Effectiveness of Respiratory Exercises on Perceived Symptoms of Fatigue among Multiple Sclerosis Patients: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Selection Criteria
- Participants: people with MS
- Intervention: respiratory physiotherapy techniques or training of the respiratory muscles.
- Comparison: control group, with or without treatment.
- Outcome: results of fatigue, before and after treatment.
- Study design: clinical trials.
2.4. Study Selection Process and Data Extraction
2.5. Risk of Bias Assessment
3. Results
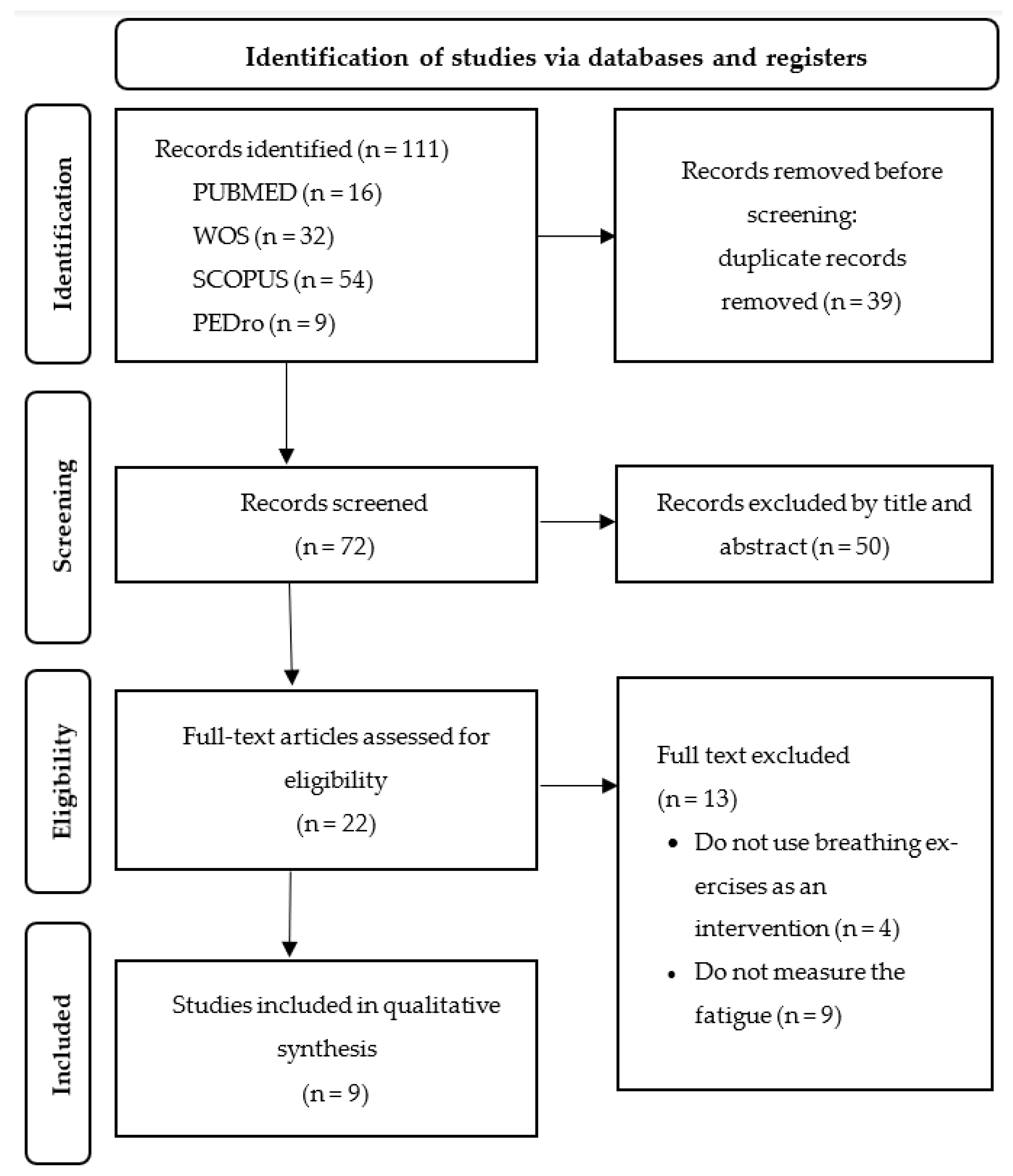
3.1. Study Selection
3.2. Study Characteristics
3.3. Evaluation or Questionnaires Used
3.4. Intervention and/or Treatments Applied
3.5. Methodogical Quality Assessment
4. Discussion
4.1. Study Limitations
4.2. Clinical Practice and Policy Implications of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of Multiple Sclerosis: Progress and Challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, H.-X.; An, Y.; Yin, L.; Sun, P.-P.; Zhao, J.-N.; Yan, J.-T. The Efficacy and Safety of Manual Therapy for Symptoms Associated with Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Integr. Complement. Med. 2022, 28, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Harirchian, M.H.; Fatehi, F.; Sarraf, P.; Honarvar, N.M.; Bitarafan, S. Worldwide Prevalence of Familial Multiple Sclerosis: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2018, 20, 43–47. [Google Scholar] [CrossRef]
- García López, F.J.; García-Merino, A.; Alcalde-Cabero, E.; de Pedro-Cuesta, J. Incidencia y Prevalencia de La Esclerosis Múltiple En España. Una Revisión Sistemática. Neurología, 2022; in press. [Google Scholar] [CrossRef]
- Goodin, D.S.; Khankhanian, P.; Gourraud, P.A.; Vince, N. The Nature of Genetic and Environmental Susceptibility to Multiple Sclerosis. PLoS ONE 2021, 16, e0246157. [Google Scholar] [CrossRef]
- Abou, L.; Qin, K.; Alluri, A.; Du, Y.; Rice, L.A. The Effectiveness of Physical Therapy Interventions in Reducing Falls among People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Bodyw. Mov. Ther. 2022, 29, 74–85. [Google Scholar] [CrossRef]
- Levy, J.; Prigent, H.; Bensmail, D. Respiratory Rehabilitation in Multiple Sclerosis: A Narrative Review of Rehabilitation Techniques. Ann. Phys. Rehabil. Med. 2018, 61, 38–45. [Google Scholar] [CrossRef]
- Royer, N.; Duboeuf, M.; Camdessanché, J.-P.; Millet, G. Prevalence of Fatigue and Its Explicative Variables among People with Multiple Sclerosis. NeuroRehabilitation 2022, 51, 509–517. [Google Scholar] [CrossRef]
- Manjaly, Z.M.; Harrison, N.A.; Critchley, H.D.; Do, C.T.; Stefanics, G.; Wenderoth, N.; Lutterotti, A.; Müller, A.; Stephan, K.E. Pathophysiological and Cognitive Mechanisms of Fatigue in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 642–651. [Google Scholar] [CrossRef]
- Razazian, N.; Kazeminia, M.; Moayedi, H.; Daneshkhah, A.; Shohaimi, S.; Mohammadi, M.; Jalali, R.; Salari, N. The Impact of Physical Exercise on the Fatigue Symptoms in Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. BMC Neurol. 2020, 20, 93. [Google Scholar] [CrossRef]
- Krupp, L.B.; Serafin, D.J.; Christodoulou, C. Multiple Sclerosis-Associated Fatigue. Expert Rev. Neurother. 2010, 10, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Mohebbirad, M.; Motaharinezhad, F.; Shahsavary, M.; Joveini, G. Effects of Sensory Interventions on Fatigue in People With Multiple Sclerosis: A Systematic Review. Int. J. MS Care 2022, 24, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Coates, K.D.; Aboodarda, S.J.; Krüger, R.L.; Martin, T.; Metz, L.M.; Jarvis, S.E.; Millet, G.Y. Multiple Sclerosis-Related Fatigue: The Role of Impaired Corticospinal Responses and Heightened Exercise Fatigability. J. Neurophysiol. 2020, 124, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Hensen, H.A.; Krishnan, A.V.; Eckert, D.J. Sleep-Disordered Breathing in People with Multiple Sclerosis: Prevalence, Pathophysiological Mechanisms, and Disease Consequences. Front. Neurol. 2018, 8, 740. [Google Scholar] [CrossRef]
- Broch, L.; Simonsen, C.S.; Flemmen, H.Ø.; Berg-Hansen, P.; Skardhamar, Å.; Ormstad, H.; Celius, E.G. High Prevalence of Fatigue in Contemporary Patients with Multiple Sclerosis. Mult. Scler. J.—Exp. Transl. Clin. 2021, 7, 2055217321999826. [Google Scholar] [CrossRef]
- Perrild, H.; Møller, A.; Pedersen, H.; Siersbæk-Nielsen, K. Undetectable Thyrotropin Levels Using Immunoradiometric Assay in L-Dopa-Treated Patients. J. Neurol. 1988, 235, 255. [Google Scholar] [CrossRef]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the Functional Impact of Fatigue: Initial Validation of the Fatigue Impact Scale. Clin. Infect. Dis. 1994, 18, S79–S83. [Google Scholar] [CrossRef]
- Téllez, N.; Río, J.; Tintoré, M.; Nos, C.; Galán, I.; Montalban, X. Does the Modified Fatigue Impact Scale Offer a More Comprehensive Assessment of Fatigue in MS? Mult. Scler. 2005, 11, 198–202. [Google Scholar] [CrossRef]
- Casuso-Holgado, M.J.; Martín-Valero, R.; Carazo, A.F.; Medrano-Sánchez, E.M.; Cortés-Vega, M.D.; Montero-Bancalero, F.J. Effectiveness of Virtual Reality Training for Balance and Gait Rehabilitation in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2018, 32, 1220–1234. [Google Scholar] [CrossRef]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Chung, L.H.; Rubio-Arias, J. Dosage and Effectiveness of Aerobic Training on Cardiorespiratory Fitness, Functional Capacity, Balance, and Fatigue in People With Multiple Sclerosis: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2021, 102, 1826–1839. [Google Scholar] [CrossRef]
- Neal, W.N.; Cederberg, K.L.; Jeng, B.; Sasaki, J.E.; Motl, R.W. Is Symptomatic Fatigue Associated With Physical Activity and Sedentary Behaviors Among Persons With Multiple Sclerosis? Neurorehabil. Neural Repair 2020, 34, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, B.; Revirajan, N.; Morris, B.; Cordano, C.; Creasman, J.; Manguinao, M.; Krysko, K.; Rutatangwa, A.; Auvray, C.; Aljarallah, S.; et al. Safety and Efficacy of Amantadine, Modafinil, and Methylphenidate for Fatigue in Multiple Sclerosis: A Randomised, Placebo-Controlled, Crossover, Double-Blind Trial. Lancet Neurol. 2021, 20, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Fabbri, M.; Poewe, W. Amantadine in the Treatment of Parkinson’s Disease and Other Movement Disorders. Lancet Neurol. 2021, 20, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Chung, L.H.; Martínez-Rodríguez, A.; Rubio-Arias, J.Á. Effects and Optimal Dosage of Resistance Training on Strength, Functional Capacity, Balance, General Health Perception, and Fatigue in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Disabil. Rehabil. 2023, 45, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.D.; Mahoney, M.C.; Fisher, N.M. Measures of Respiratory Function Correlate with Fatigue in Ambulatory Persons with Multiple Sclerosis. Disabil. Rehabil. 2015, 37, 2407–2412. [Google Scholar] [CrossRef]
- Templeman, L.; Roberts, F. Effectiveness of Expiratory Muscle Strength Training on Expiratory Strength, Pulmonary Function and Cough in the Adult Population: A Systematic Review. Physiotherapy 2020, 106, 43–51. [Google Scholar] [CrossRef]
- Ferreira, G.D.; Costa, A.C.C.; Plentz, R.D.M.; Coronel, C.C.; Sbruzzi, G. Respiratory Training Improved Ventilatory Function and Respiratory Muscle Strength in Patients with Multiple Sclerosis and Lateral Amyotrophic Sclerosis: Systematic Review and Meta-Analysis. Physiotherapy 2016, 102, 221–228. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Klefbeck, B.; Nedjad, J.H. Effect of Inspiratory Muscle Training in Patients with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2003, 84, 994–999. [Google Scholar] [CrossRef]
- Fry, D.K.; Pfalzer, L.A.; Chokshi, A.R.; Wagner, M.T.; Jackson, E.S. Randomized Control Trial of Effects of a 10-Week Inspiratory Muscle Training Program on Measures of Pulmonary Function in Persons with Multiple Sclerosis. J. Neurol. Phys. Ther. 2007, 31, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sánchez, A.M.; Matarán-Peñarrocha, G.A.; Lara-Palomo, I.; Saavedra-Hernández, M.; Arroyo-Morales, M.; Moreno-Lorenzo, C. Hydrotherapy for the Treatment of Pain in People with Multiple Sclerosis: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2012, 2012, 473963. [Google Scholar] [CrossRef]
- Bayraktar, D.; Guclu-Gunduz, A.; Yazici, G.; Lambeck, J.; Batur-Caglayan, H.Z.; Irkec, C.; Nazliel, B. Effects of Ai-Chi on Balance, Functional Mobility, Strength and Fatigue in Patients with Multiple Sclerosis: A Pilot Study. NeuroRehabilitation 2013, 33, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.D.; Udhoji, S.; Mashtare, T.L.; Fisher, N.M. A Combined Inspiratory and Expiratory Muscle Training Program Improves Respiratory Muscle Strength and Fatigue in Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2013, 94, 1964–1970. [Google Scholar] [CrossRef]
- Bulguroglu, I.; Guclu-Gunduz, A.; Yazici, G.; Ozkul, C.; Irkec, C.; Nazliel, B.; Batur-Caglayan, H.Z. The Effects of Mat Pilates and Reformer Pilates in Patients with Multiple Sclerosis: A Randomized Controlled Study. NeuroRehabilitation 2017, 41, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Grubić Kezele, T.; Babić, M.; Štimac, D. Exploring the Feasibility of a Mild and Short 4-Week Combined Upper Limb and Breathing Exercise Program as a Possible Home Base Program to Decrease Fatigue and Improve Quality of Life in Ambulatory and Non-Ambulatory Multiple Sclerosis Individuals. Neurol. Sci. 2019, 40, 733–743. [Google Scholar] [CrossRef]
- Grubić Kezele, T.; Trope, Z.; Ahel, V.; Ružić, N.; Omrčen, H.; Dudarić, L.; Fužinac-Smojver, A. Upper-Lower Limb and Breathing Exercise Program for Improving Sleep Quality and Psychological Status in Multiple Sclerosis: A Pilot Randomized Controlled Trial. Brain Impair. 2021, 24, 86–102. [Google Scholar] [CrossRef]
- Ghannadi, S.; Noormohammadpour, P.; Mazaheri, R.; Sahraian, M.A.; Mansournia, M.A.; Pourgharib Shahi, M.H.; Salmasi Fard, A.H.; Abolhasani, M. Effect of Eight Weeks Respiratory Muscle Training on Respiratory Capacity, Functional Capacity and Quality of Life on Subjects with Mild to Moderate Relapsing-Remitting Multiple Sclerosis: A Single-Blinded Randomized Controlled Trial. Mult. Scler. Relat. Disord. 2022, 68, 104208. [Google Scholar] [CrossRef]
- Huang, M.H.; Fry, D.; Doyle, L.; Burnham, A.; Houston, N.; Shea, K.; Smith, H.; Wiske, L.; Goode, J.; Khitrik, E.; et al. Effects of Inspiratory Muscle Training in Advanced Multiple Sclerosis. Mult. Scler. Relat. Disord. 2020, 37, 101492. [Google Scholar] [CrossRef]
- Gosselink, R.; Kovacs, L.; Ketelaer, P.; Carton, H.; Decramer, M. Respiratory Muscle Weakness and Respiratory Muscle Training in Severely Disabled Multiple Sclerosis Patients. Arch. Phys. Med. Rehabil. 2000, 81, 747–751. [Google Scholar] [CrossRef]
- Amedoro, A.; Berardi, A.; Conte, A.; Pelosin, E.; Valente, D.; Maggi, G.; Tofani, M.; Galeoto, G. The Effect of Aquatic Physical Therapy on Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2020, 41, 102022. [Google Scholar] [CrossRef] [PubMed]
- Soysal Tomruk, M.; Uz, M.Z.; Kara, B.; Idiman, E. Effects of Pilates Exercises on Sensory Interaction, Postural Control and Fatigue in Patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2016, 7, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.; Munari, D.; Geroin, C.; Gajofatto, A.; Benedetti, M.D.; Midiri, A.; Carla, F.; Picelli, A.; Waldner, A.; Smania, N. Sensory Integration Balance Training in Patients with Multiple Sclerosis: A Randomized, Controlled Trial. Mult. Scler. 2015, 21, 1453–1462. [Google Scholar] [CrossRef]
- Brichetto, G.; Piccardo, E.; Pedullà, L.; Battaglia, M.A.; Tacchino, A. Tailored Balance Exercises on People with Multiple Sclerosis: A Pilot Randomized, Controlled Study. Mult. Scler. 2015, 21, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Tacchino, A.; Brichetto, G.; Zaratin, P.; Battaglia, M.A.; Ponzio, M. Multiple Sclerosis and Rehabilitation: An Overview of the Different Rehabilitation Settings. Neurol. Sci. 2017, 38, 2131–2138. [Google Scholar] [CrossRef]
- Krupp, L.B. The fatigue severity scale. Arch. Neurol. 1989, 46, 1121. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, M.E.; Romero-Arenas, S.; Giráldez-García, M.A.; Colomer-Poveda, D.; Márquez, G. Acute Psychophysiological Responses during Exercise While Using Resistive Respiratory Devices: A Systematic Review. Physiol. Behav. 2022, 256, 113968. [Google Scholar] [CrossRef]
- Hoffman, M.; Augusto, V.M.; Eduardo, D.S.; Silveira, B.M.F.; Lemos, M.D.; Parreira, V.F. Inspiratory Muscle Training Reduces Dyspnea during Activities of Daily Living and Improves Inspiratory Muscle Function and Quality of Life in Patients with Advanced Lung Disease. Physiother. Theory Pract. 2021, 37, 895–905. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
| Databases and Search Terms | Initial Search Results | Records Identified |
|---|---|---|
| PUBMED (((“Multiple Sclerosis”[Mesh]) OR (“multiple sclerosis”)) OR (“Sclerosis, Multiple”) AND (randomized controlled trial [Filter])) AND ((((((“Breathing Exercises”[Mesh]) OR (“Exercise, Breathing”)) OR (“Respiratory Muscle Training”)) OR (“Muscle Training, Respiratory”)) OR (“Training, Respiratory Muscle”)) OR (“Breathing Exercises”)) | 17 | 16 |
| WEB OF SCIENCE (((“Multiple Sclerosis”[Mesh]) OR (multiple sclerosis)) OR (Sclerosis, Multiple) AND (randomized controlled trial [Filter])) AND ((((((“Breathing Exercises”[Mesh]) OR (Exercise, Breathing)) OR (Respiratory Muscle Training)) OR (Muscle Training, Respiratory)) OR (Training, Respiratory Muscle)) OR (Breathing Exercises)) | 148 | 32 |
| SCOPUS (“Multiple Sclerosis”[Mesh]) OR (multiple sclerosis) OR (Sclerosis, Multiple) AND (“Breathing Exercises”[Mesh]) OR (Exercise, Breathing) OR (Respiratory Muscle Training) OR (Muscle Training, Respiratory) OR (Training, Respiratory Muscle) OR (Breathing Exercises) | 164 | 54 |
| PEDRO (Multiple Sclerosis) AND (Breathing exercises) | 11 | 9 |
| Author, Year | Type of Study | Sample Size (Participants) | Age | Duration | Measured Variables | Aim of Results |
|---|---|---|---|---|---|---|
| Klefbeck et al., 2003 [31]. | Randomized controlled trial | 15 participants: 9 women and 6 men | Between 37 and 61 years (average age: 49) | 10 weeks | PImax, PEmax, VC, FVC, FEV1, FEV%, PEF, and fatigue | PImax and PEmax improved after 10 weeks in the GA, without changes in the CG. There were no significant differences between the groups in FSS. |
| Fry et al., 2007 [32]. | Randomized controlled trial | 46 participants: 38 women and 8 men. | Mean age: EG 50 years and CG 46.2 years. | 10 weeks | FVC, FEV1, MIP, MEP, MVV, and fatigue | FEV1 and FVC improved in the experimental group. There were no significant differences between the groups in FSS. |
| Castro-Sanchez et al., 2012 [33]. | Randomized controlled trial | 73 participants: 50 women and 23 men. EG: 36 (26 F, 10 M) and CG: 37 (24F, 13M). | Mean age: EG 46 ± 9.97 and CG 50 ± 12.31 | 20 weeks | Pain, disability, spasticity, depression, fatigue, and autonomy | Improvements in pain, spasms, fatigue, disability, autonomy, depression, and functional independence in the EG. |
| Bayraktar et al., 2013 [34]. | Non-randomized controlled trial | 18 participants: 18 women (11 EG and 7 CG). | Mean age: EG 38 years and CG 39 years | 8 weeks | Static standing balance, functional mobility, upper and lower muscle strength, and fatigue | Improvements in balance, functional mobility, muscle strength in upper and lower limbs, and fatigue in the experimental group. There were no significant differences in the CG. |
| Ray et al., 2013 [35]. | Quasi-experimental before–after trial | 21 participants: 16 women and 5 men. EG: 11 (9 F, 2 M) and CG: 10 (7 F, 3 M). | Mean age: EG 50.9 ± 5.7 and CG 56.2 ± 8.8 | 5 weeks | PImax, Pemax, fatigue, FVC, FEV1, MVV | Improvements in Pimax and Pemax and fatigue in EG. There were no significant differences in the CG. |
| Bulguroglu et al., 2017 [36]. | Randomized controlled trial | 38 participants: Mat pilates: 12 participants Pilates reformer: 13 participants CG: 13 participants. | Mean ages: Mat pilates: 45 years (39.3–45.5) Pilates reformer: 37 (29.5–40) CG: 40 (26–43) | 8 weeks | Balance, CORE stability, mobility, fatigue, and quality of life | Improvements in balance, TUG, ABC, core stability, fatigue, mental health, and quality of life in the Pilates groups, before and after. In the CG, there were no differences. |
| Grubic et al., 2019 [37]. | Randomized semicontrolled parallel group | 19 participants: 7 women and 12 men. EG: 10 (4 F, 6 M) and CG: 9 (3 F, 6 M). | Mean age: EG 53.9 ± 10.7 and CG 48.2 ± 9.3 | 4 weeks | Fatigue, quality of life | Improvements in fatigue in both the EG and CG. Improvement in quality of life only in the EG. |
| Grubic et al., 2021 [38]. | Pilot randomized controlled trial | 24 participants: 14 women and 10 men. EG: 13 (8 F, 5 M) and CG: 11 (6 F, 5 M) | Mean age: EG 50 ± 9.3 and CG 53.8 ± 11.8 | 8 weeks | Sleep quality, insomnia, psychological stress, and fatigue | Improvements in insomnia, fatigue, psychological stress, and sleepiness in EG. No change in the CG. |
| Ghannadi et al., 2022 [39]. | Single-blinded randomized controlled trial | 36 participants: 27 women and 9 men. EG: 17 (13 F, 4 M) and CG: 19 (14 F, 5 M) | Mean age: EG 36.47 ± 7.62 and CG 39.36 ± 9.83 | 8 weeks | PEmax, PImax, functionality, fatigue, quality of life | Improvements in PImax and PEmax in both groups, although higher in the EG. There were no significant changes in TUG and 6MWT. Improvement in fatigue in the EG with respect to the CG. |
| Author, Year | Intervention (EG) | Comparison (CG) | Follow Up | Scales and Measures |
|---|---|---|---|---|
| Klefbeck et al., 2003 [31] | IMT training, 10 min, 2 times/day, every 2 days, with 4 h between each session, for 10 weeks, participated from home, weekly telephone contact with help and feedback. | Breathing exercises were part of their physiotherapy session. No telephone contact to resolve doubts. | The measurements were taken at the beginning, at the end of the 10 weeks of treatment, and one month after the end of the treatment. | FSS, RPE Scale |
| Fry et al., 2007 [32] | Daily IMT exercises for 10 weeks; 3 series of 15 repetitions at 30% MIP, increasing in intensity weekly. | IMT with instructions on use and progression. Optional telephone advice. | Measurements were taken at the beginning and end of the 10 weeks of treatment. | EDSS, FSS, Borg Scale |
| Castro-Sanchez et al., 2012 [33] | Ai-Chi exercise in the pool 60 min × 2 times/week. | Conventional treatment in a therapy room. | Measurements were taken before treatment, and at 4 and 10 weeks. | Pain (VAS, PRI, PPI, MPQ), RMDQ, Spasticity (VAS spasm), MSIS-29, Beck’s depression inventory, MFIS, Barthel index |
| Bayraktar et al., 2013 [34]. | Ai-Chi exercise in the pool 60 min × 2 times/week. | Active leg exercises with abdominal exercises at home, 60 min × 2 times/week, for 8 weeks. | Measurements were taken before and after treatment. | Balance (One-leg standing test), functional mobility (TUG test, 6MWT), strength (dynamometer), fatigue (FSS) |
| Ray et al., 2013 [35] | RMT 30 min, 3 days/week, for 5 weeks. | No treatment. | Measurements were taken at baseline, every week, and between 4 and 7 days after the last session. | MFIS, 6MWT, MMSE, SF-36 |
| Bulguroglu et al., 2017 [36] | Both Pilates groups: between 1 h–1 h 30 min of Pilates 2 days/week for 8 weeks. | Home program of breathing and relaxation exercises 2 times/week for 8 weeks. | Measurements were taken before and after treatment. | Single Leg Stance, TUG, ABC, side bridge test, modified Biering–Sorensen test, trunk flexion test, prone bridge test, FSS, MSQOL-54 |
| Grubic et al., 2019 [37] | Upper limb and respiratory exercises 60 min/2 days a week at a center, and at least 20 min/3 days a week performed independently. In total, 4 weeks. | No treatment, but they attended the center twice a week for 60 min to socialize. | Measurements were taken before and after treatment. | MFIS, SF-36 |
| Grubic et al., 2021 [38] | Upper and lower limb exercises, along with breathing exercises for 60 min/2 times a week for 8 weeks. | No treatment, but they attended the center twice a week for 60 min to socialize for 8 weeks. | Measurements were taken before and after treatment. | PSQI, ISI, CORE-OM, MFIS |
| Ghannadi et al., 2022 [39] | IMT with POWERbreathe device at the beginning at 30% resistance, 15 breaths/3 series (1 min rest between sets) × 2 times a day for 8 weeks. | No treatment; they only received brochures on physical activity, education, and the importance of regular physical activity along with lifestyle modifications. | Measurements were taken before and after treatment. | 6MWT, TUG, MFIS, SF36 |
| Item (PEDRro Scale) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Klefbeck et al. [31] 2003 | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Fry et al. [32] 2007 | Y | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
| Castro-Sanchez et al. [33] 2012 | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 6 |
| Ray et al. [35] 2013 | Y | N | N | Y | N | N | N | Y | Y | Y | Y | 5 |
| Bayraktar et al. [34] 2013 | Y | N | N | N | N | N | Y | N | Y | N | Y | 3 |
| Bulguroglu et al. [36] 2017 | Y | Y | N | Y | N | N | Y | N | N | Y | Y | 5 |
| Grubic et al. [37] 2019 | Y | N | N | Y | N | N | Y | Y | Y | Y | Y | 6 |
| Grubic et al. [38] 2021 | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Ghannadi et al. [39] 2022 | N | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Álamo, L.; López-Liria, R.; Valverde-Martínez, M.Á.; Benzo-Iglesias, M.J.; Rubio-Arias, J.Á. Effectiveness of Respiratory Exercises on Perceived Symptoms of Fatigue among Multiple Sclerosis Patients: A Systematic Review. Sustainability 2023, 15, 12887. https://doi.org/10.3390/su151712887
Torres-Álamo L, López-Liria R, Valverde-Martínez MÁ, Benzo-Iglesias MJ, Rubio-Arias JÁ. Effectiveness of Respiratory Exercises on Perceived Symptoms of Fatigue among Multiple Sclerosis Patients: A Systematic Review. Sustainability. 2023; 15(17):12887. https://doi.org/10.3390/su151712887
Chicago/Turabian StyleTorres-Álamo, Lucía, Remedios López-Liria, María Ángeles Valverde-Martínez, María Jesús Benzo-Iglesias, and Jacobo Á. Rubio-Arias. 2023. "Effectiveness of Respiratory Exercises on Perceived Symptoms of Fatigue among Multiple Sclerosis Patients: A Systematic Review" Sustainability 15, no. 17: 12887. https://doi.org/10.3390/su151712887
APA StyleTorres-Álamo, L., López-Liria, R., Valverde-Martínez, M. Á., Benzo-Iglesias, M. J., & Rubio-Arias, J. Á. (2023). Effectiveness of Respiratory Exercises on Perceived Symptoms of Fatigue among Multiple Sclerosis Patients: A Systematic Review. Sustainability, 15(17), 12887. https://doi.org/10.3390/su151712887








