Elucidating the Potential of Biochar-Bentonite Composite and Kaolinite-Based Seed Balls for the Remediation of Coal Mining Impacted Heavy Metals Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sample Collection
2.3. Soil Physicochemical Characterization
2.3.1. pH and Electrical Conductivity (EC)
2.3.2. Exchangeable Nutrients, Cation Exchange Capacity (CEC), and Organic Matter
2.3.3. Heavy Metals and Soil Enzymes
2.4. Development of Bentonite-Biochar Composite-Based Seed Balls
2.5. Pot-Culture Study
2.6. Plant Analysis
2.6.1. Chlorophyll
2.6.2. Shoot and Root Biomass
2.6.3. Bioaccumulation and Translocation Factor
2.6.4. Glutathione and Proline Content
2.7. Statistical Analysis
3. Results and Discussion
3.1. Initial Soil Physicochemical Characteristics
3.2. Physicochemical Characteristics of Seed Balls
3.3. Post-Pot-Culture Soil Physicochemical Characteristics
3.3.1. pH, Available N, Organic Matter, and CEC
3.3.2. Available Nutrients, Soil Enzymes, and Soil Fertility Index (SFI)
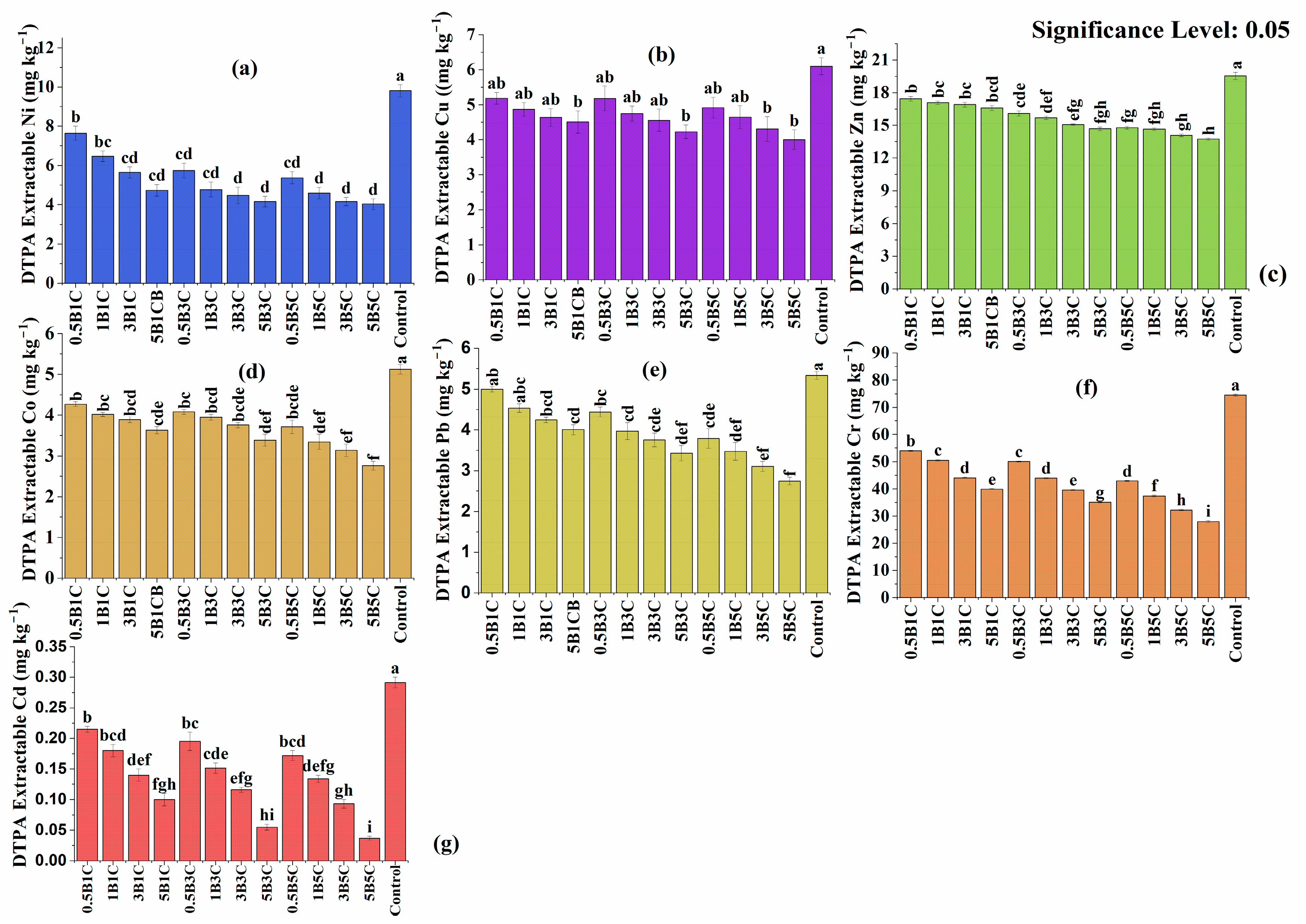
3.3.3. Heavy Metals
3.4. Plant Analysis
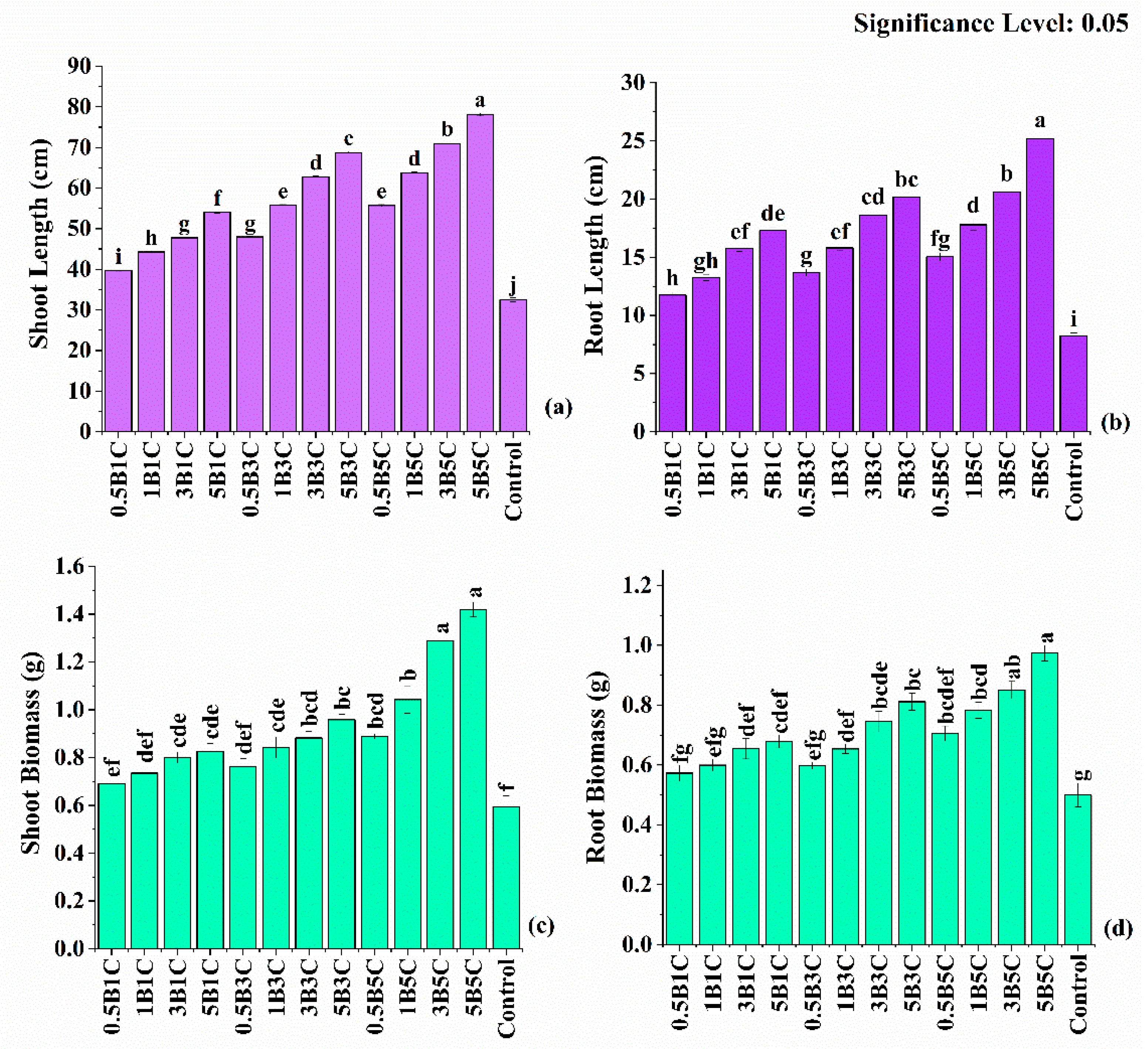
3.4.1. Soot and Root Length and Biomass
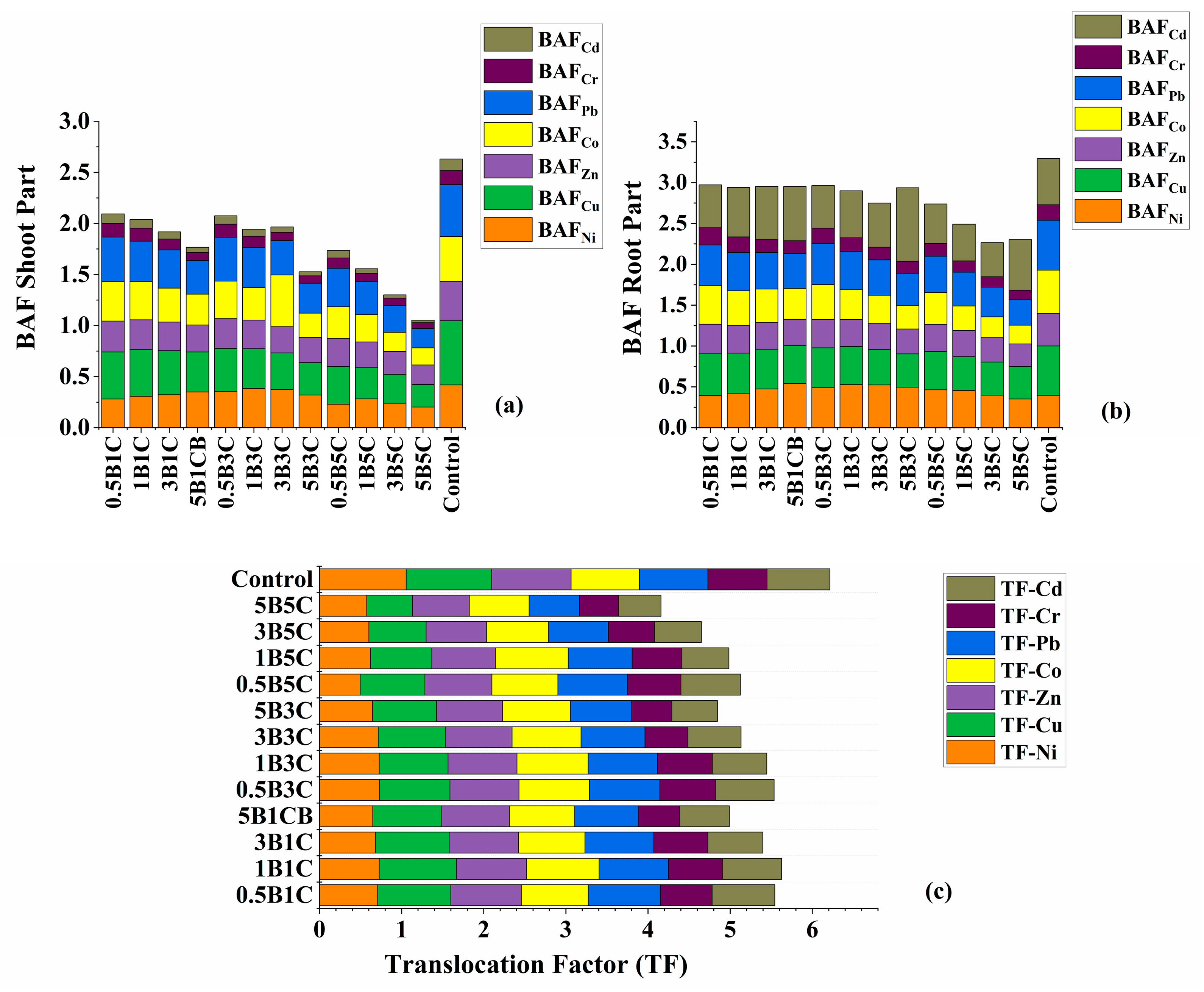
3.4.2. Translocation and Bioaccumulation Factors
3.4.3. Chlorophyll, Glutathione, and Proline Content
4. Conclusions and Future Prospects
- (i)
- The soil collected from the coal mining area was characterized as degraded and contaminated due to low organic matter and nutrient content and heavy metals such as Cr, Cd, Zn, and Co in a concentration more than the WHO limits in the soil.
- (ii)
- The application of seed balls containing Sorghum grass seeds assisted in seed germination and sapling growth in the contaminated soil.
- (iii)
- The application of seed balls containing biochar composite and kaolinite substantially improved the soil’s physicochemical properties and reduced the metals’ phytotoxicity.
- (iv)
- The increase in the biochar composite fractions in the seed balls up to 5% (w/w) of kaolinite reduced the phytoavailability of the heavy metals (specifically Cd and Cr) in the soil and increased the plant’s physiological structure.
- (v)
- The seed balls application in the test groups substantially reduced the secretion of antioxidative and stress hormones in the plant cell due to reduced abiotic stress factors and phytotoxicity.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agboola, O.; Babatunde, D.E.; Isaac Fayomi, O.S.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Yahaya, A.; Mamudu, O.A. A Review on the Impact of Mining Operation: Monitoring, Assessment and Management. Results Eng. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Koul, B.; Taak, P. Chemical Methods of Soil Remediation. In Biotechnological Strategies for Effective Remediation of Polluted Soils; Springer: Singapore, 2018. [Google Scholar]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Raja, R.; Pal, S. Remediation of Heavy Metal Contaminated Soils by Solidification/Stabilization with Fly Ash, Quick. J. Indian Chem. Soc. 2019, 96, 481–486. [Google Scholar]
- Król, A.; Mizerna, K.; Bożym, M. An Assessment of PH-Dependent Release and Mobility of Heavy Metals from Metallurgical Slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; He, Y.; Wu, Y.; Dian, B.; Zhang, J.; Li, T.; Jiang, M. Solidification/Stabilization of Soil Heavy Metals by Alkaline Industrial Wastes: A Critical Review. Environ. Pollut. 2022, 312, 120094. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Chen, L.; Yue, S.; Yang, W. Effect of Biochar on Immobilization Remediation of Cd-contaminated Soil and Environmental Quality. Environ. Res. 2022, 204, 111840. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of Bamboo and Rice Straw Biochars on the Mobility and Redistribution of Heavy Metals (Cd, Cu, Pb and Zn) in Contaminated Soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; An, S.; Zhao, J.; Yan, Y.; Zhang, D.; Wu, Z.; Shen, B.; Lyu, H. Biochar-Supported NZVI for the Removal of Cr(VI) from Soil and Water: Advances in Experimental Research and Engineering Applications. J. Environ. Manag. 2022, 316, 115211. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of Heavy Metal Contaminated Soils by Biochar: Mechanisms, Potential Risks, and Applications in China. Environ. Pollut. 2019, 251, 846–855. [Google Scholar] [CrossRef]
- Zhang, R.H.; Xie, Y.; Zhou, G.; Li, Z.; Ye, A.; Huang, X.; Xie, Y.; Shi, L.; Cao, X.; Zhang, J.; et al. The Effects of Short-Term, Long-Term, and Reapplication of Biochar on the Remediation of Heavy Metal-Contaminated Soil. Ecotoxicol. Environ. Saf. 2022, 248, 114316. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Mo, A.; Ni, J.; Xie, H.; Hu, J.; Zhu, Y.; Peng, C. Effect of Lychee Biochar on the Remediation of Heavy Metal-Contaminated Soil Using Sunflower: A Field Experiment. Environ. Res. 2020, 188, 109886. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Liu, X.; Qiu, W.; Song, Z. Effects of Fe-Mn Modified Biochar Composite Treatment on the Properties of As-Polluted Paddy Soil. Environ. Pollut. 2019, 244, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, Y.; Ding, C.; Wang, S.; Zhao, C.; Yin, W.; Wang, B.; Yang, R.; Wang, X. Remediation of Lead and Cadmium Co-Contaminated Mining Soil by Phosphate-Functionalized Biochar: Performance, Mechanism, and Microbial Response. Chemosphere 2023, 334, 138938. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Yin, H.; Li, Y.; Cai, Y.; Yan, C.; Yuan, Y.; Dang, Z. Remediation of Cu and As Contaminated Water and Soil Utilizing Biochar Supported Layered Double Hydroxide: Mechanisms and Soil Environment Altering. J. Environ. Sci. 2023, 126, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yang, W.; Xu, Y.; Wang, L.; Liang, X.; Huang, Q.; Sun, Y. Effect of Ca-Modified Biochar Coupling with Low-Cd Accumulation Maize Cultivars on Remediation of Cd Contaminated Soils and Microbial Community Composition. Soil Tillage Res. 2023, 232, 105765. [Google Scholar] [CrossRef]
- Ali, M.U.; Chand, S.; Latif, M.U. Seed Ball Technology and Its Significance. Available online: https://timesagriculture.com/seed-ball-technology-and-its-significance/ (accessed on 8 June 2023).
- USDA Forest Service. Available online: www.fs.usda.gov/Internet/FSE_DOCUMENTS (accessed on 10 June 2023).
- CMPDIL. Environmental Claerence for Expansion of New Majri UG TO OC Mine; CMPDIL: Ranchi, India, 2016. [Google Scholar]
- Maiti, S.K. Ecorestoration of the Coalmine Degraded Lands; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9788132208501. [Google Scholar]
- US EPA. Method 9045D: Soil and Waste pH, Part of Test Methods for Evaluating Solid Waste, Physical/Chemical Methods; US EPA: Washington, DC, USA, 2004; pp. 1–3. [Google Scholar]
- US EPA. EPA 9081: Cation Exchange Capacity of Soils (Sodium Acetate); US EPA: Washington, DC, USA, 1986; Volume 9, pp. 123–128. [Google Scholar]
- Hoogsteen, M.J.J.; Lantinga, E.A.; Bakker, E.J.; Groot, J.C.J.; Tittonell, P.A. Estimating Soil Organic Carbon through Loss on Ignition: Effects of Ignition Conditions and Structural Water Loss. Eur. J. Soil Sci. 2015, 66, 320–328. [Google Scholar] [CrossRef]
- US EPA. EPA 3051A: Microwave Assiststed Acid Digestion of Sedmients, Sludges, Soils, and Oils; US EPA: Washington, DC, USA, 2007; pp. 1–30. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Equilibrium Relationships of Zn2+, Fe3+, Ca2+, and H+ with EDTA and DTPA in Soils. Soil Sci. Soc. Am. J. 1969, 33, 62–68. [Google Scholar] [CrossRef]
- Roberge, M.R. Methodology of Enzymes Determination and Extraction. In Soil Enzymes; Burns, R.G., Ed.; Academic Press: New York, NY, USA, 1978; pp. 341–352. [Google Scholar]
- Turner, B.L.; Hopkins, D.W.; Haygarth, P.M.; Ostle, N. Β-Glucosidase Activity in Pasture Soils. Appl. Soil Ecol. 2002, 20, 157–162. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Medha, I.; Chandra, S.; Bhattacharya, J.; Samal, B.; Vanapalli, K.R. Development of Rice Straw-Derived Biochar-Bentonite Composite and Its Application for in Situ Sequestration of Ammonium and Phosphate Ions in the Degraded Mine Soil. Environ. Manag. 2023, 71, 1065–1086. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Chen, K.; Li, G. Spectrophotometric Determination of Chlorophylls in Different Solvents Related to the Leaf Traits of the Main Tree Species in Northeast China. IOP Conf. Ser. Earth Environ. Sci. 2021, 836, 012008. [Google Scholar] [CrossRef]
- Banerjee, R.; Goswami, P.; Pathak, K.; Mukherjee, A. Vetiver Grass: An Environment Clean-up Tool for Heavy Metal Contaminated Iron Ore Mine-Soil. Ecol. Eng. 2016, 90, 25–34. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, S.; Thangavel, P.; Li, Q.; Zheng, H.; Bai, J.; Qiu, R. Phytostabilization Potential of Jatropha Curcas l. in Polymetallic Acid Mine Tailings. Int. J. Phytoremediat. 2011, 13, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Awasthi, J.P.; Sunkar, R.; Panda, S.K. Determining Glutathione Levels in Plants. Methods Mol. Biol. 2017, 1631, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for Determination of Proline in Plants. In Plant Stress Tolerance, Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 639, pp. 1–14. ISBN 978-1-60761-701-3. [Google Scholar]
- Mukhopadhyay, S.; Maiti, S.K.; Masto, R.E. Development of Mine Soil Quality Index (MSQI) for Evaluation of Reclamation Success: A Chronosequence Study. Ecol. Eng. 2014, 71, 10–20. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Lal, R. Changes in Physical and Chemical Properties of Soil after Surface Mining and Reclamation. Geoderma 2011, 161, 168–176. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Bhattacharya, J.; Vanapalli, K.R.; Samal, B. Effect of the Co-Application of Eucalyptus Wood Biochar and Chemical Fertilizer for the Remediation of Multimetal (Cr, Zn, Ni, and Co) Contaminated Soil. Sustainability 2022, 14, 7266. [Google Scholar] [CrossRef]
- Manirakiza, E.; Ziadi, N.; St Luce, M.; Hamel, C.; Antoun, H.; Karam, A. Nitrogen Mineralization and Microbial Biomass Carbon and Nitrogen in Response to Co-Application of Biochar and Paper Mill Biosolids. Appl. Soil Ecol. 2019, 142, 90–98. [Google Scholar] [CrossRef]
- Joseph, U.E.; Toluwase, A.O.; Kehinde, E.O.; Omasan, E.E.; Tolulope, A.Y.; George, O.O.; Zhao, C.; Hongyan, W. Effect of Biochar on Soil Structure and Storage of Soil Organic Carbon and Nitrogen in the Aggregate Fractions of an Albic Soil. Arch. Agron. Soil Sci. 2020, 66, 1–12. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of Different Straw Biochars on Soil Organic Carbon, Nitrogen, Available Phosphorus, and Enzyme Activity in Paddy Soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef]
- Kharel, G.; Sacko, O.; Feng, X.; Morris, J.R.; Phillips, C.L.; Trippe, K.; Kumar, S.; Lee, J.W. Biochar Surface Oxygenation by Ozonization for Super High Cation Exchange Capacity. ACS Sustain. Chem. Eng. 2019, 7, 16410–16418. [Google Scholar] [CrossRef]
- Domingues, R.R.; Sánchez-Monedero, M.A.; Spokas, K.A.; Melo, L.C.A.; Trugilho, P.F.; Valenciano, M.N.; Silva, C.A. Enhancing Cation Exchange Capacity Ofweathered Soils Using Biochar: Feedstock, Pyrolysis Conditions and Addition Rate. Agronomy 2020, 10, 824. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F.; Besharati, H.; Khalaj, M.A. The Effects of Biochar on Soil Nutrients Status, Microbial Activity and Carbon Sequestration Potential in Two Calcareous Soils. Biochar 2021, 3, 105–116. [Google Scholar] [CrossRef]
- Kalu, S.; Simojoki, A.; Karhu, K.; Tammeorg, P. Long-Term Effects of Softwood Biochar on Soil Physical Properties, Greenhouse Gas Emissions and Crop Nutrient Uptake in Two Contrasting Boreal Soils. Agric. Ecosyst. Environ. 2021, 316, 107454. [Google Scholar] [CrossRef]
- Pandey, B.; Suthar, S.; Chand, N. Effect of Biochar Amendment on Metal Mobility, Phytotoxicity, Soil Enzymes, and Metal-Uptakes by Wheat (Triticum aestivum) in Contaminated Soils. Chemosphere 2022, 307, 135889. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Guo, D.; Arockiam Jeyasundar, P.G.S.; Li, Y.; Xiao, R.; Du, J.; Li, R.; Zhang, Z. Application of Wood Biochar in Polluted Soils Stabilized the Toxic Metals and Enhanced Wheat (Triticum aestivum) Growth and Soil Enzymatic Activity. Ecotoxicol. Environ. Saf. 2019, 184, 109635. [Google Scholar] [CrossRef] [PubMed]
- Tanzeem-Ul-haq, H.S.; Rasool, B.; Ehtisham-Ul-haque, S.; Saif, S.; Zafar, S.; Younis, T.; Akhtar, I.; Jafri, L.; Iqbal, N.; Masood, N.; et al. Chitosan with Bentonite and Biochar in Ni-Affected Soil Reduces Grain Ni Concentrations, Improves Soil Enzymes and Grain Quality in Lentil. Minerals 2021, 11, 11. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, K.; Jeong, T.U.; Ahn, K.H. Influence of Pyrolysis Temperature on Characteristics and Phosphate Adsorption Capability of Biochar Derived from Waste-Marine Macroalgae (Undaria pinnatifida Roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bolan, N.S.; Tsang, D.C.W.; Hou, D. Green Immobilization of Toxic Metals Using Alkaline Enhanced Rice Husk Biochar: Effects of Pyrolysis Temperature and KOH Concentration. Sci. Total Environ. 2020, 720, 137584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; You, L.C.; Lyu, H.H.; Liu, Y.X.; He, L.L.; Hu, Y.D.; Luo, F.C.; Yang, S.M. Role of Biochar-Mineral Composite Amendment on the Immobilization of Heavy Metals for Brassica chinensis from Naturally Contaminated Soil. Environ. Technol. Innov. 2022, 28, 102622. [Google Scholar] [CrossRef]
- Vrînceanu, N.O.; Motelică, D.M.; Dumitru, M.; Calciu, I.; Tănase, V.; Preda, M. Assessment of Using Bentonite, Dolomite, Natural Zeolite and Manure for the Immobilization of Heavy Metals in a Contaminated Soil: The Copșa Mică Case Study (Romania). Catena 2019, 176, 336–342. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Effect of Biochar on Pb, Cd and Cr Availability and Maize Growth in Artificial Contaminated Soil. Ann. Agric. Sci. 2019, 64, 95–102. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; de Jesus Santos, J.C.; Ganassali Junior, L.F.; Fontes, P.T.N.; Araújo, J.d.S.; Gonzaga, T.A.S. Copper Uptake, Physiological Response, and Phytoremediation Potential of Brassica juncea under Biochar Application. Int. J. Phytoremediat. 2022, 24, 474–482. [Google Scholar] [CrossRef]
- Wu, D.; Peng, W.; Bao, L.; Yu, X.; Dong, X.; Lai, M.; Liang, Z.; Xie, S.; Jacobs, D.F.; Zeng, S. Biochar Alleviating Heavy Metals Phytotoxicity in Sludge-Amended Soil Varies with Plant Adaptability. Environ. Res. 2022, 215, 114248. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 155–166. [Google Scholar]
- Adejumo, S.A.; Owoseni, O.; Mur, L.A.J. Low Light Intensity and Compost Modified Biochar Enhanced Maize Growth on Contaminated Soil and Minimized Pb Induced Oxidative Stress. J. Environ. Chem. Eng. 2021, 9, 104764. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wang, X.; Naveed, M.; Mustafa, A.; Ashraf, S.; Samreen, T.; Nadeem, S.M.; Jamil, M. Biochar Mediated-Alleviation of Chromium Stress and Growth Improvement of Different Maize Cultivars in Tannery Polluted Soils. Int. J. Environ. Res. Public Health 2021, 18, 4461. [Google Scholar] [CrossRef]
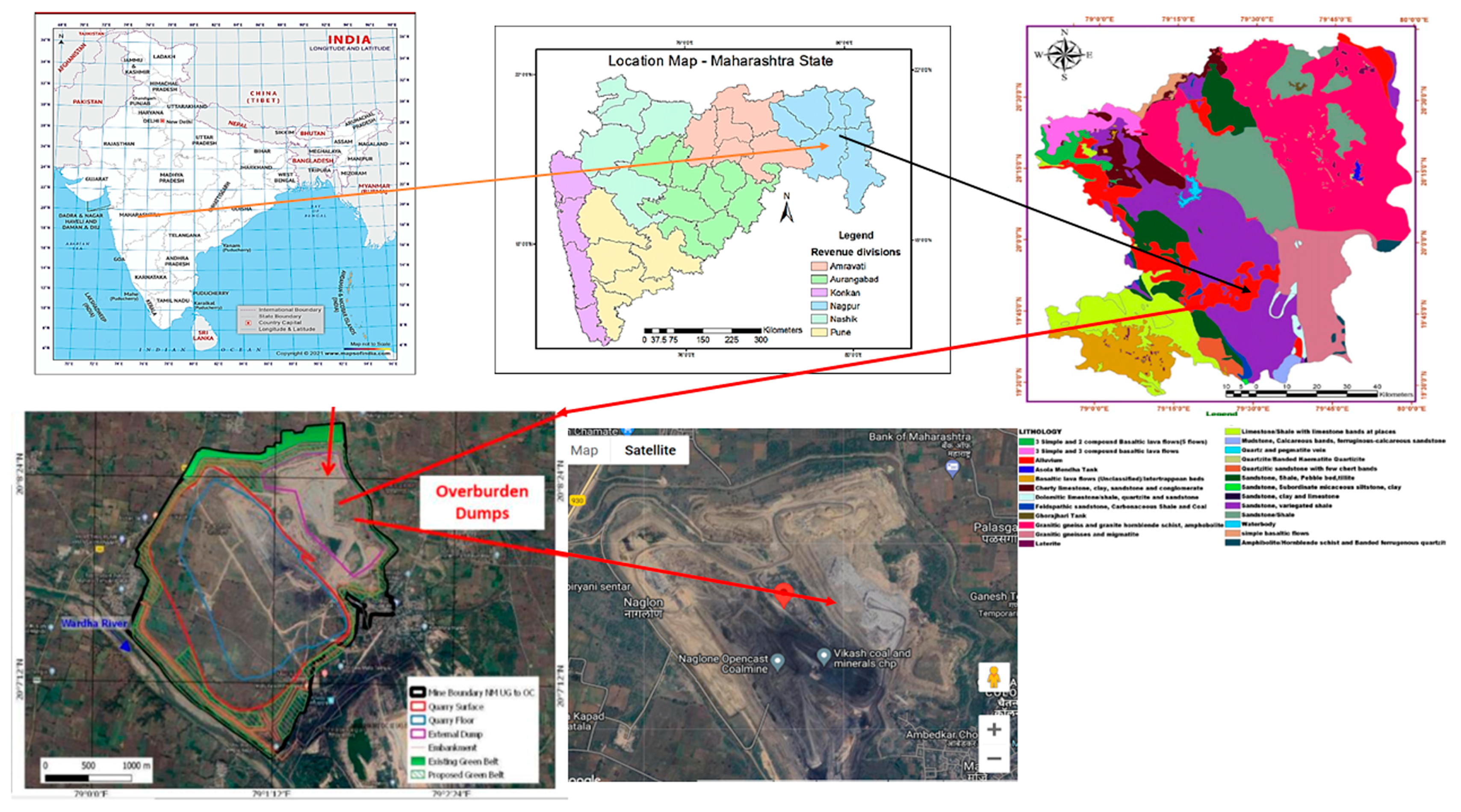
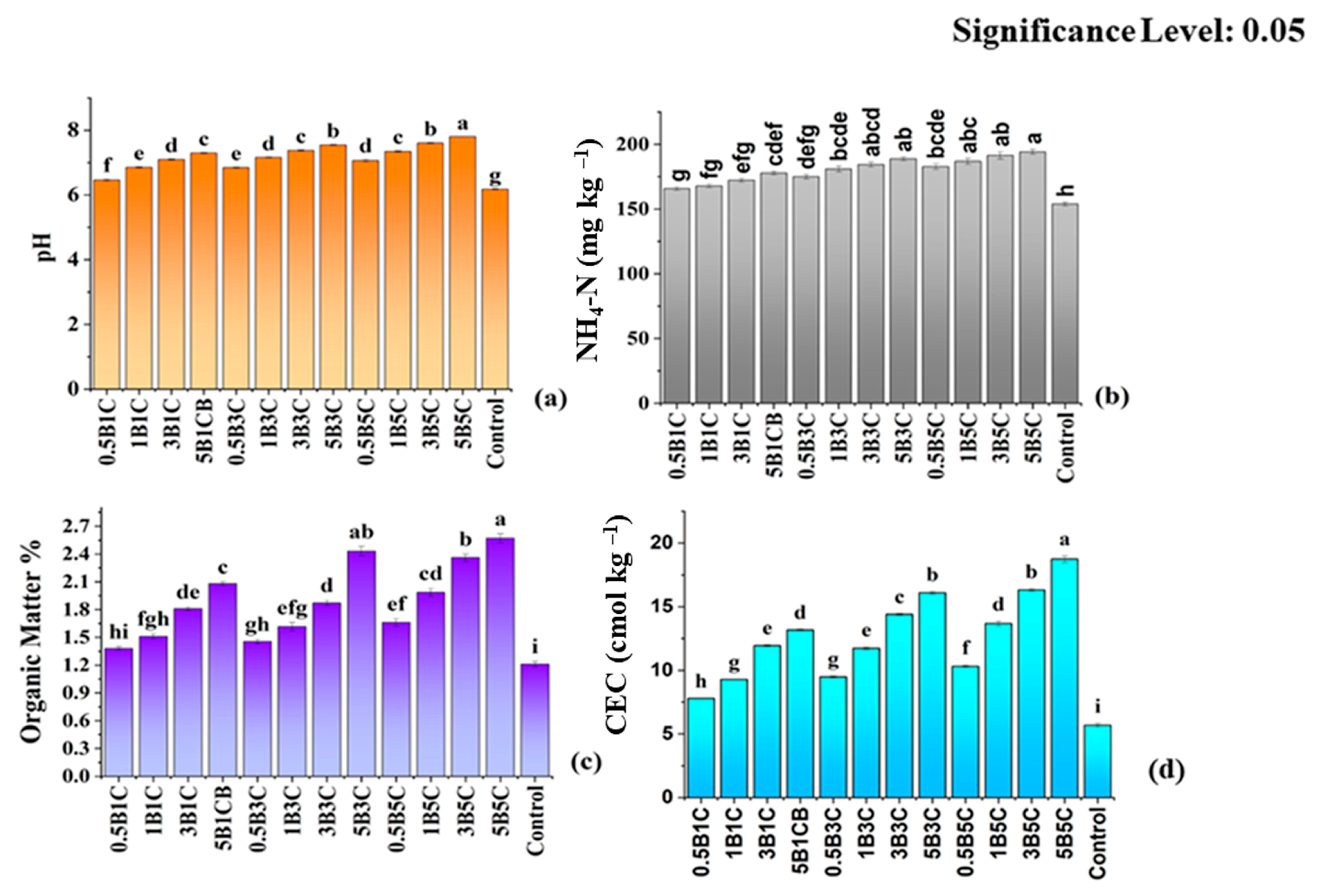
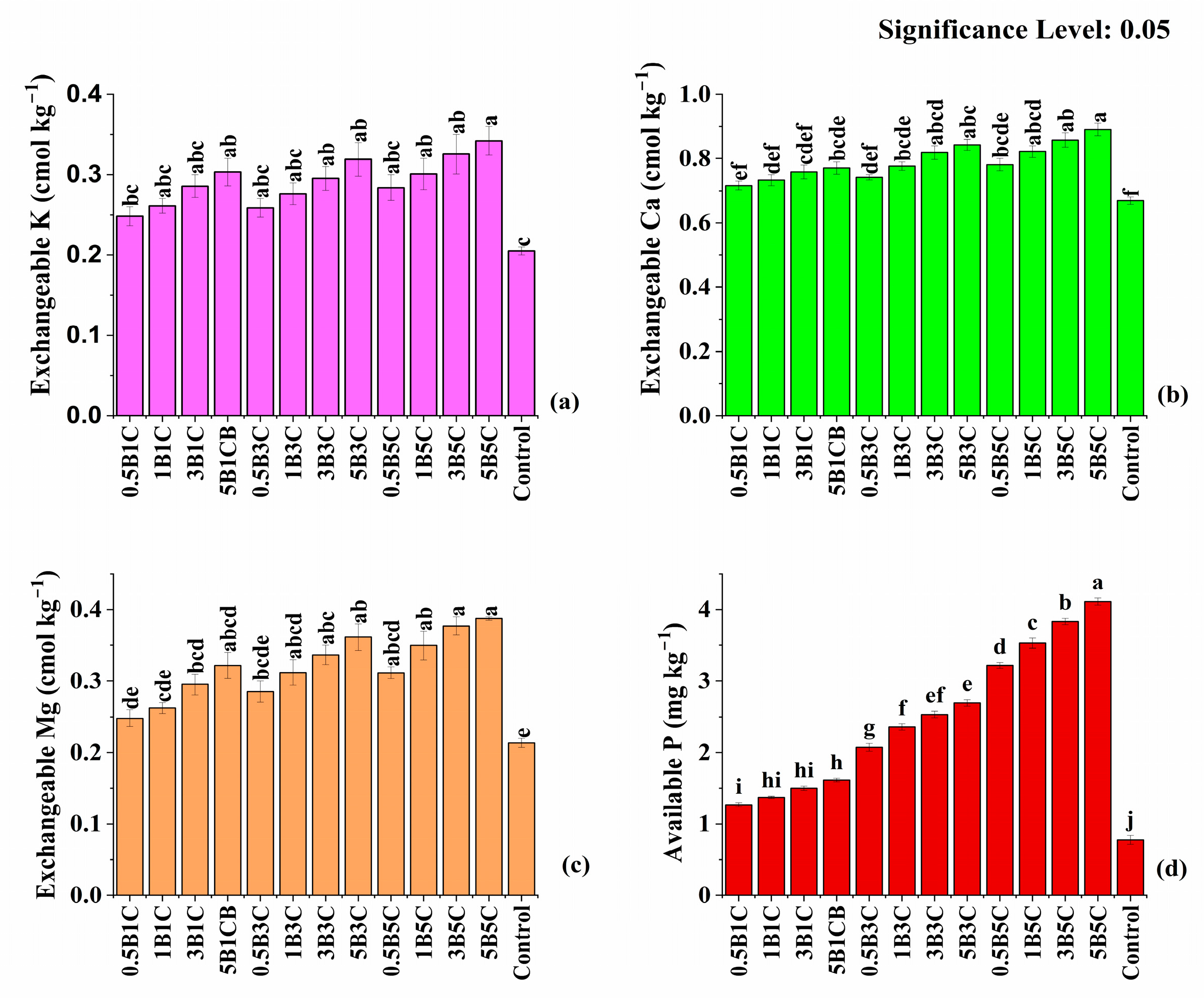
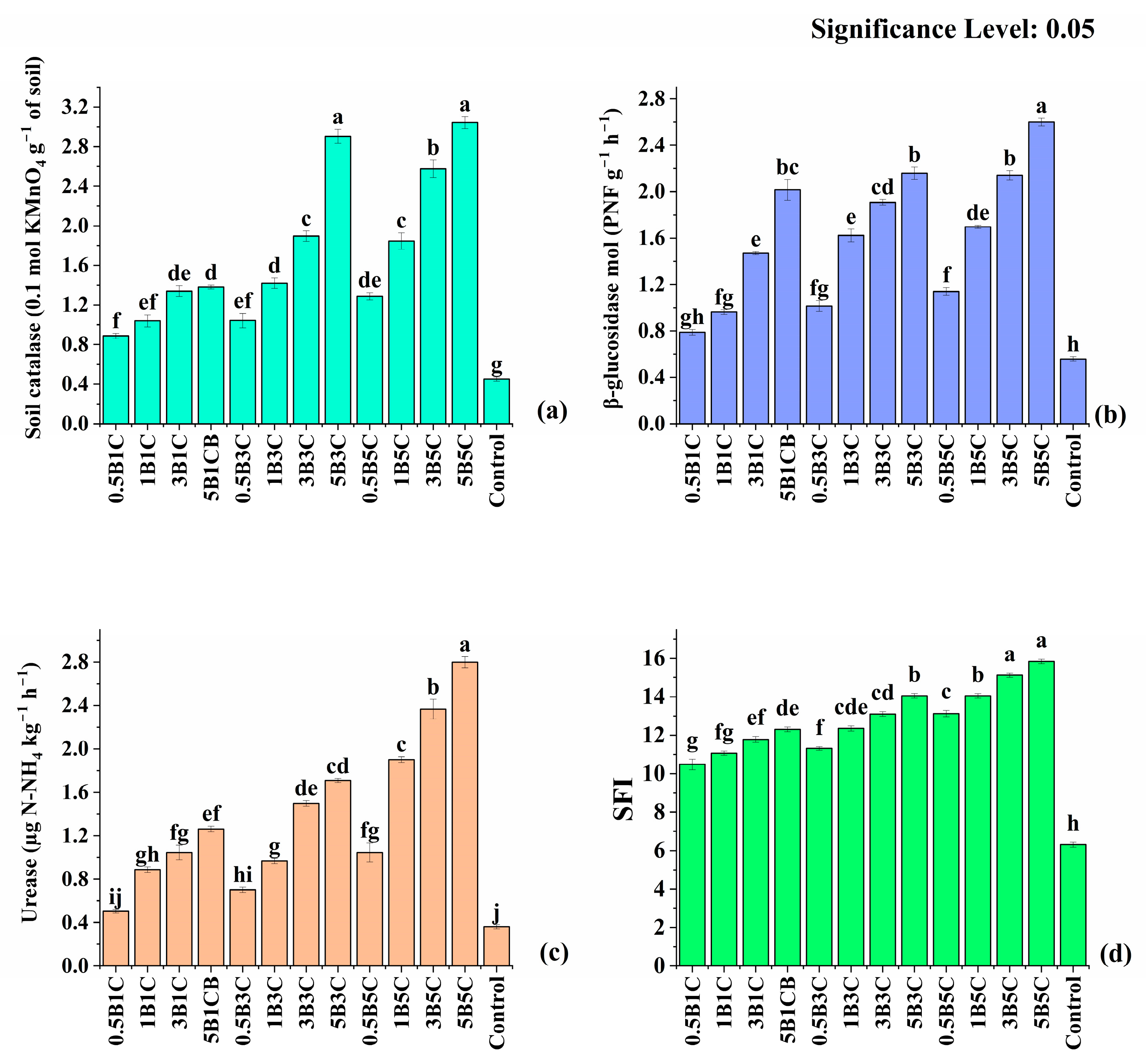

| Soil Parameter | Values | ||
|---|---|---|---|
| Particle size | Sand | 52% | Loam Texture |
| Silt | 40% | ||
| Clay | 8% | ||
| pH | 6.16 ± 0.11 | ||
| EC (µS cm−1) | 102.87 ± 5.24 | ||
| Total organic carbon (%) | 0.722 ± 0.08 | ||
| Organic Matter (%) | 1.24 ± 0.11 | ||
| Exchangeable Na (mg kg−1) | 122.89 ± 4.19 | ||
| Exchangeable K (mg kg−1) | 83.26 ± 2.30 | ||
| Exchangeable Ca (mg kg−1) | 131.42 ± 3.79 | ||
| Exchangeable Mg (mg kg−1) | 24.86 ± 0.91 | ||
| Available P (mg kg−1) | 0.72 ± 0.02 | ||
| Available N (mg kg−1) | 152.38 ± 5.01 | ||
| Cation exchange capacity, CEC (cmol kg−1) | 5.83 ± 0.24 | ||
| Manganese, Mn (mg kg−1) | 291.90 ± 8.05 | ||
| Nickel, Ni (mg kg−1) | 49.97 ± 1.55 | ||
| Copper, Cu (mg kg−1) | 20.30 ± 0.56 | ||
| Zinc, Zn (mg kg−1) | 62.19 ± 1.72 | ||
| Cobalt, Co (mg kg−1) | 22.11 ± 0.86 | ||
| Lead, Pb (mg kg−1) | 24.82 ± 1.02 | ||
| Chromium. Cr (mg kg−1) | 133.82 ± 5.13 | ||
| Cadmium, Cd (mg kg−1) | 1.14 ± 0.03 | ||
| DTPA-extractable Mn (mg kg−1) | 75.66 ± 3.83 | ||
| DTPA-extractable Ni (mg kg−1) | 9.49 ± 0.36 | ||
| DTPA-extractable Cu (mg kg−1) | 5.19 ± 0.21 | ||
| DTPA-extractable Zn (mg kg−1) | 19.20 ± 1.53 | ||
| DTPA-extractable Co (mg kg−1) | 5.01 ± 0.17 | ||
| DTPA-extractable Pb (mg kg−1) | 5.43 ± 0.21 | ||
| DTPA-extractable Cr (mg kg−1) | 41.83 ± 1.23 | ||
| DTPA-extractable Cd (mg kg−1) | 0.30 ± 0.03 | ||
| Soil catalase (0.1 mol KMnO4 g−1 of soil) | 0.432 ± 0.015 | ||
| β–glucosidase (mol PNF g−1 h−1) | 0.539 ± 0.014 | ||
| Urease (µg N-NH4 kg−1 h−1) | 0.231 ± 0.077 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medha, I.; Chandra, S.; Bhattacharya, J. Elucidating the Potential of Biochar-Bentonite Composite and Kaolinite-Based Seed Balls for the Remediation of Coal Mining Impacted Heavy Metals Contaminated Soil. Sustainability 2023, 15, 12900. https://doi.org/10.3390/su151712900
Medha I, Chandra S, Bhattacharya J. Elucidating the Potential of Biochar-Bentonite Composite and Kaolinite-Based Seed Balls for the Remediation of Coal Mining Impacted Heavy Metals Contaminated Soil. Sustainability. 2023; 15(17):12900. https://doi.org/10.3390/su151712900
Chicago/Turabian StyleMedha, Isha, Subhash Chandra, and Jayanta Bhattacharya. 2023. "Elucidating the Potential of Biochar-Bentonite Composite and Kaolinite-Based Seed Balls for the Remediation of Coal Mining Impacted Heavy Metals Contaminated Soil" Sustainability 15, no. 17: 12900. https://doi.org/10.3390/su151712900
APA StyleMedha, I., Chandra, S., & Bhattacharya, J. (2023). Elucidating the Potential of Biochar-Bentonite Composite and Kaolinite-Based Seed Balls for the Remediation of Coal Mining Impacted Heavy Metals Contaminated Soil. Sustainability, 15(17), 12900. https://doi.org/10.3390/su151712900






