Hydrogen-Based Direct Reduction of Iron Oxides: A Review on the Influence of Impurities
Abstract
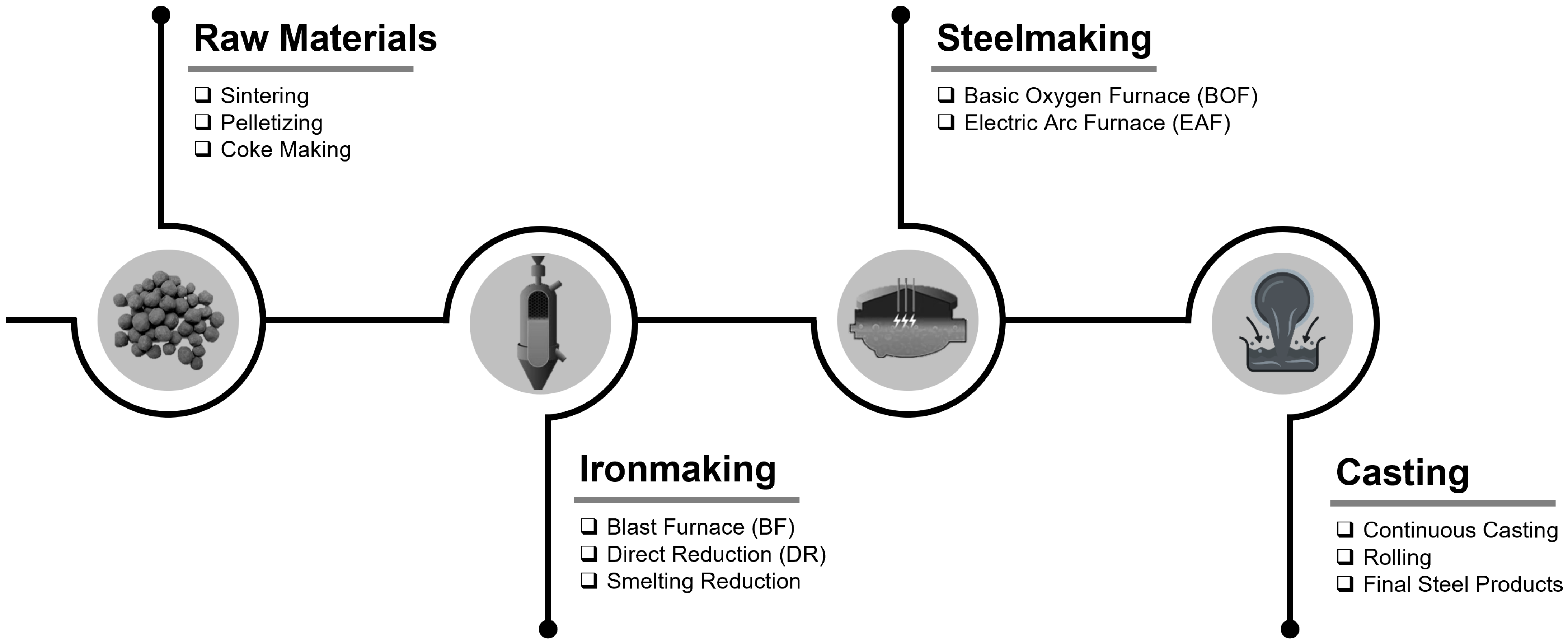
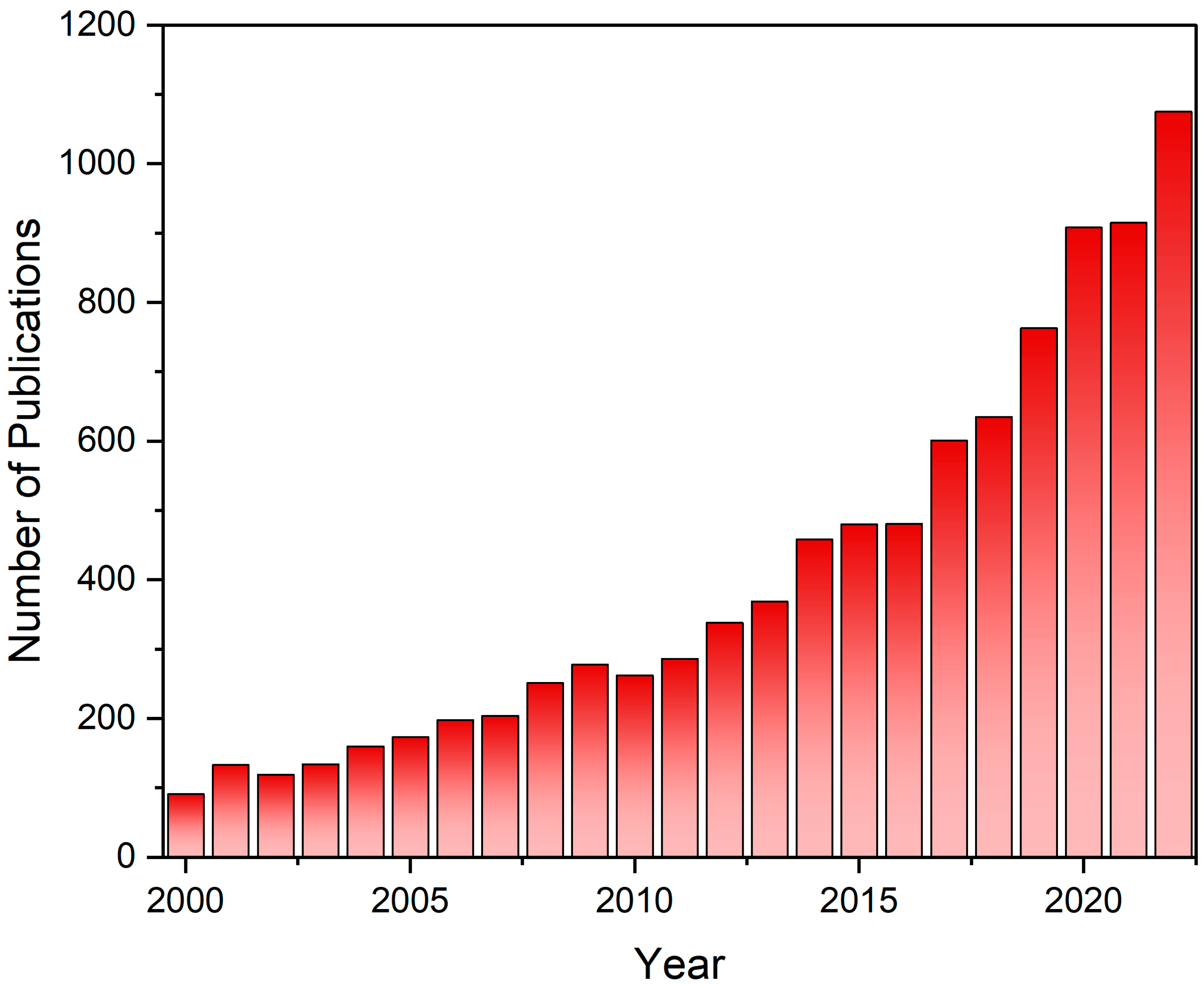
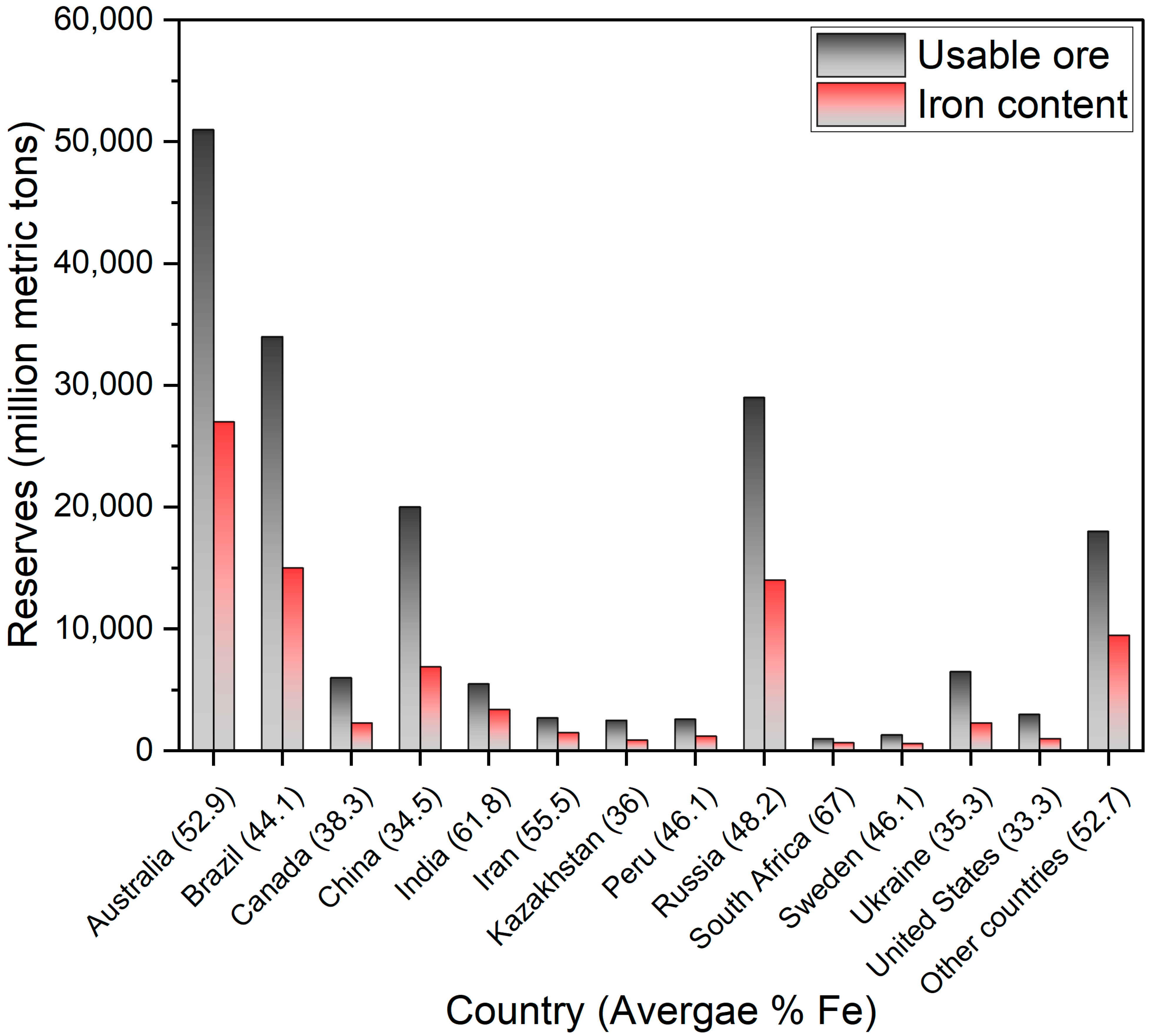
1. Introduction
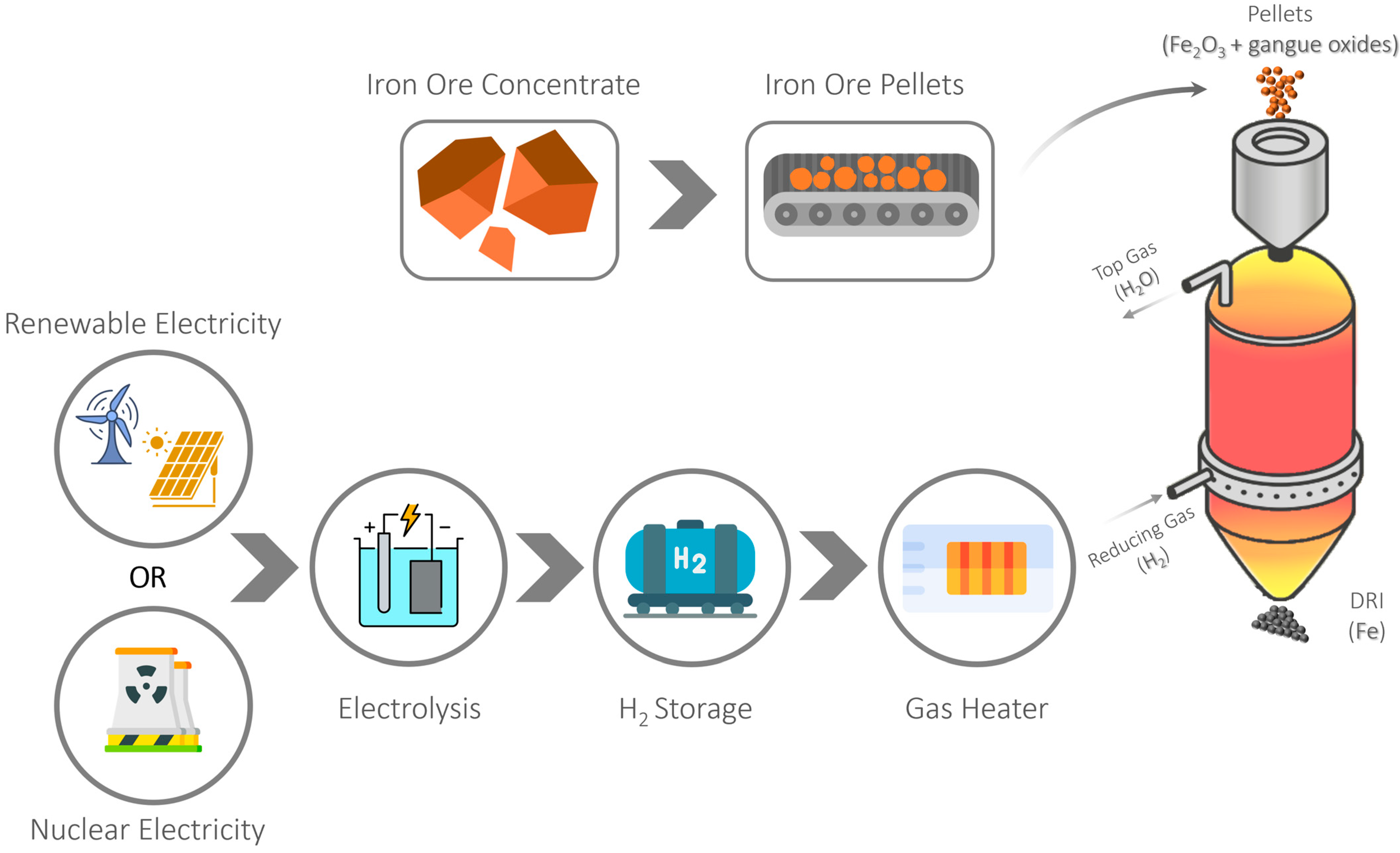
2. Hydrogen as a Reducing Agent in the Direct Reduction Process
3. Research and Development Programs
3.1. ULCOS Project
3.2. HYBRIT Project
3.3. COURSE50 Project
4. The Kinetics of Iron Oxide Reduction with H2
4.1. The Properties of the Starting Material
4.2. Experimental Conditions
4.3. The Modeling of Reduction Kinetics
5. The Role of Impurities on the Reduction of Iron Oxides
5.1. The Effect of Individual Impurities on the Reduction of Iron Ore Using Hydrogen as a Reductant
- SiO2
- Al2O3
- CaO
- MgO
- MnO2
5.2. The Effect of the Presence of Multiple Impurities on the Reduction of Iron Ore Using Hydrogen as a Reductant
5.3. The Effect of Impurities on the Reduction of Iron Ore Using Carbon Monoxide as a Reductant
6. Considerations for Future Work
- The experimental setup for reduction experiments may introduce certain inherent challenges including measurement inaccuracies caused by instability in the mass measurements, the incubation time required for changing the furnace atmosphere from inert to reducing, and temperature stabilization.
- The reduction of iron oxides occurs through a series of successive reactions. However, most of the previous investigations examined the sample micro-structure after almost complete reduction. Therefore, the impact of impurities on each step is unclear.
- The differences in the characteristics of the starting material cause variations in the reduction behavior. These differences include the mineralogy of iron oxides, the utilization of either industrial or synthetic samples, and variations in the pelletizing and sintering procedures leading to diverse shapes and porosity fractions.
7. Concluding Remarks
Funding
Conflicts of Interest
References
- Raabe, D.; Tasan, C.C.; Olivetti, E.A. Strategies for improving the sustainability of structural metals. Nature 2019, 575, 64–74. [Google Scholar] [CrossRef]
- Association, W.S. Steel’s Contribution to a Low Carbon Future and Climate Resilient Societies; World Steel Association: Brussels, Belgium, 2020; pp. 1–6. [Google Scholar]
- Raabe, D. The Materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023, 123, 2436–2608. [Google Scholar] [CrossRef]
- Jaimes, W.; Maroufi, S. Sustainability in steelmaking. Curr. Opin. Green Sustain. Chem. 2020, 24, 42–47. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J.; Zhang, Z.; Manovic, V. Inherent potential of steelmaking to contribute to decarbonisation targets via industrial carbon capture and storage. Nat. Commun. 2018, 9, 4422. [Google Scholar] [CrossRef]
- Chen, Y.; Zuo, H. Review of hydrogen-rich ironmaking technology in blast furnace. Ironmak. Steelmak. 2021, 48, 749–768. [Google Scholar] [CrossRef]
- Yang, X.D.; Zhang, D.C.; Liu, K.; Jing, F.F.H.L. Substituted pelleting for sintering—An important approach of energy-saving, low-carbon and emission reduction before ironmaking. J. Eng. Stud. 2017, 9, 44–52. [Google Scholar] [CrossRef]
- Kasai, A.; Matsui, Y. Lowering of thermal reserve zone temperature in blast furnace by adjoining carbonaceous material and iron ore. ISIJ Int. 2004, 44, 2073–2078. [Google Scholar] [CrossRef]
- Patisson, F.; Mirgaux, O. Hydrogen ironmaking: How it works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- Schenk, J.L. Recent status of fluidized bed technologies for producing iron input materials for steelmaking. Particuology 2011, 9, 14–23. [Google Scholar] [CrossRef]
- Kim, S.-H.; Zhang, X.; Ma, Y.; Filho, I.R.S.; Schweinar, K.; Angenendt, K.; Vogel, D.; Stephenson, L.T.; El-Zoka, A.A.; Mianroodi, J.R. Influence of microstructure and atomic-scale chemistry on the direct reduction of iron ore with hydrogen at 700 C. Acta Mater. 2021, 212, 116933. [Google Scholar] [CrossRef]
- Yuan, B.; Kongstein, O.E.; Haarberg, G.M. Electrowinning of iron in aqueous alkaline solution using a rotating cathode. J. Electrochem. Soc. 2008, 156, D64. [Google Scholar] [CrossRef]
- Wang, R.R.; Zhao, Y.Q.; Babich, A.; Senk, D.; Fan, X.Y. Hydrogen direct reduction (H-DR) in steel industry—An overview of challenges and opportunities. J. Clean. Prod. 2021, 329, 129797. [Google Scholar] [CrossRef]
- Ariyama, T.; Takahashi, K.; Kawashiri, Y.; Nouchi, T. Diversification of the ironmaking process toward the long-term global goal for carbon dioxide mitigation. J. Sustain. Metall. 2019, 5, 276–294. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Brooks, G.; Rhamdhani, M.A. Decarbonisation and hydrogen integration of steel industries: Recent development, challenges and technoeconomic analysis. J. Clean. Prod. 2023, 395, 136391. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrogen Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Heidari, A.; Niknahad, N.; Iljana, M.; Fabritius, T. A Review on the Kinetics of Iron Ore Reduction by Hydrogen. Materials 2021, 14, 7540. [Google Scholar] [CrossRef]
- Ma, Y.; Filho, I.R.S.; Bai, Y.; Schenk, J.; Patisson, F.; Beck, A.; van Bokhoven, J.A.; Willinger, M.G.; Li, K.; Xie, D. Hierarchical nature of hydrogen-based direct reduction of iron oxides. Scr. Mater. 2022, 213, 114571. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Reduction of iron oxides with hydrogen—A review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Rissman, J.; Bataille, C.; Masanet, E.; Aden, N.; Morrow, W.R., III; Zhou, N.; Elliott, N.; Dell, R.; Heeren, N.; Huckestein, B. Technologies and policies to decarbonize global industry: Review and assessment of mitigation drivers through 2070. Appl. Energy 2020, 266, 114848. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Birat, J.-P.; Patisson, F.; Mirgaux, O. Hydrogen steelmaking, part 2: Competition with other net-zero steelmaking solutions–geopolitical issues. Matériaux Tech. 2021, 109, 307. [Google Scholar] [CrossRef]
- Fan, Z.; Friedmann, S.J. Low-carbon production of iron and steel: Technology options, economic assessment, and policy. Joule 2021, 5, 829–862. [Google Scholar] [CrossRef]
- Rechberger, K.; Spanlang, A.; Conde, A.S.; Wolfmeir, H.; Harris, C. Green hydrogen-based direct reduction for low-carbon steelmaking. Steel Res. Int. 2020, 91, 2000110. [Google Scholar] [CrossRef]
- Yilmaz, C.; Wendelstorf, J.; Turek, T. Modeling and simulation of hydrogen injection into a blast furnace to reduce carbon dioxide emissions. J. Clean. Prod. 2017, 154, 488–501. [Google Scholar] [CrossRef]
- Usui, T.; Kawabata, H.; Ono-Nakazato, H.; Kurosaka, A. Fundamental experiments on the H2 gas injection into the lower part of a blast furnace shaft. ISIJ Int. 2002, 42, S14–S18. [Google Scholar] [CrossRef] [PubMed]
- Yearbook, S.S. Concise Version, World Steel Association; Economics Committee: Brussels, Belgium, 2019; p. 42. [Google Scholar]
- Patisson, F.; Mirgaux, O.; Birat, J.-P. Hydrogen steelmaking. Part 1: Physical chemistry and process metallurgy. Matériaux Tech. 2021, 109, 303. [Google Scholar] [CrossRef]
- Lyu, B.; Wang, G.; Yang, F.; Zuo, H.; Xue, Q.; Wang, J. Kinetic Analysis of Isothermal and Non-Isothermal Reduction of Iron Ore Fines in Hydrogen Atmosphere. Metals 2022, 12, 1754. [Google Scholar] [CrossRef]
- Agency, I.E. Iron and Steel Technology Roadmap: Towards More Sustainable Steelmaking; OECD Publishing: Washington, DC, USA, 2020. [Google Scholar]
- Meijer, K.; Denys, M.; Lasar, J.; Birat, J.-P.; Still, G.; Overmaat, B. ULCOS: Ultra-low CO2 steelmaking. Ironmak. Steelmak. 2009, 36, 249–251. [Google Scholar] [CrossRef]
- Quader, M.A.; Ahmed, S.; Dawal, S.Z.; Nukman, Y. Present needs, recent progress and future trends of energy-efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) program. Renew. Sustain. Energy Rev. 2016, 55, 537–549. [Google Scholar] [CrossRef]
- Nuber, D.; Eichberger, H.; Rollinger, B. Circored fine ore direct reduction. Millen. Steel. 2006, 2006, 37–40. [Google Scholar]
- Hornby, S. Mini-Mill Burdening for Maximum Efficiency and Yield? Iron Steel Technol. 2014, 12, 50–62. [Google Scholar]
- Pei, M.; Petäjäniemi, M.; Regnell, A.; Wijk, O. Toward a fossil free future with HYBRIT: Development of iron and steelmaking technology in Sweden and Finland. Metals 2020, 10, 972. [Google Scholar] [CrossRef]
- Nishioka, K.; Ujisawa, Y.; Tonomura, S.; Ishiwata, N.; Sikstrom, P. Sustainable aspects of CO2 ultimate reduction in the steelmaking process (COURSE50 Project), part 1: Hydrogen reduction in the blast furnace. J. Sustain. Metall. 2016, 2, 200–208. [Google Scholar] [CrossRef]
- Onoda, M.; Matsuzaki, Y.; Chowdhury, F.A.; Yamada, H.; Goto, K.; Tonomura, S. Sustainable aspects of ultimate reduction of CO2 in the steelmaking process (COURSE50 Project), part 2: CO2 capture. J. Sustain. Metall. 2016, 2, 209–215. [Google Scholar] [CrossRef]
- Hessling, O.; Fogelström, J.B.; Kojola, N.; Sichen, D. The Effect of the Endothermic Reaction Nature on the Iron Ore Pellet Reduction Using Hydrogen. Metall. Mater. Trans. B 2022, 53, 1258–1268. [Google Scholar] [CrossRef]
- Hidayat, T.; Shishin, D.; Jak, E.; Decterov, S.A. Thermodynamic reevaluation of the Fe–O system. Calphad 2015, 48, 131–144. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Seetharaman, S. Fundamentals of Metallurgy; Taylor & Francis: Oxfordshire, UK, 2005. [Google Scholar]
- Dhawan, N.; Manzoor, U.; Agrawal, S. Hydrogen reduction of low-grade banded iron ore. Min. Eng. 2022, 187, 107794. [Google Scholar] [CrossRef]
- Hayes, P.C. Analysis of product morphologies and reaction mechanisms on gaseous reduction of iron oxides. Steel Res. Int. 2011, 82, 480–493. [Google Scholar] [CrossRef]
- Kazemi, M.; Pour, M.S.; Sichen, D. Experimental and modeling study on reduction of hematite pellets by hydrogen gas. Metall. Mater. Trans. B 2017, 48, 1114–1122. [Google Scholar] [CrossRef]
- Kazemi, M.; Sichen, D. Effect of Experimental Conditions on Cementite Formation during Reduction of Iron Ore Pellets. Metall. Mater. Trans. B 2016, 47, 3519–3526. [Google Scholar] [CrossRef]
- Strangway, P.K. Kinetics of Reduction of Iron Oxide by Reformed Natural Gas. Master’s Thesis, Toronto University, Toronto, ON, Canada, 1964. [Google Scholar]
- Bahgat, M.; Khedr, M.H. Reduction kinetics, magnetic behavior and morphological changes during reduction of magnetite single crystal. Mater. Sci. Eng. B 2007, 138, 251–258. [Google Scholar] [CrossRef]
- Szekely, J.; El-Tawil, Y. The reduction of hematite pellets with carbon monoxide-hydrogen mixtures. Metall. Trans. B 1976, 7, 490–492. [Google Scholar] [CrossRef]
- Edstrom, J.O. The mechanism of reduction of iron oxides. J. Iron Steel Inst. 1953, 175, 289. [Google Scholar]
- Teplov, O.A. Kinetics of the low-temperature hydrogen reduction of magnetite concentrates. Russ. Metall. 2012, 2012, 8–21. [Google Scholar] [CrossRef]
- Corbari, R.; Fruehan, R.J. Reduction of iron oxide fines to wustite with CO/CO2 gas of low reducing potential. Metall. Mater. Trans. B 2010, 41, 318–329. [Google Scholar] [CrossRef]
- Kawasaki, E.; Sanscrainte, J.; Walsh, T.J. Kinetics of reduction of iron oxide with carbon monoxide and hydrogen. AIChE J. 1962, 8, 48–52. [Google Scholar] [CrossRef]
- El-Geassy, A.A. Gaseous reduction of Fe2O3 compacts at 600 to 1050 C. J. Mater. Sci. 1986, 21, 3889–3900. [Google Scholar] [CrossRef]
- Bradshaw, A.V. Rate-controlling factors in gas-solid reactions of metallurgical interest. Trans. Inst. Min. Met. Dec. 1970, 79C, C281–C294. [Google Scholar]
- Warner, N.A. Reduction kinetics of hematite+ influence of gaseous diffusion. Trans. Metall. Soc. AIME 1964, 230, 163. [Google Scholar]
- Valipour, M.S.; Hashemi, M.Y.M.; Saboohi, Y. Mathematical modeling of the reaction in an iron ore pellet using a mixture of hydrogen, water vapor, carbon monoxide and carbon dioxide: An isothermal study. Adv. Powder Technol. 2006, 17, 277–295. [Google Scholar] [CrossRef]
- Tsay, Q.T.; Ray, W.H.; Szekely, J. The modeling of hematite reduction with hydrogen plus carbon monoxide mixtures: Part II. The direct reduction process in a shaft furnace arrangement. AIChE J. 1976, 22, 1072–1079. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Peng, H.; Jiang, T. Action rules of H2 and CO in gas-based direct reduction of iron ore pellets. J. Cent. South Univ. 2012, 19, 2291–2296. [Google Scholar] [CrossRef]
- Bonalde, A.; Henriquez, A.; Manrique, M. Kinetic analysis of the iron oxide reduction using hydrogen-carbon monoxide mixtures as reducing agent. ISIJ Int. 2005, 45, 1255–1260. [Google Scholar] [CrossRef]
- Zuo, H.; Wang, C.; Dong, J.; Jiao, K.; Xu, R. Reduction kinetics of iron oxide pellets with H2 and CO mixtures. Int. J. Miner. Metall. Mater. 2015, 22, 688–696. [Google Scholar] [CrossRef]
- Ghadi, A.Z.; Valipour, M.S.; Vahedi, S.M.; Sohn, H.Y. A review on the modeling of gaseous reduction of iron oxide pellets. Steel Res. Int. 2020, 91, 1900270. [Google Scholar] [CrossRef]
- Nakiboglu, F.; St John, D.H.; Hayes, P.C. The gaseous reduction of solid calciowustites in CO/CO2 and H2/H2O gas mixtures. Metall. Trans. B 1986, 17, 375–381. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VI, USA, 2023. [Google Scholar]
- Li, P.; Li, Y.; Yu, J.; Gao, P.; Han, Y. Kinetics and microstructural changes during fluidized reduction of magnetite with hydrogen at low temperatures. Int. J. Hydrogen Energy 2022, 47, 31140–31151. [Google Scholar] [CrossRef]
- Mao, X.; Garg, P.; Hu, X.; Li, Y.; Nag, S.; Kundu, S.; Zhang, J. Kinetic analysis of iron ore powder reaction with hydrogen—Carbon monoxide. Int. J. Miner. Metall. Mater. 2022, 29, 1882–1890. [Google Scholar] [CrossRef]
- Li, S.; Gu, H.; Huang, A.; Zou, Y.; Yang, S.; Fu, L. Thermodynamic analysis and experimental verification of the direct reduction of iron ores with hydrogen at elevated temperature. J. Mater. Sci. 2022, 57, 10419–20434. [Google Scholar] [CrossRef]
- Ayyandurai, A.; Pal, J. Kinetics of Carbon Oxidation During Induration of Hematite Ore Pellet. Min. Metall. Explor. 2022, 39, 2551–2560. [Google Scholar] [CrossRef]
- Meshram, A.; Govro, J.; OMalley, R.J.; Sridhar, S.; Korobeinikov, Y. Modeling Isothermal Reduction of Iron Ore Pellet Using Finite Element Analysis Method: Experiments & Validation. Metals 2022, 12, 2026. [Google Scholar]
- Turkdogan, E.T.; Vinters, J.V. Reducibility of iron ore pellets and effect of additions. Can. Metall. Q. 1973, 12, 9–21. [Google Scholar] [CrossRef]
- Scharm, C.; Küster, F.; Laabs, M.; Huang, Q.; Volkova, O.; Reinmöller, M.; Guhl, S.; Meyer, B. Direct reduction of iron ore pellets by H2 and CO: In-situ investigation of the structural transformation and reduction progression caused by atmosphere and temperature. Min. Eng. 2022, 180, 107459. [Google Scholar] [CrossRef]
- Wang, H.T.; Sohn, H.Y. Effect of CaO and SiO2 on swelling and iron whisker formation during reduction of iron oxide compact. Ironmak. Steelmak. 2011, 38, 447–452. [Google Scholar] [CrossRef]
- Turcotte, S.; Marquis, H.; Dancy, T. The use of direct reduced iron in the electric arc furnace. In Electric Furnace Steelmaking; AIME, Iron Steel Society: New York, NY, USA, 1985; pp. 115–126. Available online: https://www.ltu.se/cms_fs/1.13387!/efs_chap_10.pdf (accessed on 21 August 2023).
- Gyllenram, R.; Arzpeyma, N.; Wei, W.; Jönsson, P.G. Driving investments in ore beneficiation and scrap upgrading to meet an increased demand from the direct reduction-EAF route. Miner. Econ. 2022, 35, 203–220. [Google Scholar] [CrossRef]
- Shehata, K. Study of the Final Stages of Reduction of Iron Oxides. Trans. Inst. Min. Met. 1973, 82. [Google Scholar]
- Bahgat, M.; Halim, K.S.A.; Nasr, M.I.; El-Geassy, A.A. Morphological changes accompanying gaseous reduction of SiO2 doped wüstite compacts. Ironmak. Steelmak. 2008, 35, 205–212. [Google Scholar] [CrossRef]
- Kim, W.-H.; Lee, Y.-S.; Suh, I.-K.; Min, D.-J. Influence of CaO and SiO2 on the reducibility of wüstite using H2 and CO gas. ISIJ Int. 2012, 52, 1463–1471. [Google Scholar] [CrossRef]
- Shigematsu, N.; Iwai, H. Effect of the Addition of SiO2 and SiO2-CaO on the Reduction of Dense Wustite at High Temperatures. Tetsu Hagané 1993, 79, 920–926. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sayama, S.; Nishida, K. Expansion and Contraction during Hydrogen Reduction of Green and Pre-heated Hematite Compacts Containing Foreign Oxides. Trans. Iron Steel Inst. Jpn. 1981, 21, 870–878. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamamoto, M.; Kotanigawa, T.; Nishida, K. Some aspects on porous properties of iron oxides containing foreign oxides reduced by hydrogen. Metall. Trans. B 1981, 12, 691–697. [Google Scholar] [CrossRef]
- Sato, K.; Suzuki, S.; Sawamura, Y.; Ono, K. Relation between Sinter Microstructures and Sinter Properties during Reduction. Tetsu Hagané 1982, 68, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Higuchi, K.; Hosotani, Y.; Ohshio, A.; Kasama, S. Influence of Micro Pore on Reducibility and Permeability of Sinter above 1000 °C in Blast Furnace. Tetsu Hagané 1998, 84, 702–708. [Google Scholar] [CrossRef][Green Version]
- Naito, M.; Murayama, T.; Usui, T. Kinetic analysis on gaseous reduction of agglomerates, Part 3, application of gaseous reduction models for agglomerates to Blast Furnace Analysis. Tetsu Hagané 1994, 80, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Ono, Y. Experimental Study on the Relation between Microstructures of Constituent Minerals and Pores and Reducibility of Sinter. Tetsu Hagané 1986, 72, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Heerema, R.H. Influence of artificially induced porosity on strength and reduction behavior of hematite compacts. ISIJ Int. 2005, 45, 574–581. [Google Scholar] [CrossRef][Green Version]
- Shigematsu, N.; Iwai, H. Effect of the Addition of Al2O3 and Al2O3-Ca0 on the Reduction of Dense Wustite with H2. Tetsu Hagané 1987, 73, 2243–2250. [Google Scholar] [CrossRef]
- Fischer, W.A.; Hoffmann, A. Das Zustandsschaubild Eisenoxydul-Aluminiumoxyd. Archiv. Für. Das. Eisenhüttenwesen 1956, 27, 343–346. [Google Scholar] [CrossRef]
- Schürmann, E.; Bannenberg, N. Metall-Schlackengleichgewichte im System Eisen-Aluminium-Sauerstoff als Grundlage der Aluminiumdesoxidation von Stahlschmelzen. Archiv. Für. Das. Eisenhüttenwesen 1984, 55, 409–414. [Google Scholar] [CrossRef]
- Atlas, L.M.; Sumida, W.K. Solidus, Subsolidus, and Subdissociation Phase Equilibria in the System Fe-Al-O. J. Am. Ceram. Soc. 1958, 41, 150–160. [Google Scholar] [CrossRef]
- Elrefaie, F.A.; Smeltzer, W.W. Thermodynamics of the system iron-aluminum-oxygen between 1073 K and 1573 K. Metall. Mater. Trans. B 1983, 14, 85–93. [Google Scholar] [CrossRef]
- Novokhatskii, I.A.; Belov, B.F.; Gorokh, A.V.; Savinskaya, A.A. The phase diagram for the system ferrous oxide-alumina. Russ. J. Phys. Chem. 1965, 39, 1498–1500. [Google Scholar]
- Rosenbach, K.; Schmitz, J.A. Untersuchungen im Dreistoffsystem Eisen (II)-oxid–Chrom (III)-oxid–Tonerde. Archiv. Für. Das. Eisenhüttenwesen 1974, 45, 843–847. [Google Scholar] [CrossRef]
- Oelsen, W.; Heynert, G. The reactions between iron-manganese melts and the melts of their aluminates. Arch. Eisenhuttenwes 1955, 26, 567–575. [Google Scholar]
- Lindwall, G.; Liu, X.L.; Ross, A.; Fang, H.; Zhou, B.-C.; Liu, Z.-K. Thermodynamic modeling of the aluminum–iron–oxygen system. Calphad 2015, 51, 178–192. [Google Scholar] [CrossRef]
- Shishin, D.; Prostakova, V.; Jak, E.; Decterov, S.A. Critical assessment and thermodynamic modeling of the Al-Fe-O system. Metall. Mater. Trans. B 2016, 47, 397–424. [Google Scholar] [CrossRef]
- Prostakova, V.; Shishin, D.; Shevchenko, M.; Jak, E. Thermodynamic optimization of the Al2O3–FeO–Fe2O3–SiO2 oxide system. Calphad 2019, 67, 101680. [Google Scholar] [CrossRef]
- Seth, B.B.L.; Ross, H.U. The Effect of Lime on The Reducibility of Iron-Oxide Agglomerates. Can. Metall. Q. 1963, 2, 15–30. [Google Scholar] [CrossRef]
- Notini, U. J. Iron Steel Inst. 1958, 189. Available online: https://mcmaster.primo.exlibrisgroup.com/discovery/openurl?institution=01OCUL_MU&vid=01OCUL_MU:OMNI&rft.aulast=Notini&rft.epage=326&url_ver=Z39.88-2004&rft.date=1958&rfr_id=info:sid%2Fliteratum:tandf&rft.spage=322&rft.btitle=J.%20Iron%20Steel%20Inst.,%20 (accessed on 21 August 2023).
- Philbrook, W.O. A Study of the Reducibility of Ores and Blast Furnace Sinter. Blast Furnace and Steel Plant. August 1943. Available online: https://books.google.ca/books?id=TpIAAAAAMAAJ&pg=PA114&lpg=PA114&dq=%22A+study+of+the+reducibility+of+ores+and+blast+furnace+sinter%22&source=bl&ots=O16tjfWNHO&sig=ACfU3U3ZtmKQF-y0zur3kTrf1TZQfFxocg&hl=en&sa=X&ved=2ahUKEwj1i4TknfmAAxXVkIkEHV3RAeYQ6AF6BAg (accessed on 21 August 2023).
- White, J. The Physical Chemistry of Open-Hearth Slags. J. Iron Steel Inst. 1943, 148, 579–694. [Google Scholar]
- Strangway, P.K.; Ross, H.U. The effect of calcium carbonate on the reducibility of iron-oxide agglomerates. Can. Metall. Q. 1965, 4, 97–111. [Google Scholar] [CrossRef]
- Iguchi, Y.; Fukunaga, M.; Hirao, J. Dependence of the Reduction Rate on the Temperature and the α-γ Transformation in Wustite Containing CaO. Trans. Jpn. Inst. Met. 1983, 24, 115–124. [Google Scholar] [CrossRef]
- Williams, R.V. Control and Analysis in Iron and Steelmaking; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hsieh, L.-H.; Ja, W. Effect of raw material composition on the mineral phases in lime-fluxed iron ore sinter. ISIJ Int. 1993, 33, 462–473. [Google Scholar] [CrossRef]
- Härkki, J. Über den Einfluss von Magnesiumoxyd-Zusatz auf die Reduktion des Wüstits, Technische Universität Helsinki. Institut für Prozessmetallurgie, 1978. Available online: https://kansalliskirjasto.finna.fi/kansalliskirjastofikka/Record/fikka.3353156 (accessed on 21 August 2023).
- Bahgat, M.; Halim, K.S.A.; Nasr, M.I.; El-Geassy, A.H.A. Reduction Behaviour of Wüstite Doped with MgO. Steel Res. Int. 2007, 78, 443–450. [Google Scholar] [CrossRef]
- Pan, F.; Zhu, Q.; Du, Z.; Sun, H. Migration Behavior of the MgO and its Influence on the Reduction of Fe3O4–MgO Sinter. ISIJ Int. 2018, 58, 652–659. [Google Scholar] [CrossRef]
- Zheng, H.; Spreitzer, D.; Wolfinger, T.; Schenk, J.; Xu, R. Effect of prior oxidation on the reduction behavior of magnetite-based iron ore during hydrogen-induced fluidized bed reduction. Metall. Mater. Trans. B 2021, 52, 1955–1971. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Nasr, M.I.; Omar, A.A.; Mousa, E.A. Isothermal reduction behaviour of MnO2 doped Fe2O3 compacts with H2 at 1073–1373 K. Ironmak. Steelmak. 2008, 35, 531–538. [Google Scholar] [CrossRef]
- Ellingham, H.J.T. Reducibility of oxides and sulphides in metallurgical processes. J. Soc. Chem. Ind. 1944, 63, 125–160. [Google Scholar]
- ISO 11323:2010; Iron Ore and Direct Reduced Iron—Vocabulary. ISO: Geneva, Switzerland, 2010.
- Cores, A.; Babich, A.; Muñiz, M.; Ferreira, S.; Mochon, J. The influence of different iron ores mixtures composition on the quality of sinter. ISIJ Int. 2010, 50, 1089–1098. [Google Scholar] [CrossRef]
- Kumar, C.U.; Ramana, R.V.; Ali, S.; Das, A.K.; Kumar, A.; De, A.K.; Mukherjee, T. Quality of sinter in the light of blast performance. Tata Search. 1995, 20–25. [Google Scholar]
- Iguchi, Y.; Inouye, M. On the rate of the reduction of wustite, magnetite, and hematite containing Al2O3, CaO, and MgO. Tetsu Hagané 1979, 65, 1692–1701. [Google Scholar] [CrossRef][Green Version]
- Iguchi, Y.; Iida, M.; Inouye, M. Pore Radius Distributions of Iron Reduced from Hematite Containing Several Foreign Oxides. Tetsu Hagané 1979, 65, 24–33. [Google Scholar] [CrossRef][Green Version]
- Bleifuss, R.L. Calcium as a cause of catastrophic swelling of pellets during reduction. Trans. Soc. Min. Eng. Aime 1970, 247, 225–231. [Google Scholar]
- Raghavan, V. Fe-Mg-O (Iron-Magnesium-Oxygen). J. Phase Equilibria Diffus. 2010, 31, 368. [Google Scholar] [CrossRef][Green Version]
- Shigematsu, N.; Iwai, H. Effects of Initiation of Reduction and Oxide Additives on Morphology of Iron Nuclei in the Reduction of Wustite with Hydrogen at 670 °C. Tetsu Hagané 1993, 79, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Fukumoto, Y.; Shimizu, M. Effect of SiO2 and Al2O3 on high temperature reduction of iron ore. Tetsu Hagané 2001, 87, 327–334. [Google Scholar] [CrossRef]
- Paananen, T.; Heinänen, K.; Härkki, J. Degradation of iron oxide caused by alumina during reduction from magnetite. ISIJ Int. 2003, 43, 597–605. [Google Scholar] [CrossRef]
- Wang, P.; Wang, C.; Wang, H.; Long, H.; Zhou, T. Effects of SiO2, CaO and basicity on reduction behaviors and swelling properties of fluxed pellet at different stages. Powder Technol. 2022, 396, 477–489. [Google Scholar] [CrossRef]
- El-Geassy, A.A. Gaseous reduction of MgO-doped Fe2O3 compacts with carbonmonoxide at 1173–1473 K. ISIJ Int. 1996, 36, 1328–1337. [Google Scholar] [CrossRef][Green Version]

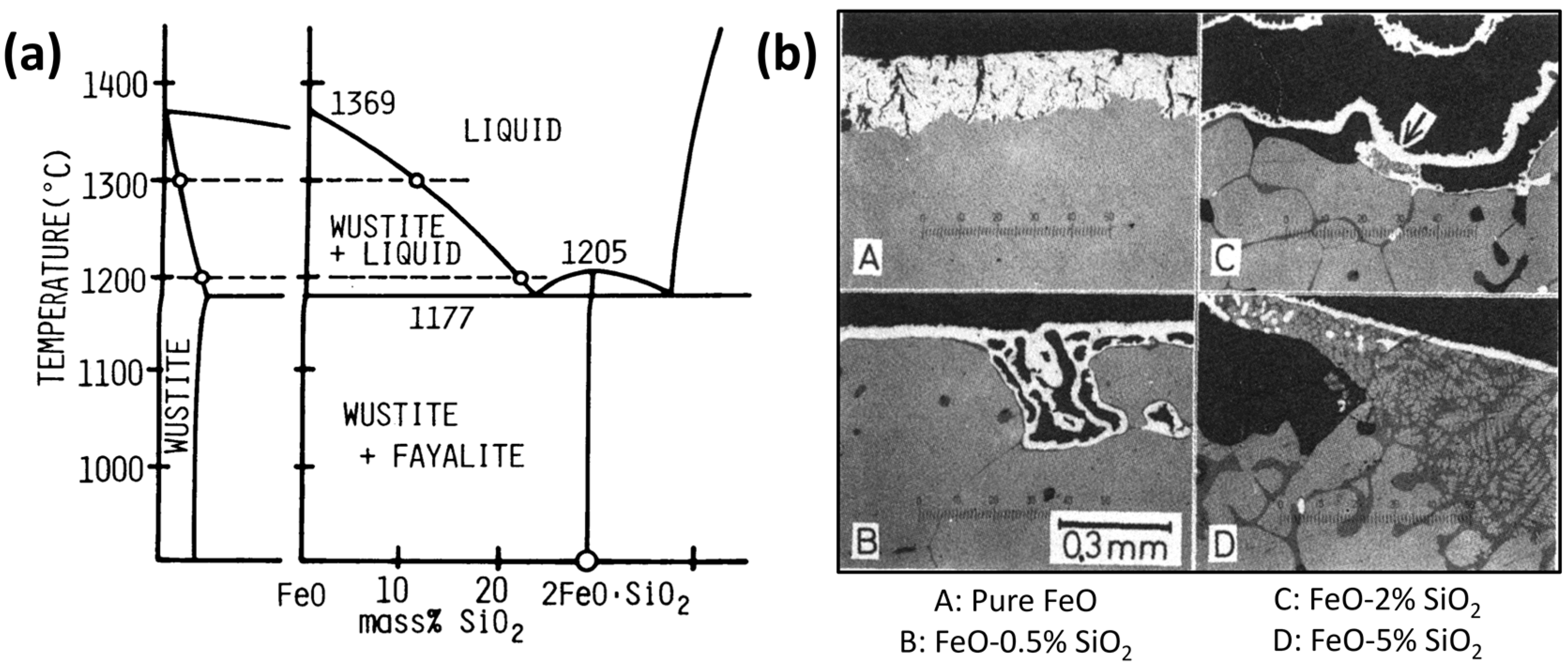
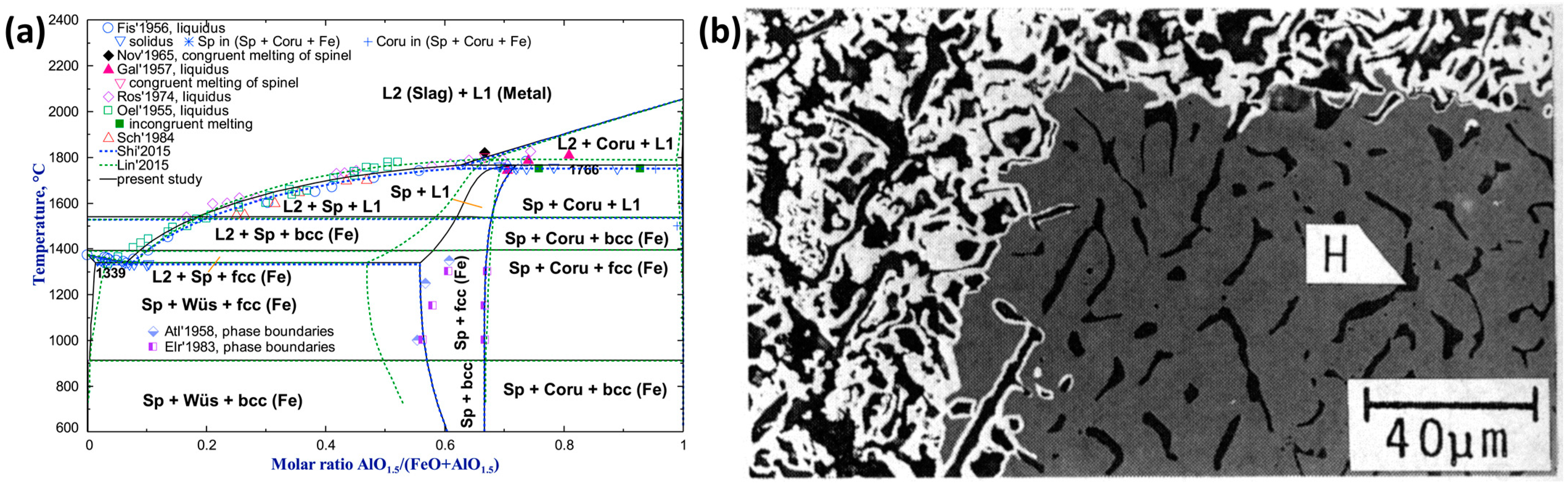
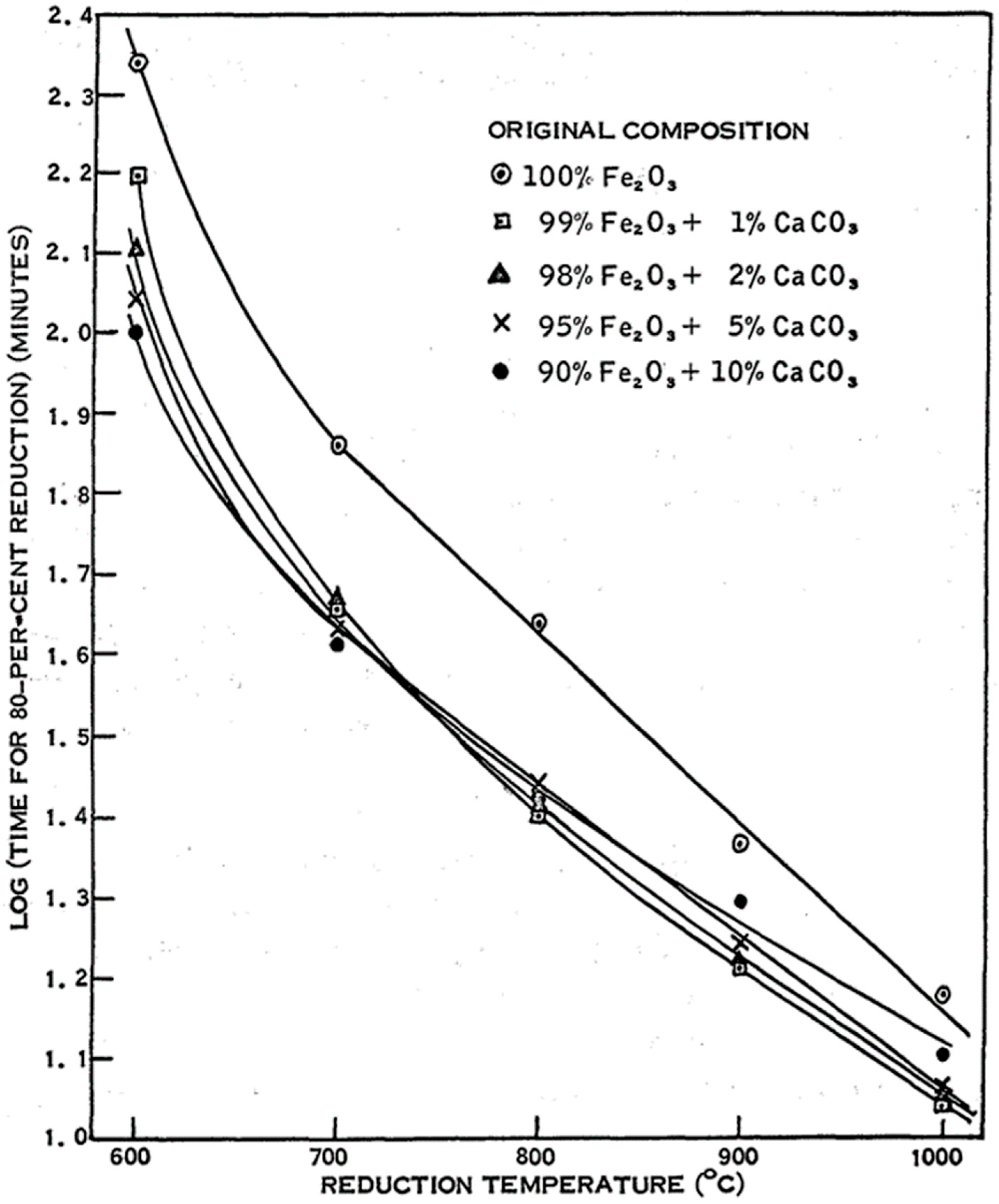
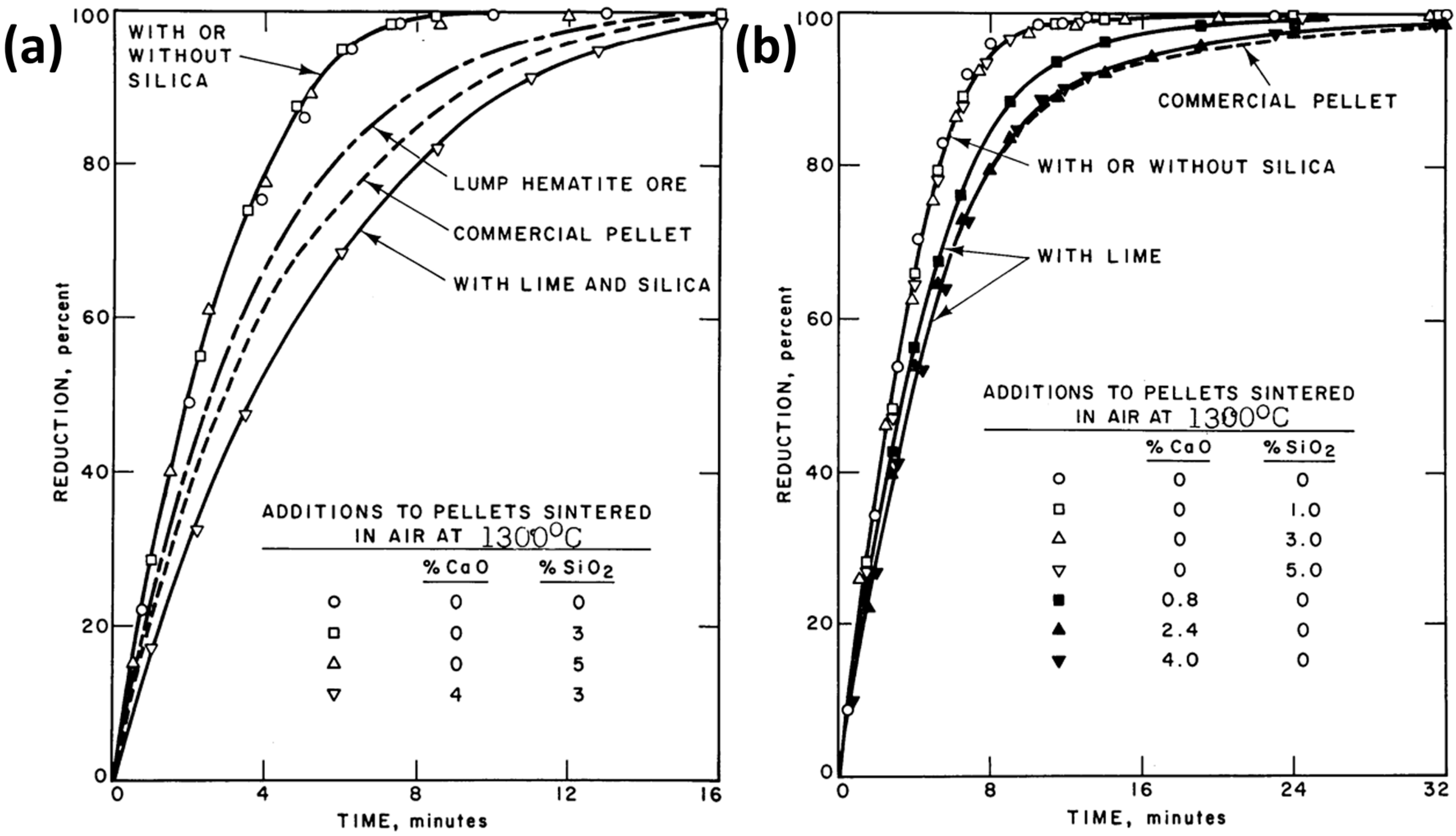
| Reference | Reducing Agent(s) | Temperature (°C) | Raw Materials | Main Findings |
|---|---|---|---|---|
| [38] | H2 | 600–900 | Commercial hematite pellets | Significant increase in the reduction rate was observed with increasing temperature. Different incubation times were observed depending on the unsteady conditions of gas composition and/or temperature before the reduction reaction begins. Heat transfer in the pellet was found to be the rate-controlling step. |
| [44] | H2 | 800–850 | Industrial hematite pellets, Pure hematite pellets | High density of pure hematite pellets (porosity < 20%) limited the mass transfer of reducing gas; thereby changing the reduction mechanism. A layer-by-layer reduction mechanism was noted for the dense pure hematite pellets. whereas this mechanism was not followed in the reduction of industrial pellets. The presence of layers with different phases in industrial pellets (observed by SEM) suggests that the reaction’s progress was controlled by both the internal diffusion of H2 and chemical reactions within the layers. |
| [45] | H2-CO | 700–950 | Industrial hematite pellets | Examining the partially reduced samples confirmed the simultaneous progress of reduction reactions and cementite formation. The reaction temperature and gas atmosphere were the major contributing factors to cementite formation. The activity of carbon determines the carburizing process, and it decreases as the CO2 concentration increases. However, the higher CO2 levels enhanced the formation of cementite, indicating that the cementite formation kinetics were affected by CO2 as well. The highest level of cementite (24–30%) was achieved when there was an absence of CO2 in the reducing gas at 850 °C. The introduction of 1% CO2 led to a substantial decrease in the Fe3C content (8%). However, subsequent increase in the CO2 concentration to 3 and 10% exhibited an increase in the quantity of cementite to 15 and 22%, respectively. It appears that having CO2 in the gas mix suppresses C deposition and hence promotes Fe3C formation. At lower reaction temperatures, noticeable carbon deposition was observed on the pellets, which hindered the gas diffusion into the pellet. |
| [29] | H2 | 800–1100 | Commercial iron ore fines (<100 µm) | Higher temperatures led to an increased reduction rate at the initial and final stages of reduction. As the reduction proceeded, the apparent activation energy (Ea) increased. Consequently, it was concluded that gaseous diffusion and interfacial chemical reaction were the rate-controlling steps in the initial stages, whereas solid diffusion and interfacial chemical reaction controlled the later stages. The conclusions regarding the rate-controlling steps were based on the relationship between Ea and the reduction mechanism, as defined in [46]. |
| [47] | H2 | 900–1100 | Magnetite single crystals | Irrespective of the temperature, the highest reduction rates were observed at the early stages, followed by a decrease until the end of reduction. The low reduction rates at 900 and 950 °C caused longer reaction times, which resulted in the formation of a dense metallic layer around wüstite grains. This in turn hindered full reduction. |
| [48] | H2-CO Pure H2 Pure CO | 750–900 | Analytical grade hematite | Increasing the H2 content of the reducing gas resulted in much shorter reduction times. The mean pore diameter of the specimens increased as the reduction proceeded. This was more pronounced in pure and CO-rich gas mixtures. That being said, producing a loose and permeable structure did not lead to an increase in the effective diffusivity when compared to H2-rich gas mixtures. This was related to the higher molecular weight of the CO-rich gas mixtures. |
| Foreign Oxide | Solubility in Iron Oxides | Effect |
|---|---|---|
| Al2O3 | Highly soluble in hematite and magnetite but almost insoluble in wüstite [114]. | Leads to the formation of micro-pores, and as the quantity of foreign oxide increases, the pore radius decreases [114]. |
| CaO | Limited solubility in wüstite Limited solubility in hematite [114] and slightly greater solubility in magnetite (~1:3) was reported in [115]. | Results in the formation of macro-pores, and as the quantity of foreign oxide increases, the pore radius increases [114]. |
| MgO | Forms continuous solid solution with wüstite and does not exhibit limited solubility [114]. Insignificant solubility in hematite was reported in [116]. | Minimal influence on the pore radius [114]. |
| SiO2 | Almost insoluble in any iron oxides [114]. | No effect [114]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakeri, A.; Coley, K.S.; Tafaghodi, L. Hydrogen-Based Direct Reduction of Iron Oxides: A Review on the Influence of Impurities. Sustainability 2023, 15, 13047. https://doi.org/10.3390/su151713047
Zakeri A, Coley KS, Tafaghodi L. Hydrogen-Based Direct Reduction of Iron Oxides: A Review on the Influence of Impurities. Sustainability. 2023; 15(17):13047. https://doi.org/10.3390/su151713047
Chicago/Turabian StyleZakeri, Ali, Kenneth S. Coley, and Leili Tafaghodi. 2023. "Hydrogen-Based Direct Reduction of Iron Oxides: A Review on the Influence of Impurities" Sustainability 15, no. 17: 13047. https://doi.org/10.3390/su151713047
APA StyleZakeri, A., Coley, K. S., & Tafaghodi, L. (2023). Hydrogen-Based Direct Reduction of Iron Oxides: A Review on the Influence of Impurities. Sustainability, 15(17), 13047. https://doi.org/10.3390/su151713047







