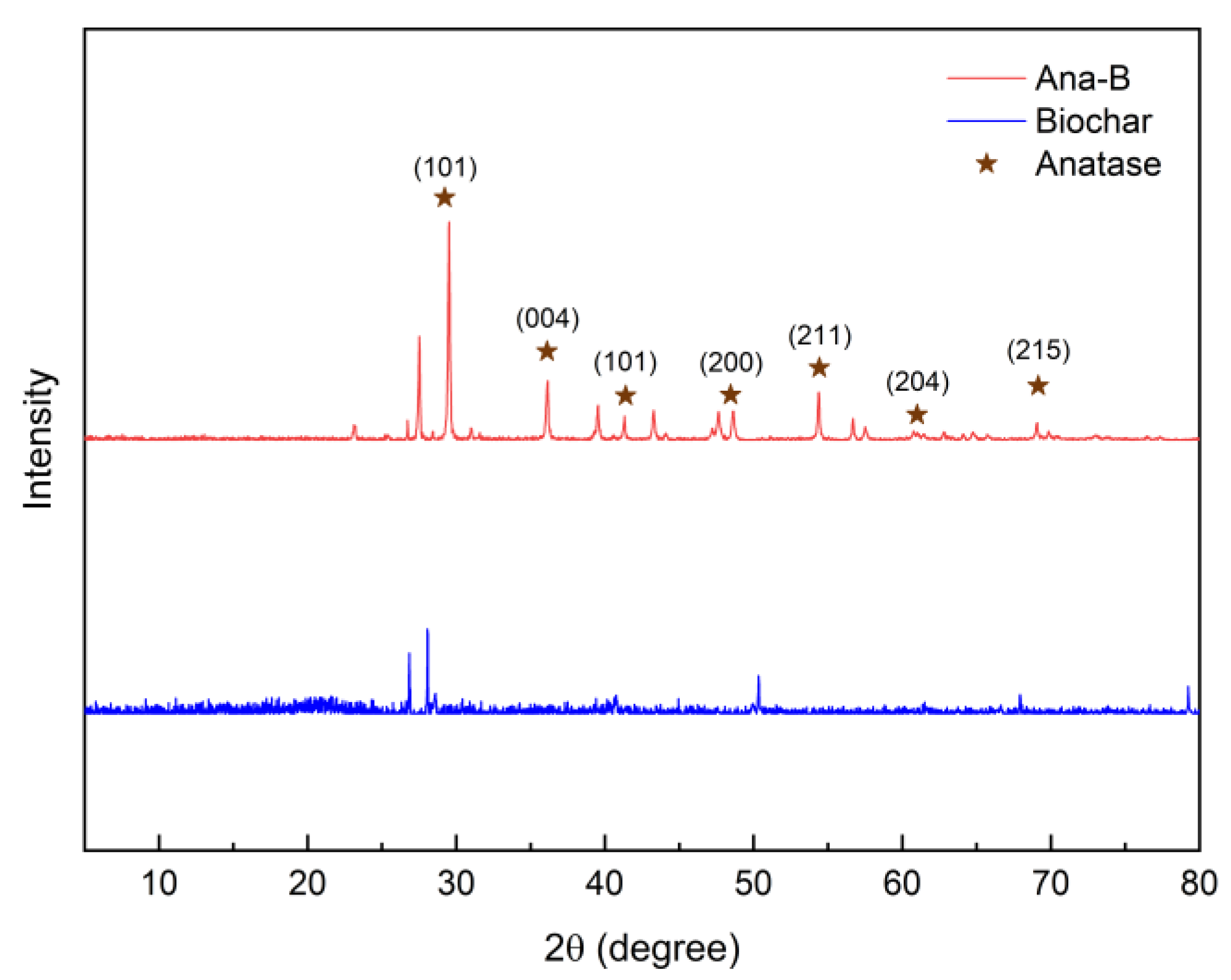
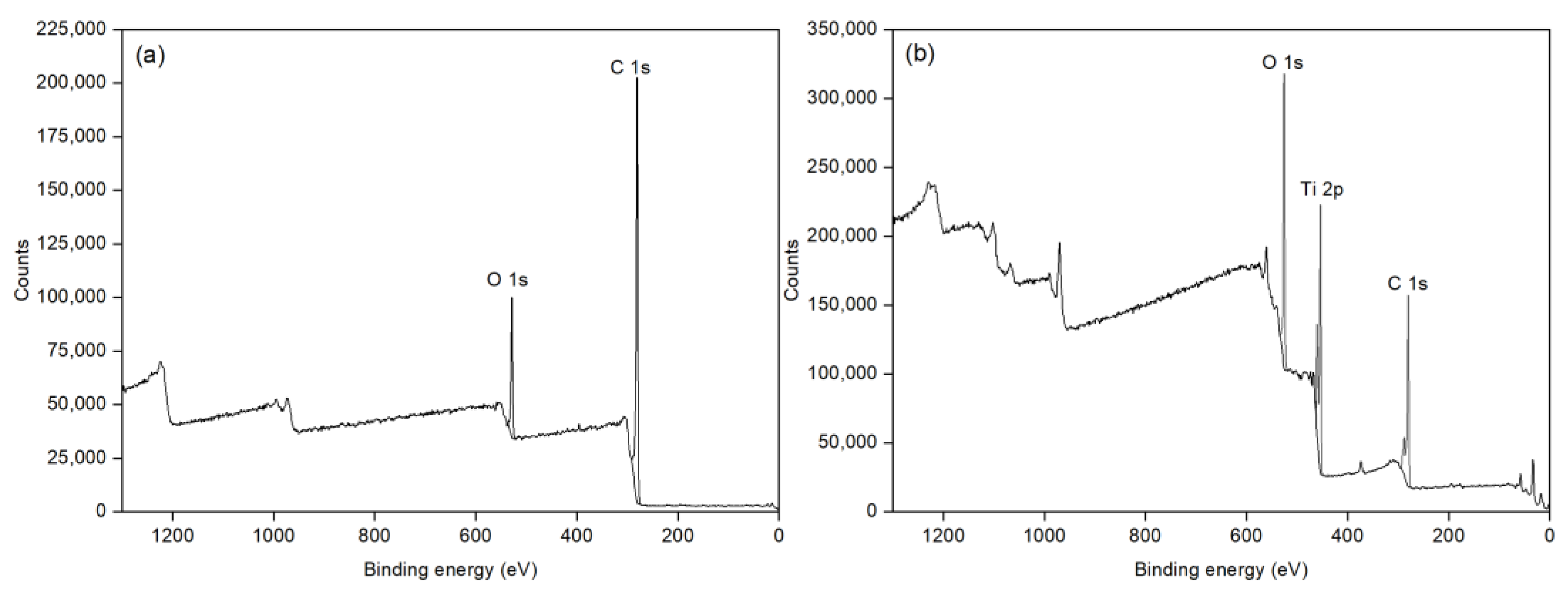
3.1. Characteristics of Ana-B
3.2. Electricity Generation Performance of the MFC
3.3. Study on the Cr (VI) Degradation Performance of the MFC
3.4. Effect of the Solution Initial Concentration
3.5. Effect of Solution pH
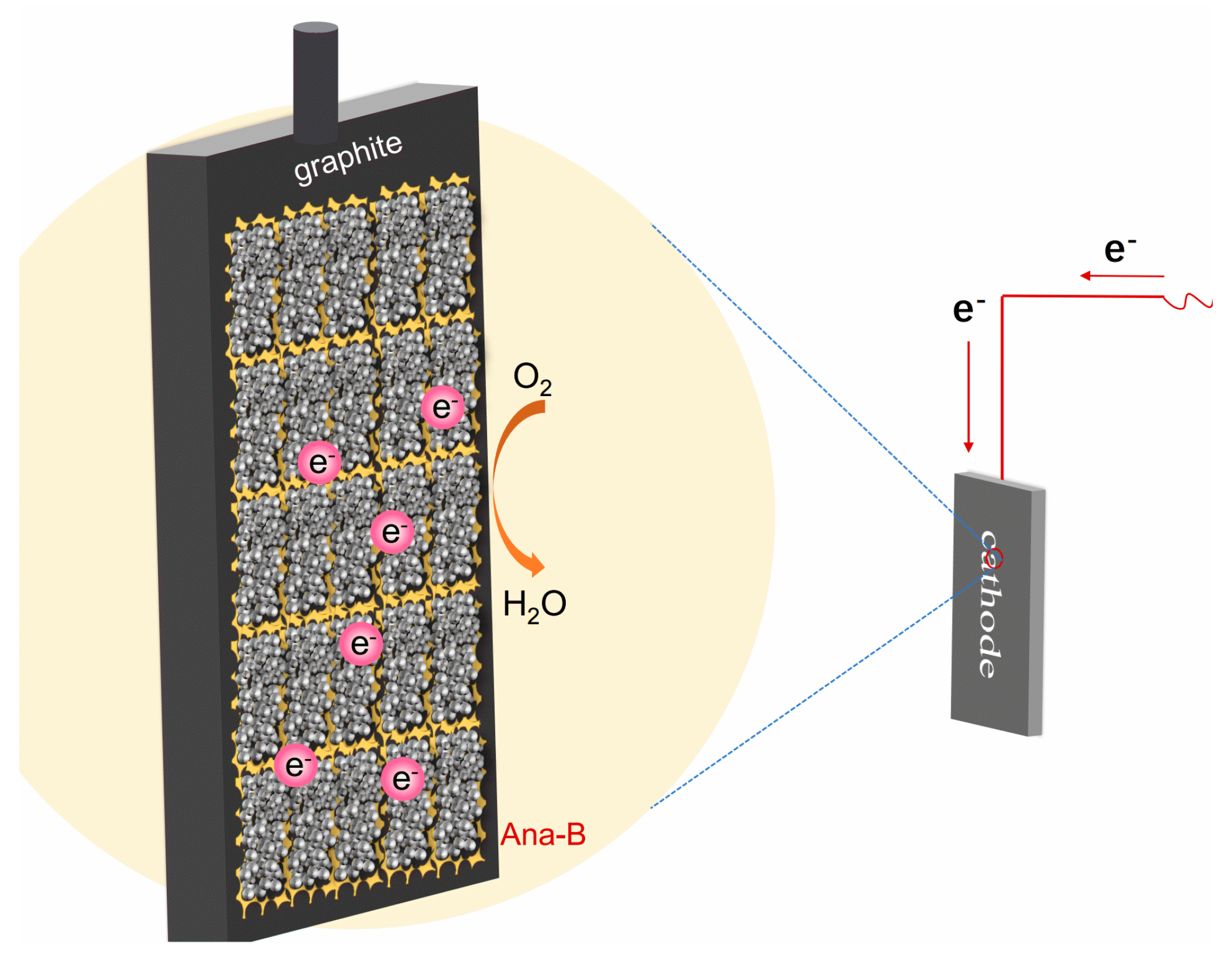
3.6. Mechanism of the Ana-B Cathode Catalysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ombaka, L.M.; McGettrick, J.D.; Oseghe, E.O.; Al-Madanat, O.; Rieck, B.F.; Msagati, T.A.M.; Davies, M.L.; Bredow, T.; Bahnemann, D.W. Photocatalytic H2 production and degradation of aqueous 2-chlorophenol over B/N-graphene-coated Cu0/TiO2: A DFT, experimental and mechanistic investigation. J. Environ. Manag. 2022, 311, 114822. [Google Scholar]
- AlSalka, Y.; Al-Madanat, O.; Hakki, A. TiO2-based photocatalytic hydrogen production: How to transfer it to an applicable approach. Appl. Catal. A 2023, 662, 119287. [Google Scholar]
- Gao, Q.; Tao, D.W.; Qi, Z.B.; Liu, Y.F.; Guo, J.; Yu, Y. Amidoxime functionalized PVDF-based chelating membranes enable synchronous elimination of heavy metals and organic contaminants from wastewater. J. Environ. Manag. 2022, 318, 115643. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Ghosh, N.; Mandal, C.; Das, K.; Dey, N.; Adak, M.K. Responses of the maize plant to chromium stress with reference to antioxidation activity. Brazilian. J. Plant. Physiol. 2012, 24, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, A.; Mishra, S.P.; Mohapatra, P.; Parida, S. One-step solvothermal synthesis of TiO2-reduced graphene oxide nanocomposites with enhanced visible light photoreduction of Cr (VI). J. Nanopart. Res. 2017, 19, 206. [Google Scholar]
- Qin, X.M.; Bai, L.; Tan, Y.Z.; Li, L.; Song, F.; Wang, Y.Z. β-Cyclodextrin-crosslinked polymeric adsorbent for simultaneous removal and stepwise recovery of organic dyes and heavy metal ions: Fabrication, performance and mechanisms. Chem. Eng. J. 2019, 372, 1007–1018. [Google Scholar]
- Martinkosky, L.; Barkley, J.; Sabadell, G.; Gough, H.; Davidson, S. Earthworms (Eisenia fetida) demonstrate potential for use in soil bioremediation by increasing the degradation rates of heavy crude oil hydrocarbons. Sci. Total. Environ. 2017, 580, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Maden, T.; Şahin, M. Modelling of The PEM Type Fuel Cells. Turk. J. Mater 2019, 4, 1–10. [Google Scholar]
- Lovley, D. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 487–508. [Google Scholar]
- Li, W.; Yu, H.; Zhen, H. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energ. Environ. SCI. 2014, 7, 911–924. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Ke, Z.; Wang, Q.; Zhang, C.; Wang, Y.; Ren, J.; Ren, G. Efficient organic contaminant and Cr (VI) synchronous re-moving by one-step modified molybdenite cathode microbial fuel cells. Environ. Sci. Pollut. Res. 2023, 30, 4423–4434. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.L.; Reguera, G.; Beyenal, H.; Lu, A.H.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Katuri, K.P.; Kalathil, S.; Ragab, A.; Bian, B.; Alqahtani, M.F.; Pant, D.; Saikaly, P.E. Dual-function electrocatalytic and macro-porous hollow-fiber cathode for converting waste streams to valuable resources using microbial electrochemical systems. Adv. Mater. 2018, 30, e1707072. [Google Scholar] [CrossRef] [PubMed]
- Badi, N.; Theodore, A.M.; Alghamdi, S.A.; Al-Aoh, H.A.; Lakhouit, A.; Roy, A.S.; Ignatiev, A. Fabrication and Characterization of Flexible Solid Polymers Electrolytes for Supercapacitor Application. Polymers 2022, 14, 3837. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.M.; Liu, G.; Fu, B. Conversion of sewage sludge into environmental catalyst and microbial fuel cell electrode material: A review. Sci. Total Environ. 2019, 666, 525–539. [Google Scholar]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Zhou, M.; Chi, M.; Luo, J. An overview of electrode materials in microbial fuel cells. J. Power. Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Rahman, S.; Balushi, N.J.; Nayak, J.K.; Al-Mamun, A.; Al-Abri, M.; Alawi, M.; Sana, A. A review on semiconductor photocathode in bio electrochemical systems: Mechanism, limitation, and environmental application. Mater. Today Sustain. 2023, 22, 100349. [Google Scholar]
- Chen, K.F.; Jiang, L.T.; Yang, J.Q.; Wang, X.M.; An, Y.; Yang, D.X.; Wei, Q.Y.; Wang, Y.L.; Wang, R.J.; Yang, Y.W.; et al. Enhanced electrochemical performance in microbial fuel cell with carbon nanotube/NiCoAl-layered double hydroxide nanosheets as air-cathode. Int. J. Hydrogen Energy 2021, 46, 36466–36476. [Google Scholar] [CrossRef]
- Xu, H.T.; Wang, L.G.; Wen, Q.; Chen, Y.; Qi, L.J.; Huang, J.X.; Tang, Z.S. A 3D porous NCNT sponge anode modified with chitosan and polyaniline for high-performance microbial fuel cell. Bioelectrochemistry 2019, 129, 144–153. [Google Scholar] [CrossRef]
- Oh, S.; Min, B.; Logan, B. Cathode performance as a factor in electricity generation in microbial fuel cells. Environ. Sci. Technol. 2004, 38, 4900–4904. [Google Scholar] [CrossRef]
- Chen, B.; Brueckner, T.M.; Altarawneh, R.M.; Pickup, P.G. Composition Dependence of Ethanol Oxidation at Ruthenium. J. Electrochem. Soc. 2018, 165, J3019–J3025. [Google Scholar] [CrossRef]
- Hang, H.; Altarawneh, R.M.; Brueckner, T.M.; Pickup, P.G. Pt/Ru–Sn Oxide/Carbon Catalysts for Ethanol Oxidation. J. Electrochem. Soc. 2020, 167, 054518. [Google Scholar] [CrossRef]
- Lu, G.L.; Zhu, Y.L.; Lu, L.; Xu, K.L.; Wang, H.M.; Jin, Y.H.; Ren, Z.J.; Liu, Z.N.; Zhang, W. Iron-rich nanoparticle encapsulated, nitrogen doped porous carbon materials as efficient cathode electrocatalyst for microbial fuel cells. J. Power. Sources 2016, 315, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.P.; Ding, H.R.; Li, Y.; Lu, A.H. Natural hematite as a low-cost and earth-abundant cathode material for performance improvement of microbial fuel cells. Catalysts 2016, 6, 157. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.H.; Li, Y.; Jin, S.; Wang, X.; Wu, X.L.; Zeng, C.P.; Li, Y.; Ding, H.R.; Hao, R.X.; Lv, M.; et al. Growth of non-phototrophic microorganisms using solar energy through mineral photocatalysis. Nat Commun. 2012, 3, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlámalová, M.; Lásková, P.; Vinarčíková, M.; Zukalová, M. Inherent electrochemical activity of TiO2 (anatase, rutile) enhances the charge capacity of cathodes of lithium-sulfur batteries. J. Solid State Electrochem. 2022, 26, 639–647. [Google Scholar] [CrossRef]
- Ren, G.P.; Sun, Y.; Lu, A.H.; Li, Y.; Ding, H.R. Boosting electricity generation and Cr (VI) reduction based on a novel silicon solar cell coupled double-anode (photoanode/bioanode) microbial fuel cell. J. Power. Sources 2018, 408, 46–50. [Google Scholar] [CrossRef]
- Sovik, D.; Swati, D.; Ghangrekar, M. Application of TiO2 and Rh as cathode catalyst to boost the microbial electrosynthesis of organic compounds through CO2 sequestration. Process. Biochem. 2021, 101, 237–246. [Google Scholar]
- Zhang, Q.; Liu, L. Cathodes of membrane and packed manganese dioxide/titanium dioxide/graphitic carbon nitride/granular activated carbon promoted treatment of coking wastewater in microbial fuel cell. Bioresource Technol. 2021, 321, 124442. [Google Scholar] [CrossRef]
- Cai, T.; Huang, M.H.; Huang, Y.X.; Zheng, W. Enhanced performance of microbial fuel cells by electrospinning carbon nanofibers hybrid carbon nanotubes composite anode. Int. J. Hydrog. Energy. 2019, 44, 3088–3098. [Google Scholar] [CrossRef]
- Chang, H.; Gustave, W.; Yuan, Z. One-step fabrication of binder-free air cathode for microbial fuel cells by using balsa wood biochar. Environ. Technol. 2020, 18, 100615. [Google Scholar]
- Ramya, M.; Harsha, V.K.; Senthil, K.P. Metal mixed biochar electrodes for the generation of electricity with high power density in microbial fuel cell. Sustain. Energy Technol. Assess. 2022, 53, 102549. [Google Scholar]
- Yuan, Y.; Yuan, T.; Wang, D.; Tang, J.H.; Zhou, S.G. Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in a microbial fuel cell. Bioresour. Technol. 2013, 144, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Zhao, Q.L.; Ding, J.; Wang, K.; Jiang, J.Q. Development of an MFC-powered BEF system with novel Fe–Mn–Mg/CF composite cathode to degrade refractory pollutants. J. Clean. Prod. 2021, 326, 129348. [Google Scholar] [CrossRef]
- Qu, K.J.; Huang, L.; Hu, S.Y.; Liu, C.; Yang, Q.Y.; Liu, L.H.; Li, K.; Zha, Z.P.; Wang, Z.X. TiO2 supported on rice straw biochar as an adsorptive and photocatalytic composite for the efficient removal of ciprofloxacin in aqueous matrices. J. Environ. Chem. Eng. 2023, 11, 109430. [Google Scholar] [CrossRef]
- Li, S.N.; Ho, S.H.; Hua, T.; Zhou, Q.X.; Li, F.X.; Tang, J.C. Sustainable biochar as electrocatalysts for the oxygen reduction reaction in microbial fuel cells. Green. Energy. Environ. 2020, 6, 644–659. [Google Scholar] [CrossRef]
- Wang, R.; Wan, S.; Liu, B. Denitrification in perspective of carbon neutralization: CO2 emission reduction and electricity generation by Fe-anode and bio-cathode MFC. J. Water Process Eng. 2022, 48, 102868. [Google Scholar]
- Singh, H.; Zhuang, S.Q.; Ingis, B.J.; Nunna, B.B.; Lee, E.S. Carbon-based catalysts for oxygen reduction reaction: A review on degradation mechanisms. Carbon 2019, 151, 160–174. [Google Scholar]
- Feng, X.Y.; Wang, P.F.; Hou, J.; Qian, J.; Ao, Y.H.; Wang, C. Significantly enhanced visible light photocatalytic efficiency of phosphorus doped TiO2 with surface oxygen vacancies for ciprofloxacin degradation: Synergistic effect and intermediates analysis. J. Hazard. Mater. 2018, 351, 196–205. [Google Scholar] [PubMed]
- Cheng, H.; Jing, Z.H.; Yang, L.; Lu, A.H.; Ren, G.P.; Liu, J. Sunlight-triggered synergy of hematite and Shewanella oneidensis MR-1 in Cr (VI) removal. Geochim. Cosmochim. Acta. 2021, 305, 19–32. [Google Scholar] [CrossRef]
- Juliastuti, S.R.; Darmawan, R.; Hendrianie, N.; Prakoso, G.A.; Bachtiar, T.A. Influence of Shewanella oneidensis MR-1 bacterial metabolism process on waste treatment of Cr and Mn metals in reactor microbial fuel cell (MFC). IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 12089. [Google Scholar] [CrossRef] [Green Version]
- Hidayat, A.R.P.; Widyanto, A.R.; Zulfa, L.L.; Asranudin, A.; Sugiarso, D.; Putro, H.S.; Purnomo, A.S.; Prasetyoko, D.; Priyangga, A.; Atmaja, L.; et al. Mechanism adsorption–reduction into the incorporation of microbial fuel cell–metal organic framework and overview of hydrodynamics effects for enhanced reduction of Cr(VI). J. Water Process Eng. 2022, 49, 103095. [Google Scholar]
- Liu, L.; Yuan, Y.; Li, F.B.; Feng, C.H. In-situ Cr (VI) reduction with electrogenerated hydrogen peroxide driven by iron-reducing bacteria. Biores Technol. 2011, 102, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, M.; Zhou, M. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard Mater. 2019, 373, 820–834. [Google Scholar]
- Talooki, E.F.; Ghorbani, M.; Rahimnejad, M.; Lashkenari, M.S. Evaluation of a visible light-responsive polyaniline nanofiber−cadmium sulfide quantum dots photocathode for simultaneous hexavalent chromium reduction and electricity generation in photo-microbial fuel cell. J. Electroanal. Chem. 2020, 873, 114469. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Mi, Y. Cu (II) and Cr (VI) Removal in Tandem with Electricity Generation via Dual-Chamber Microbial Fuel Cells. J. Sustain. 2023, 15, 2388. [Google Scholar]
- Bhowmick, G.D.; Das, S.; Ghangrekar, M.M.; Mitra, A.; Banerjee, R. Improved wastewater treatment by combined system of microbial fuel cell with activated carbon/TiO2 cathode catalyst and membrane bioreactor. J. Inst. Eng. Ser. A 2019, 100, 675–682. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Yang, F. Destruction of tetracycline hydrochloride antibiotics by FeOOH/TiO2 granular activated carbon as expanded cathode in low-cost MBR/MFC coupled system. J. Membrane Sci. 2017, 525, 202–209. [Google Scholar] [CrossRef]
- Liu, C.; Min, Y.; Zhang, A.; Si, Y.; Chen, J.; Yu, H.Q. Electrochemical treatment of phenol-containing wastewater by facet-tailored TiO2: Efficiency, characteristics and mechanisms. Water Res. 2019, 165, 114980. [Google Scholar]
- Zhao, J.; Cheng, L.; Wang, J.; Liu, Y.Y.; Yang, J.; Xu, Q.Z.; Chen, R.S.; Ni, H.W. Heteroatom-doped carbon nanofilm embedded in highly ordered TiO2 nanotube arrays by thermal nitriding with enhanced electrochemical activity. J. Electroanal. Chem. 2019, 852, 113513. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Zhu, Z.Y.; Shen, B.X.; Liu, L.N. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Huang, W. Research progress on the biochar production and its applications in enhancing electron transport and catalysis performance. Res. Environ. Sci. 2021, 34, 1157–1167. [Google Scholar]
- Liang, B.; Li, K.; Liu, Y.; Kang, X. Nitrogen and phosphorus dual-doped carbon derived from chitosan: An excellent cathode catalyst in microbial fuel cell. Chem. Eng. J. 2019, 358, 1002–1011. [Google Scholar]

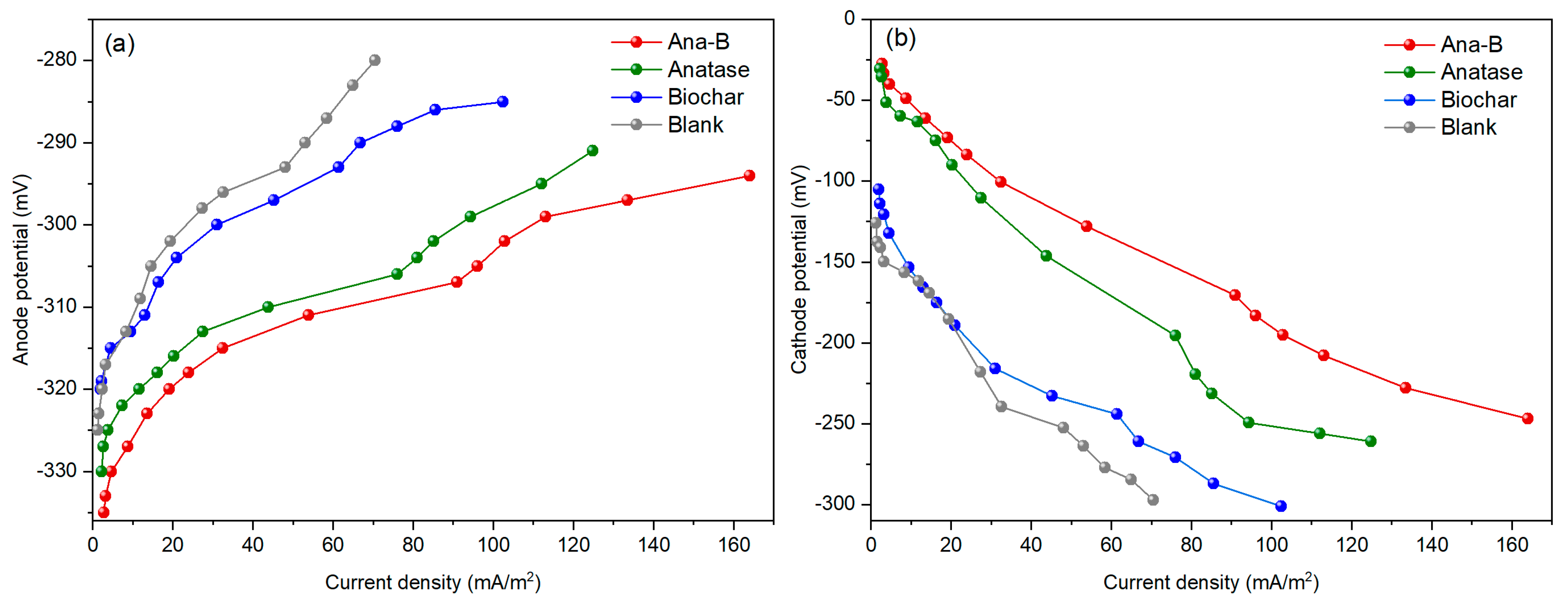
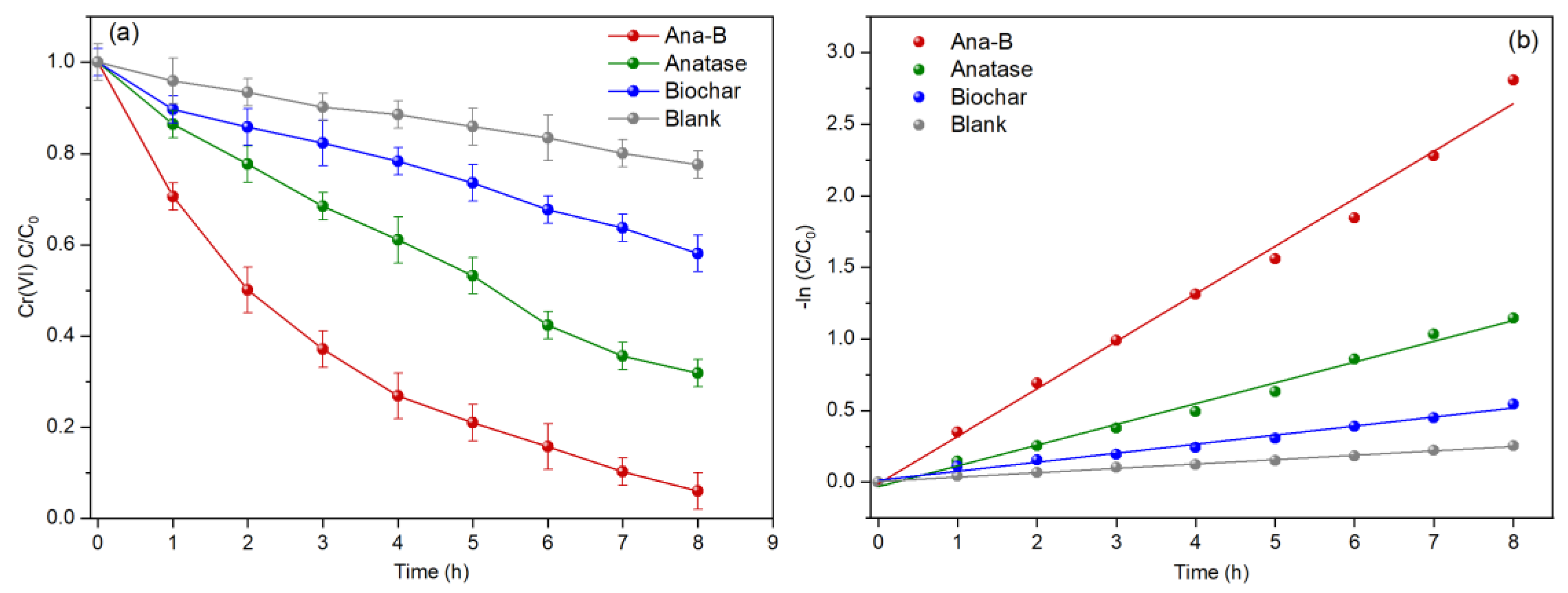
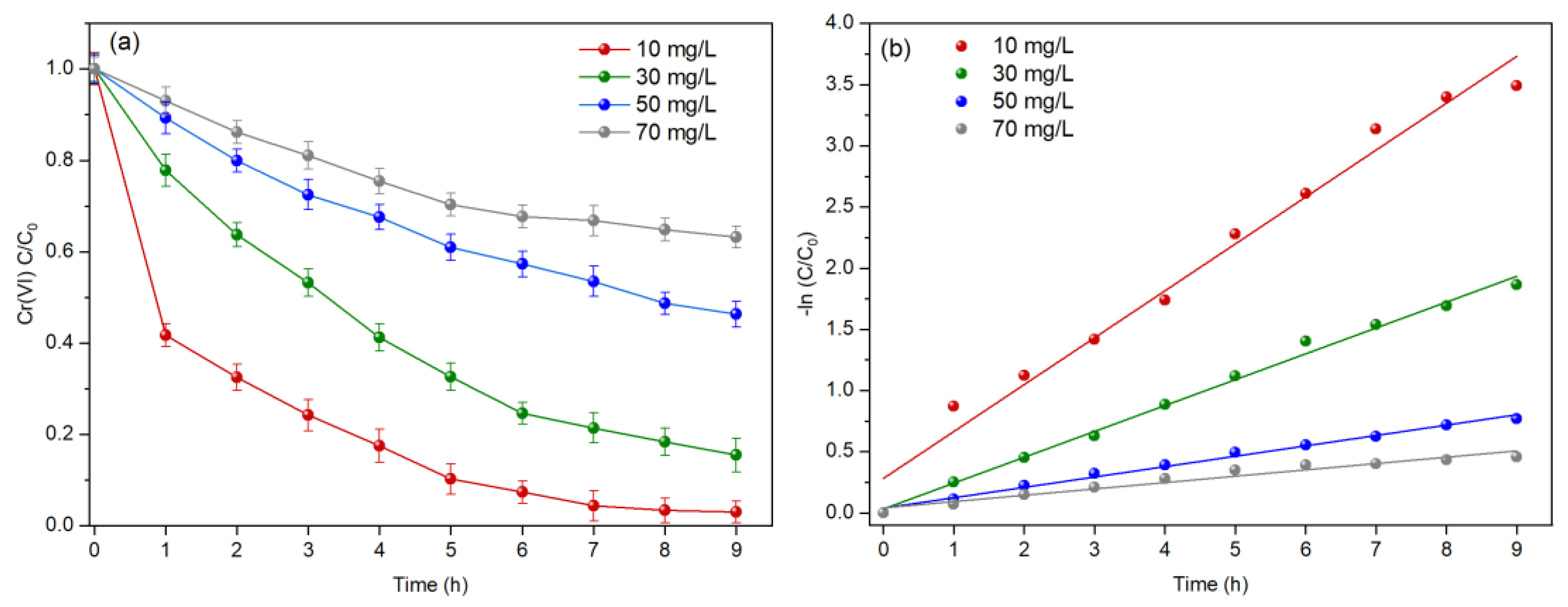
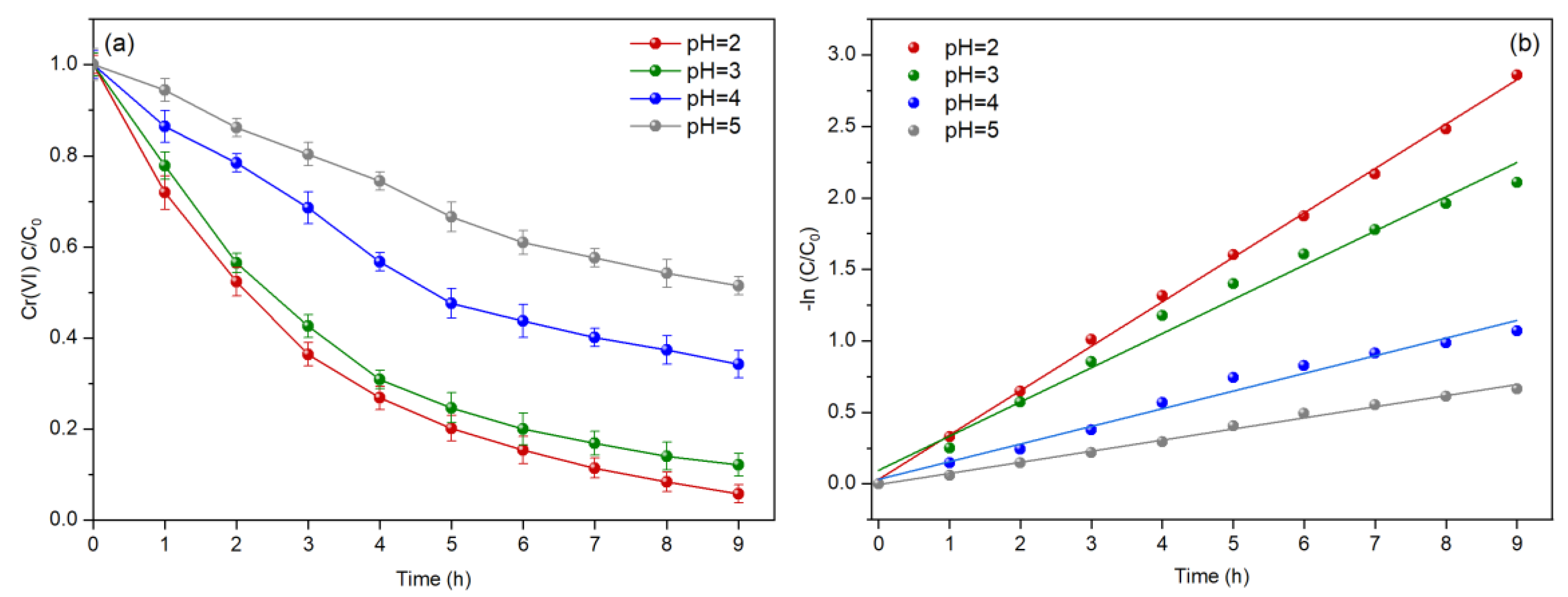
| Systems | Open Circuit Voltage (mV) | Maximum Power Density (mW/m2) | Limiting Current Density (mA/m2) |
|---|---|---|---|
| Ana-B | 311.34 | 10.34 | 164.00 |
| Anatase | 246.00 | 7.22 | 124.81 |
| Biochar | 201.47 | 5.94 | 102.52 |
| Blank | 160 | 2.83 | 70.57 |
| Systems | Fitting Equation | Reaction Rate Constant (K/d−1) | R2 |
|---|---|---|---|
| Ana-B | y = Intercept + Slope * x | 0.3318 ± 0.0116 | 0.9915 |
| Anatase | y = Intercept + Slope * x | 0.145 ± 0.0054 | 0.99 |
| Biochar | y = Intercept + Slope * x | 0.0631 ± 0.0028 | 0.9871 |
| Blank | y = Intercept + Slope * x | 0.0306 ± 0.0008 | 0.9951 |
| Systems | Fitting Equation | Reaction Rate Constant (K/d−1) | R2 |
|---|---|---|---|
| Ana-B | y = Intercept + Slope * x | 0.3832 ± 0.0187 | 0.98125 |
| Anatase | y = Intercept + Slope * x | 0.2113 ± 0.0055 | 0.9945 |
| Biochar | y = Intercept + Slope * x | 0.0847 ± 0.0027 | 0.9915 |
| Blank | y = Intercept + Slope * x | 0.0518 ± 0.0039 | 0.9548 |
| Systems | Fitting Equation | Reaction Rate Constant (K/d−1) | R2 |
|---|---|---|---|
| Ana-B | y = Intercept + Slope * x | 0.3107 ± 0.00385 | 0.9863 |
| Anatase | y = Intercept + Slope * x | 0.2392 ± 0.0105 | 0.9847 |
| Biochar | y = Intercept + Slope * x | 0.1235 ± 0.0059 | 0.9818 |
| Blank | y = Intercept + Slope * x | 0.0777 ± 0.0022 | 0.9935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Miao, Q.; Shi, X.; Zheng, P.; Li, H. Accelerating Electricity Generation and Cr (VI) Removal Using Anatase–Biochar-Modified Cathode Microbial Fuel Cells. Sustainability 2023, 15, 12276. https://doi.org/10.3390/su151612276
Cui X, Miao Q, Shi X, Zheng P, Li H. Accelerating Electricity Generation and Cr (VI) Removal Using Anatase–Biochar-Modified Cathode Microbial Fuel Cells. Sustainability. 2023; 15(16):12276. https://doi.org/10.3390/su151612276
Chicago/Turabian StyleCui, Xinglan, Qingdong Miao, Xinyue Shi, Peng Zheng, and Hongxia Li. 2023. "Accelerating Electricity Generation and Cr (VI) Removal Using Anatase–Biochar-Modified Cathode Microbial Fuel Cells" Sustainability 15, no. 16: 12276. https://doi.org/10.3390/su151612276






