Assessment of Gus Expression Induced by Anti-Sense OsPPO Gene Promoter and Antioxidant Enzymatic Assays in Response to Drought and Heavy Metal Stress in Transgenic Arabidopsis thaliana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construct Preparation
2.2. Plant Growth
2.3. Drought
2.4. Heavy Metals
Cu and Ni Treatment
2.5. GUS Staining
2.6. Enzymatic Assay
2.6.1. Extract Preparations
2.6.2. Catalase (CAT)
2.6.3. Ascorbate Peroxidase
2.6.4. The Reaction Mixture for Peroxidase
2.7. RNA Isolation and cDNA Synthesis
2.8. Expression Analysis of OsPPO Promoter via Real-Time PCR (RT-PCR)
2.9. Statistical Analysis
3. Results
3.1. GUS Staining
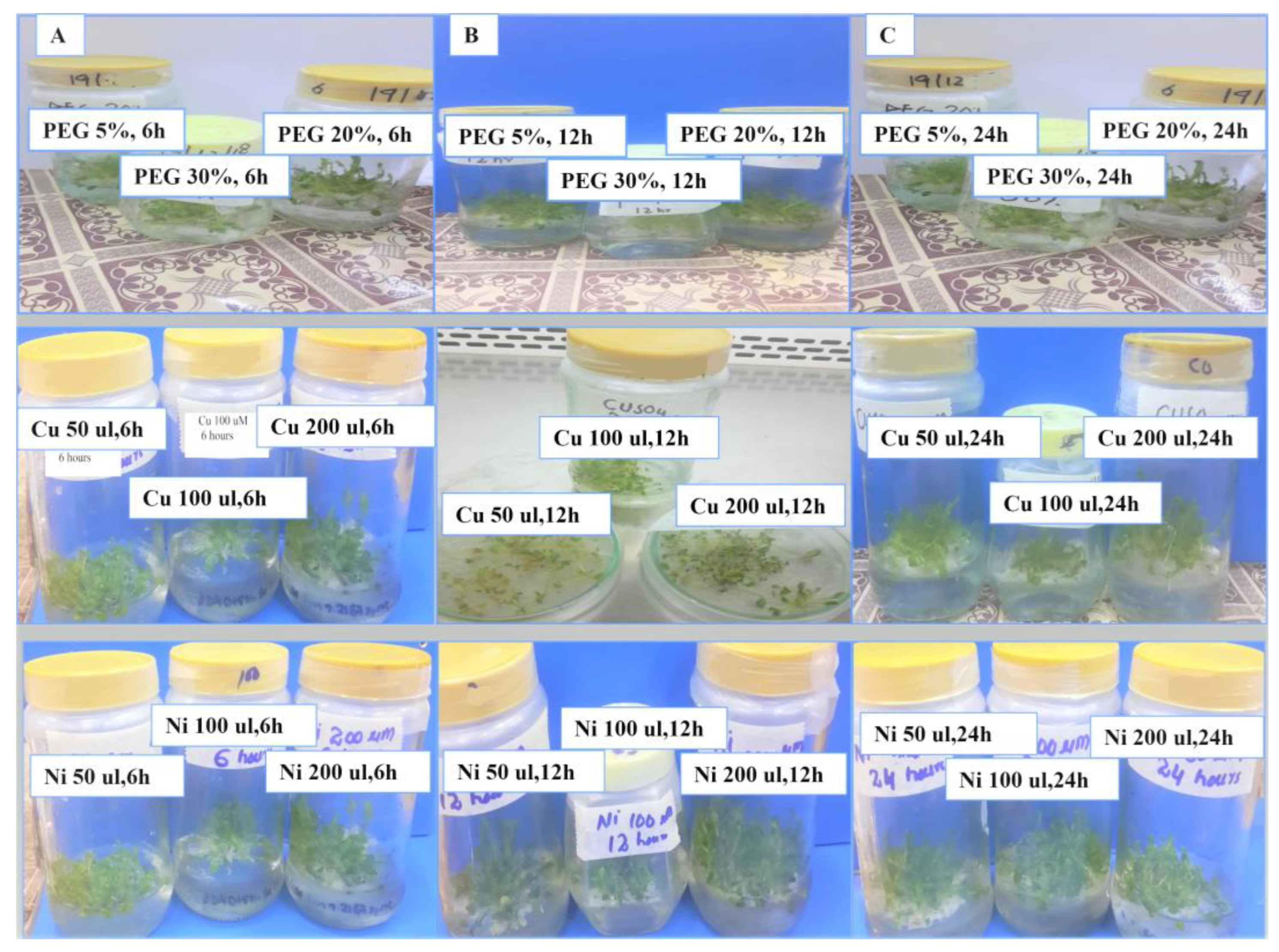
3.1.1. Expression of OsPPOGUS in Response to PEG
3.1.2. Expression of OsPPOGUS in Response to Heavy Metal Stress
3.2. OsPPOGUS Response to Drought Treatment
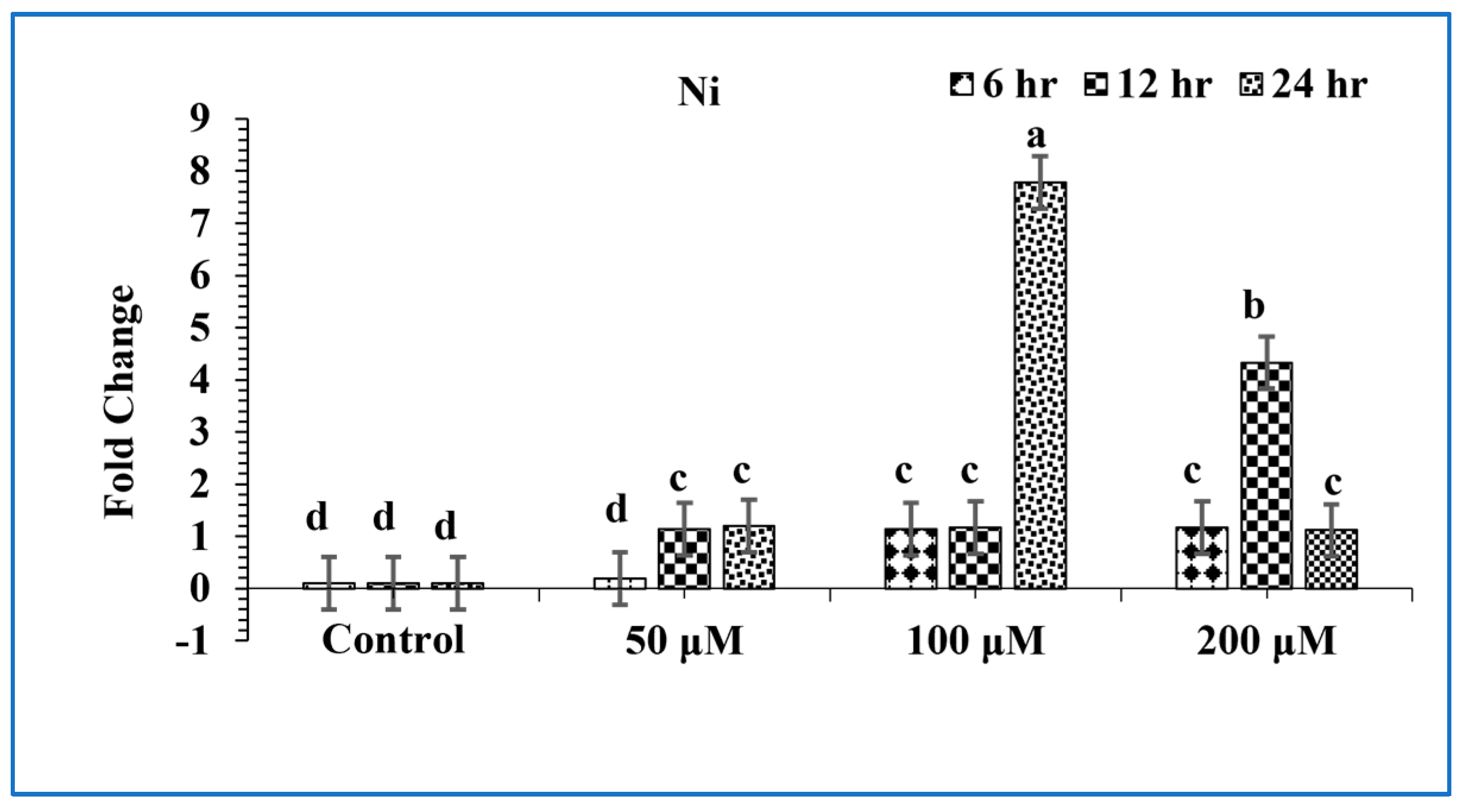
3.3. Induction of OsPPOGUS in Response to Ni Treatment
3.4. OsPPOGUS Response to Cu Treatment
3.5. Quantification of ROS and Antioxidant Assay in A. thaliana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Z.; Yuan, X.; Li, L.; Zeng, M.; Yang, J.; Tang, H.; Duan, C. Genome-wide analysis of the ATP-binding cassette (ABC) transporter family in Zea mays L. and its response to heavy metal stresses. Int. J. Mol. Sci. 2022, 23, 2109. [Google Scholar] [CrossRef]
- Haider, S.; Raza, A.; Iqbal, J.; Shaukat, M.; Mahmood, T. Analyzing the regulatory role of heat shock transcription factors in plant heat stress tolerance: A brief appraisal. Mol. Biol. Rep. 2022, 49, 5771–5785. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.L.; Reis, A.F.D.B.; Mazzafera, P.; Favarin, J.L. Determination of the Water Potential Threshold at Which Rice Growth Is Impacted. Plants 2018, 7, 48. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate Trends and Global Crop Production Since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Na-noparticles Induced Genotoxicity, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cu-cumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef]
- Kogan, F.; Guo, W.; Yang, W. Drought and food security prediction from NOAA new generation of operational satellites. Geomat. Nat. Hazards Risk 2019, 10, 651–666. [Google Scholar] [CrossRef]
- Skuras, D.; Psaltopoulos, D. A broad overview of the main problems derived from climate change that will affect agricultural production in the Mediterranean area. Build. Resil. Adapt. Clim. Chang. Agric. Sect. 2012, 23, 217. [Google Scholar]
- Sperry, J.S.; Hacke, U.; Oren, R.; Comstock, J.P. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002, 25, 251–263. [Google Scholar] [CrossRef]
- Northup, A.P.; Keitt, T.H.; Farrior, C.E. Cavitation-resistant junipers cease transpiration earlier than cavitation-vulnerable oaks under summer dry conditions. Ecohydrology 2022, 15, e2337. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Tripathi, A.; Raghubanshi, A.; Singh, J. Functional traits indicate a continuum of tree drought strategies across a soil water availability gradient in a tropical dry forest. For. Ecol. Manag. 2020, 482, 118740. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Detto, M.; Pacala, S.W. Predicting shifts in the functional composition of tropical forests under increased drought and CO2 from trade-offs among plant hydraulic traits. Ecol. Lett. 2018, 22, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, Y.; Li, Z.; Xie, X.; Gong, E.S.; Tian, J.; Si, X.; Wang, Y.; Gao, N.; Shu, C.; et al. Effects of high hydrostatic pressure and thermal processing on anthocyanin content, polyphenol oxidase and β-glucosidase activities, color, and antioxidant activities of blueberry (Vaccinium spp.) puree. Food Chem. 2020, 342, 128564. [Google Scholar] [CrossRef] [PubMed]
- Ullah, Z.; Gul, F.; Iqbal, J.; Abbasi, B.A.; Kanwal, S.; Chalgham, W.; Mahmood, T. Biogenic Synthesis of Multifunctional Silver Oxide Nanoparticles (Ag2ONPs) Using Parieteria alsinaefolia Delile Aqueous Extract and Assessment of Their Di-verse Biological Applications. Microorganisms 2023, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.S.; Ali, E.F.; Mahfouz, S.A. Comparison between different fertilization sources, irrigation frequency and their combinations on the growth and yield of the coriander plant. Aust. J. Basic Appl. Sci. 2012, 6, 600–615. [Google Scholar]
- Kuromori, T.; Fujita, M.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Inter-tissue and inter-organ signaling in drought stress response and phenotyping of drought tolerance. Plant J. 2022, 109, 342–358. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Hoveizeh, N.F.; Kadkhodaei, S.; Vaculík, M. Comparative morphological, physiological and molecular analyses of drought-stressed strawberry plants affected by SiO2 and SiO2-NPs foliar spray. Sci. Horti-Cult. 2023, 309, 111686. [Google Scholar] [CrossRef]
- Hessini, K.; Wasli, H.; Al-Yasi, H.M.; Ali, E.F.; Issa, A.A.; Hassan, F.A.; Siddique, K.H. Graded moisture deficit effect on secondary metabolites, antioxidant, and inhibitory enzyme activities in leaf extracts of Rosa damascena Mill. var. trigentipetala. Horticulturae 2022, 8, 177. [Google Scholar] [CrossRef]
- Tighe-Neira, R.; Gonzalez-Villagra, J.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Impact of nanoparticles and their ionic counterparts derived from heavy metals on the physiology of food crops. Plant Physiol. Biochem. 2022, 172, 14–23. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc Oxide and Silicone Nanoparticles to Improve the Re-sistance Mechanism and Annual Productivity of Salt-Stressed Mango Trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Sun, L.; Song, F.; Guo, J.; Zhu, X.; Liu, S.; Liu, F.; Li, X. Nano-ZnO-Induced Drought Tolerance is Associated with Melatonin Synthesis and Metabolism in Maize. Int. J. Mol. Sci. 2020, 21, 782. [Google Scholar] [CrossRef]
- Demirevska, K.; Zasheva, D.; Dimitrov, R.; Simova-Stoilova, L.; Stamenova, M.; Feller, U. Drought stress effects on Rubisco in wheat: Changes in the Rubisco large subunit. Acta Physiol. Plant. 2009, 31, 1129–1138. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; El-Mageed, T.A.A.; El-Mageed, S.A.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of Silicon on Plant–Pathogen Interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress, and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Abeed, A.H.A.; Salama, F.M. Attenuating Effect of an Extract of Cd-Hyperaccumulator Solanum nigrum on the Growth and Physio-chemical Changes of Datura innoxia under Cd Stress. J. Soil Sci. Plant Nutr. 2022, 22, 4868–4882. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, R.; Zhang, M.; Xia, G.; Liu, S. Functional analysis of long non-coding RNAs involved in alkaline stress responses in wheat. J. Exp. Bot. 2022, 73, 5698–5714. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.Q.; Xu, M.J.; Zhang, C.M.; Gao, J.; Li, C.G.; Xing, K.; Qin, S. Antifungal volatile organic compounds from Strep-tomyces setonii WY228 control black spot disease of sweet potato. Appl. Environ. Microbiol. 2022, 88, e02317-21. [Google Scholar] [CrossRef]
- Miao, S.; Li, F.; Han, Y.; Yao, Z.; Xu, Z.; Chen, X.; Liu, J.; Zhang, Y.; Wang, A. Identification of OSCA gene family in Solanum habrochaites and its function analysis under stress. BMC Genom. 2022, 23, 547. [Google Scholar] [CrossRef] [PubMed]
- Gozari, M.; Hafezieh, M.; Tamadoni Jahromi, S.; Pourmozaffar, S.; Moradi, Y. Evaluation of inhibitory effect of secondary metabolites of Streptomyces sp. Strain SC 190 on polyphenoloxidase activity to prevent melanosis in Pacific white shrimp (Litopenaeus vannamei). J. Aquat. Ecol. 2020, 10, 22–32. [Google Scholar]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2020, 181, 112588. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Bravo, R.; Nederpel, C.; Naranjo, S.; Kim, H.K.; Rodríguez-López, M.J.; Chen, G.; Glauser, G.; Leiss, K.A.; Klinkhamer, P.G. Ultravio-let radiation modulates both constitutive and inducible plant defenses against thrips but is dose and plant genotype de-pendent. J. Pest Sci. 2021, 94, 69–81. [Google Scholar] [CrossRef]
- Shariatipour, N.; Heidari, B.; Ravi, S.; Stevanato, P. Genomic analysis of ionome-related QTLs in Arabidopsis thaliana. Sci. Rep. 2021, 11, 19194. [Google Scholar] [CrossRef] [PubMed]
- El-Flaah, R.F.; El-Said, R.A.; Nassar, M.A.; Hassan, M.; Abdelaal KA, A. Effect of rhizobium, nano silica and ascorbic acid on morpho-physiological characters and gene expression of POX and PPO in faba bean (Vicia faba L.) Under salinity stress conditions. Fresenius Environ. Bull. 2021, 30, 5751–5764. [Google Scholar]
- Alzandi, A.A.; Naguib, D.M. Effect of hydropriming on Trigonella foenum callus growth, biochemical traits and phyto-chemical components under PEG treatment. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 141, 179–190. [Google Scholar] [CrossRef]
- Moazami-Goodarzi, K.; Mortazavi, S.; Heidari, B.; Norouzi, P. Optimization of agrobacterium-mediated transformation of sugar beet: Glyphosate and insect pests resistance associated genes. Agron. J. 2020, 112, 4558–4567. [Google Scholar] [CrossRef]
- Akhtar, W.; Aziz, E.; Koiwa, H.; Mahmood, T. Characterization of rice polyphenol oxidase promoter in transgenic Arabidopsis thaliana. Turk. J. Bot. 2017, 41, 223–233. [Google Scholar] [CrossRef]
- Akhtar, W. Expression Profiling of Reporter Genes Induced by Rice Polyphenol Oxidase Promoter in Transgenic Model Plant. Ph.D. Thesis, Quaid-i-Azam University, Islamabad, Pakistan, 2016. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant secondary metabolites as a tool to investigate biotic stress tolerance in plants: A review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-W.; Wang, C.-K.; Huang, X.-Y.; Hu, D.-G. Anthocyanin stability and degradation in plants. Plant Signal. Behav. 2021, 16, 1987767. [Google Scholar] [CrossRef] [PubMed]
- Oñate-Sánchez, L.; Vicente-Carbajosa, J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res. Notes 2008, 1, 93. [Google Scholar] [CrossRef]
- Hakiman, M.; Maziah, M. Non enzymatic and enzymatic antioxidant activities in aqueous extract of different Ficus del-toidea accessions. J. Med. Plants Res. 2009, 3, 120–131. [Google Scholar]
- Grilo, L.F.; Martins, J.D.; Cavallaro, C.H.; Nathanielsz, P.W.; Oliveira, P.J.; Pereira, S.P. Development of a 96-well based assay for kinetic determination of catalase enzymatic-activity in biological samples. Toxicol. In Vitro 2020, 69, 104996. [Google Scholar] [CrossRef]
- Gomes, M.P.; Kitamura, R.S.A.; Marques, R.Z.; Barbato, M.L.; Zámocký, M. The Role of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Catalase) in the Tolerance of Lemna minor to Antibiotics: Implications for Phytoremediation. Antioxidants 2022, 11, 151. [Google Scholar] [CrossRef]
- Gao, K.; Khan, W.U.; Li, J.; Huang, S.; Yang, X.; Guo, T.; Guo, B.; Wu, R.; An, X. Identification and Validation of Reliable Reference Genes for Gene Expression Studies in Koelreuteria paniculata. Genes 2022, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Javid, M.G.; Soltani, E.; Wojtyla, L.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Peng, J.; Dong, B.; Wang, D.; Zhao, Y.; Meng, H.; Zhou, H. Analysis of differential metabolites of sunflower induced by virulent Verticillium dahlia V33 and hypovirulent Gibellulopsis nigrescens Vn-1. J. Phytopathol. 2022, 170, 349–358. [Google Scholar] [CrossRef]
- Attia, M.S.; Abdelaziz, A.M.; Al-Askar, A.A.; Arishi, A.A.; Abdelhakim, A.M.; Hashem, A.H. Plant Growth-Promoting Fungi as Biocontrol Tool against Fusarium Wilt Disease of Tomato Plant. J. Fungi 2022, 8, 775. [Google Scholar] [CrossRef]
- Vidović, M.; Battisti, I.; Pantelić, A.; Morina, F.; Arrigoni, G.; Masi, A.; Jovanović, S.V. Desiccation Tolerance in Ramonda serbica Panc.: An Integrative Transcriptomic, Proteomic, Metabolite and Photosynthetic Study. Plants 2022, 11, 1199. [Google Scholar] [CrossRef]
- Vásquez, C.; Dávila, M.; Méndez, N.; Jiménez, M.A.; Sandoval, M.F.; Alcalá, F.J. Oxidative Enzymes in Coconut cultivars in response to Raoiella indica feeding. Afr. J. Biotechnol. 2016, 15, 1755–1762. [Google Scholar]
- Muhammad, F.; Raza, M.A.S.; Iqbal, R.; Zulfiqar, F.; Aslam, M.U.; Yong, J.W.H.; Altaf, M.A.; Zulfiqar, B.; Amin, J.; Ibrahim, M.A. Ameliorating Drought Effects in Wheat Using an Exclusive or Co-Applied Rhizobacteria and ZnO Nanoparticles. Biology 2022, 11, 1564. [Google Scholar] [CrossRef]
- Berumen-Varela, G.; Martínez-González, M.E.; Palomino-Hermosillo, Y.A.; Jiménez-Zurita, J.O.; Peña-Sandoval, G.R.; Balois-Morales, R. Expression profile of EXP, Succ-CoA and ALDH genes in soursop (Annona muricata L.) fruits during ripening in response to refrigeration conditions. Indian J. Biotechnol. 2020, 19, 254–262. [Google Scholar]
- Kumari, S.; Khanna, R.R.; Nazir, F.; Albaqami, M.; Chhillar, H.; Wahid, I.; Khan, M.I.R. Bio-Synthesized Nanoparticles in Developing Plant Abiotic Stress Resilience: A New Boon for Sustainable Approach. Int. J. Mol. Sci. 2022, 23, 4452. [Google Scholar] [CrossRef] [PubMed]
- Einhardt, A.M.; Ferreira, S.; Hawerroth, C.; Valadares, S.V.; Rodrigues, F. Nickel potentiates soybean resistance against infection by Phakopsora pachyrhizi. Plant Pathol. 2020, 69, 849–859. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.-L.; Li, J.; Chen, M.; An, R.-D. Comparative study on the bioaccumulation of lead, cadmium and nickel and their toxic effects on the growth and enzyme defence strategies of a heavy metal accumulator, Hydrilla verticillata (L.f.) Royle. Environ. Sci. Pollut. Res. 2020, 27, 9853–9865. [Google Scholar] [CrossRef]










| Chemical Name | Concentration | Volume |
|---|---|---|
| Potassium phosphate buffer | 25 mM (pH 7.8) | 1.4 mL |
| EDTA 100 mM | 0.4 mM | 16 µL |
| Ascorbic Acid 200 mM | 1 mM | 20 µL |
| Polyvinyl pyrmildone | 2% | 0.08 gm |
| Water | 2.5 mL | |
| Total | 4 mL |
| Chemical Name | Concentration | Volume |
|---|---|---|
| Potassium phosphate buffer | 25 mM (pH 7.0) | 357 µL |
| Ascorbic acid (50 mM) | 0.25 mM | 6 µL |
| EDTA (100 mM) | 0.1 mM | 1.5 µL |
| H2O2 (100 mM) | 10 mM | 110 µL |
| Enzyme extract | 0.05 mL | 52 µL |
| Water | 487 µL | |
| Total | 1 mL |
| Chemical Name | Concentration | Volume |
|---|---|---|
| Sodium phosphate buffer | 100 mM (pH 7.8) | 1.5 mL |
| Poly phenylenediamine | 4% | 1 mL |
| H2O2 | 1% | 1 mL |
| Enzyme extract | 50 µL | |
| Water | 450 µL | |
| H2SO4 | 5 N | 1 mL |
| Total | 5 mL |
| Primer | Primer Sequence |
|---|---|
| GUS F: | 5′ CGGCAGAGAAGGTACTGGAA 3′ |
| GUS R: | 5′ ATATCCAGCCATGCACACTG 3′ |
| Actintac F: | 5′ GATGAAGATACTCACAGAAAGA 3′ |
| Actintac R: | 5′ GTGGTTTCATGAATGCCAGCA 3′ |
| Variable | Minimum | Maximum | Mean | Std. Deviation | Mean Squares of Error | Significance |
|---|---|---|---|---|---|---|
| Ni | 0.100 | 7.780 | 1.628 | 2.247 | 4.905 | 0.46129 |
| Cu | 0.100 | 15.030 | 3.323 | 4.285 | 6.502 | 0.03743 |
| PEG | 0.030 | 1.900 | 0.279 | 0.520 | 0.255 | 0.43417 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, Z.; Iqbal, J.; Abbasi, B.A.; Akhtar, W.; Kanwal, S.; Ali, I.; Chalgham, W.; El-Sheikh, M.A.; Mahmood, T. Assessment of Gus Expression Induced by Anti-Sense OsPPO Gene Promoter and Antioxidant Enzymatic Assays in Response to Drought and Heavy Metal Stress in Transgenic Arabidopsis thaliana. Sustainability 2023, 15, 12783. https://doi.org/10.3390/su151712783
Ullah Z, Iqbal J, Abbasi BA, Akhtar W, Kanwal S, Ali I, Chalgham W, El-Sheikh MA, Mahmood T. Assessment of Gus Expression Induced by Anti-Sense OsPPO Gene Promoter and Antioxidant Enzymatic Assays in Response to Drought and Heavy Metal Stress in Transgenic Arabidopsis thaliana. Sustainability. 2023; 15(17):12783. https://doi.org/10.3390/su151712783
Chicago/Turabian StyleUllah, Zakir, Javed Iqbal, Banzeer Ahsan Abbasi, Wasim Akhtar, Sobia Kanwal, Iftikhar Ali, Wadie Chalgham, Mohamed A. El-Sheikh, and Tariq Mahmood. 2023. "Assessment of Gus Expression Induced by Anti-Sense OsPPO Gene Promoter and Antioxidant Enzymatic Assays in Response to Drought and Heavy Metal Stress in Transgenic Arabidopsis thaliana" Sustainability 15, no. 17: 12783. https://doi.org/10.3390/su151712783
APA StyleUllah, Z., Iqbal, J., Abbasi, B. A., Akhtar, W., Kanwal, S., Ali, I., Chalgham, W., El-Sheikh, M. A., & Mahmood, T. (2023). Assessment of Gus Expression Induced by Anti-Sense OsPPO Gene Promoter and Antioxidant Enzymatic Assays in Response to Drought and Heavy Metal Stress in Transgenic Arabidopsis thaliana. Sustainability, 15(17), 12783. https://doi.org/10.3390/su151712783










