Utilization of Food Waste for the Development of Composite Bread
Abstract
:1. Introduction
2. Material and Method
2.1. Pr °Curement of Raw Material
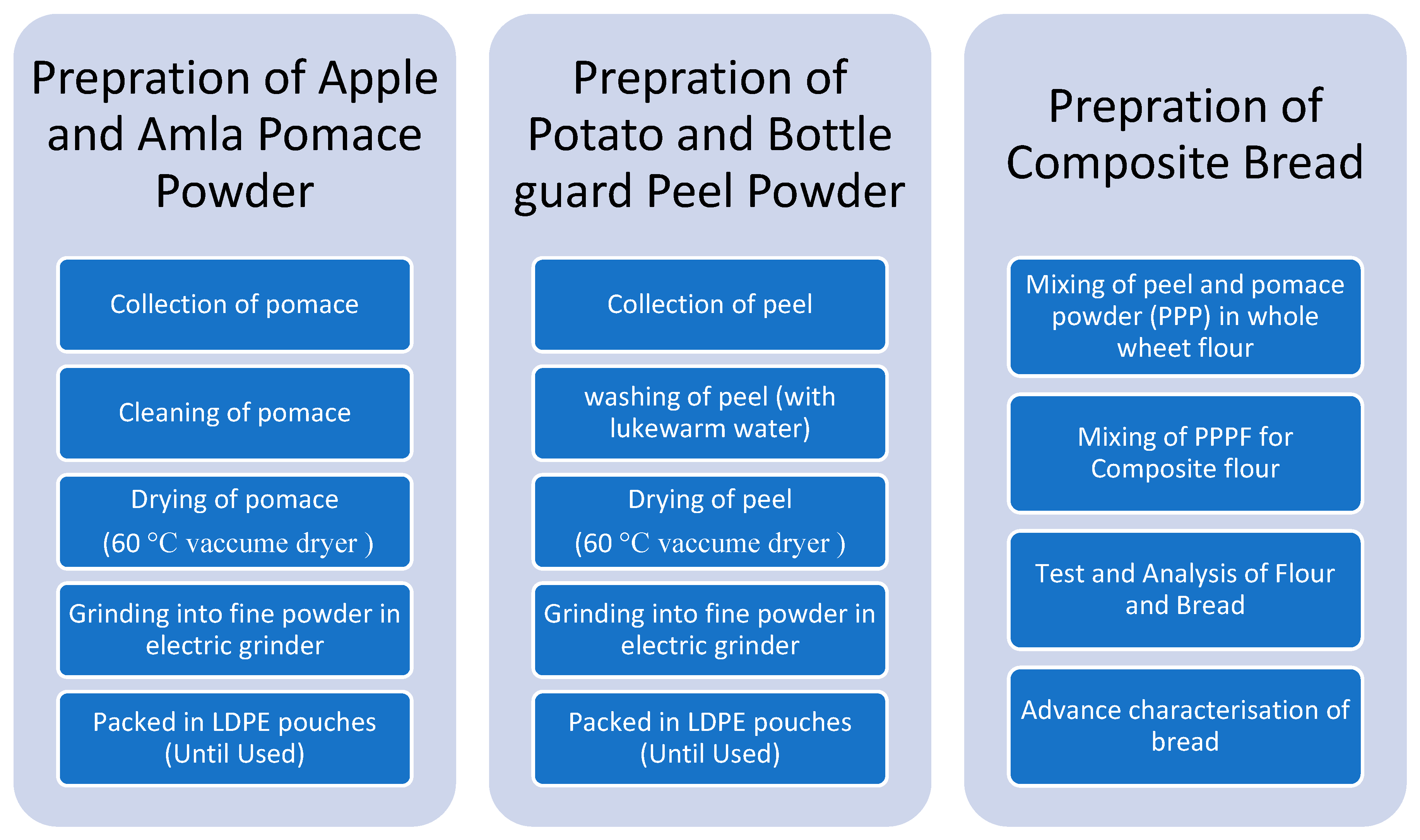
2.2. Preparation of Peel and Pomace Powder (PPP)
2.3. Preparation of Peel and Pomace Powder Composite Flour
2.4. Preparation of Bread
2.5. Nutritional Evaluations
2.5.1. Total Phenolic Content Analysis
2.5.2. Ascorbic Acid Analysis
2.5.3. Total Sugar Analysis
2.6. Functional Analysis
2.6.1. Water Absorption Capacity
2.6.2. Oil Absorption Capacity
2.6.3. Swelling Capacity
2.6.4. Emulsion Capacity
2.6.5. Foam Capacity
2.7. Physical Analysis
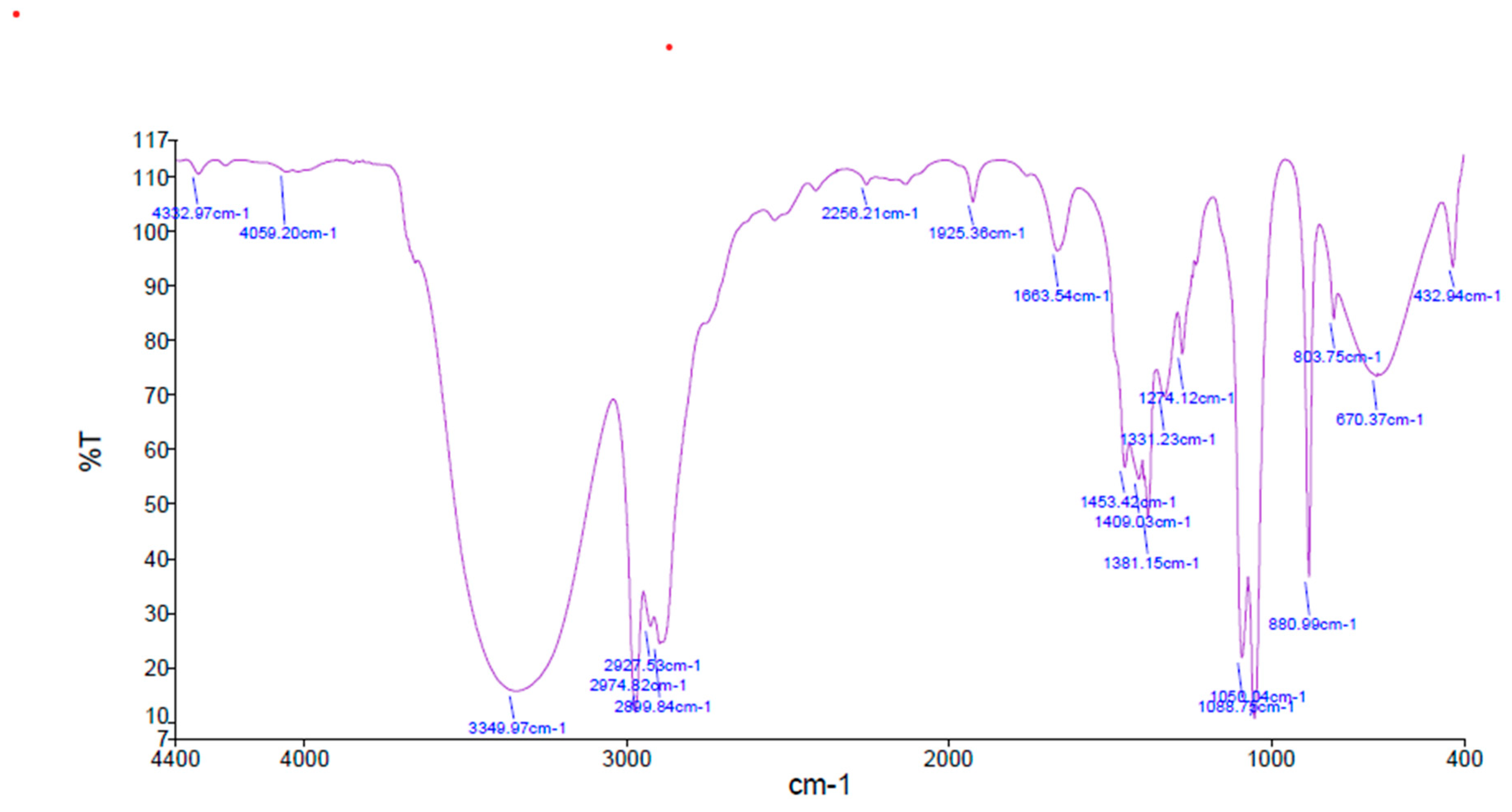
2.8. FTIR Analysis
2.9. GC–MS Analysis
2.10. XRD Analysis
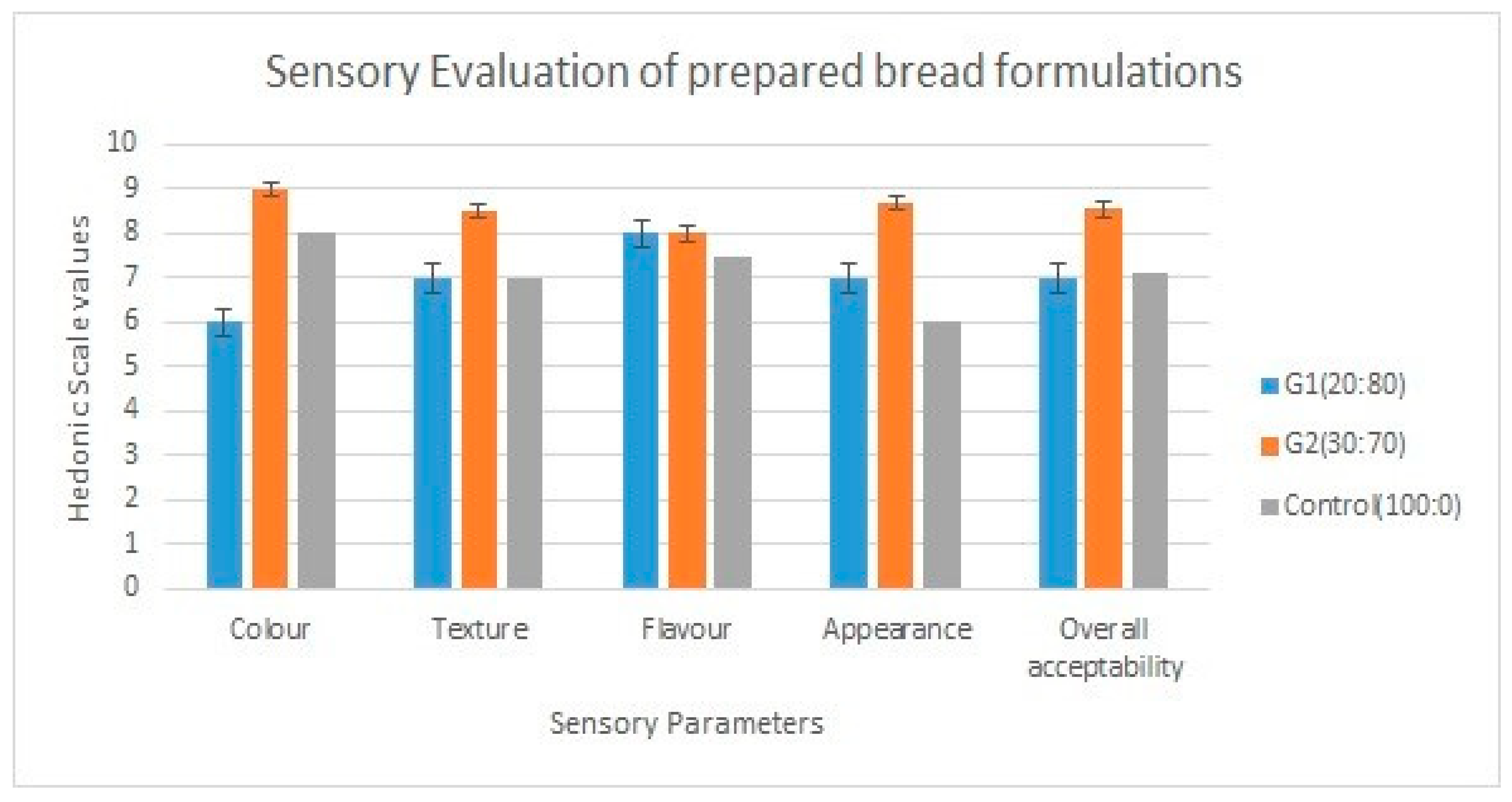
2.11. Sensory Analysis
2.12. Statistical Analysis
Hypothesis Conducted
3. Results and Discussion
3.1. Nutritional Analysis Result
3.2. Functional Analysis
3.2.1. Water Absorption Capacity (WAC)
3.2.2. Oil Absorption Capacity (OAC)
3.2.3. Swelling Capacity Results
3.2.4. Emulsion Capacity Results
3.2.5. Foaming Capacity Results
3.3. Physical Analysis Results
3.4. FTIR Analysis Results
3.5. GC-MS Analysis Results
3.6. XRD Analysis Result
3.7. Sensory Evaluation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morone, P.; Koutinas, A.; Gathergood, N.; Arshadi, M.; Matharu, A. Food waste: Challenges and opportunities for enhancing the emerging bio-economy. J. Clean. Prod. 2019, 221, 10–16. [Google Scholar] [CrossRef]
- Forbes, H.; Quested, T.; O’Connor, C. Food Waste Index Report 2021; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Yun, Y.M.; Lee, M.K.; Im, S.W.; Marone, A.; Trably, E.; Shin, S.R.; Kim, M.G.; Cho, S.K.; Kim, D.H. Biohydrogen production from food waste: Current status, limitations, and future perspectives. Bioresour. Technol. 2018, 248, 79–87. [Google Scholar] [CrossRef]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of high value-added compounds from pineap-ple, melon, watermelon and pumpkin processing by-products: An overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Gaurav, N. Current scenario and future perspectives of nanotechnology in sustainable agriculture and food production. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 99–121. [Google Scholar]
- Shashikant, M.; Bains, A.; Chawla, P.; Sharma, M.; Kaushik, R.; Kandi, S.; Kuhad, R.C. In-vitro antimicrobial and anti-inflammatory activity of modified solvent evaporated ethanolic extract of Calocybe indica: GCMS and HPLC characterization. Int. J. Food Microbiol. 2022, 376, 109741. [Google Scholar] [CrossRef] [PubMed]
- Cauvain, S.P. Other cereals in breadmaking. In Technology of Breadmaking; Springer: Berlin/Heidelberg, Germany, 2007; pp. 371–388. ISBN 0387385630. [Google Scholar]
- Sridhar, A.; Kapoor, A.; Senthil, P.; Ponnuchamy, M.; Balasubramanian, S.; Prabhakar, S. Conversion of food waste to energy: A focus on sustainability and life cycle assessment. Fuel 2021, 302, 121069. [Google Scholar] [CrossRef]
- Kumar, V.; Jaiswal, K.K.; Tomar, M.S.; Rajput, V.; Upadhyay, S.; Nanda, M.; Vlaskin, M.S.; Kumar, S.; Kurbatova, A. Production of high value-added biomolecules by microalgae cultivation in wastewater from anaerobic digestates of food waste: A review. Biomass Convers. Biorefinery 2021, 13, 9625–9642. [Google Scholar] [CrossRef]
- Lister, C.E.; Lancaster, J.E.; Walker, J.R.L. Phenylalanine ammonia-lyase (PAL) activity and its relationship to anthocyanin and flavonoid levels in New Zealand-grown apple cultivars. J. Am. Soc. Hortic. Sci. 1996, 121, 281–285. [Google Scholar] [CrossRef]
- Tulasi, G.; Deepika, U.; Venkateshwarlu, P.; Santhosh, V.; Srilatha, P. Development of millet based instant soup mix and Pulav mix. IJCS 2020, 8, 832–835. [Google Scholar] [CrossRef]
- Gajera, R.R.; Joshi, D.C.; Ravani, A. Processing potential of bottle gourd (L. siceraria) Fruits: An overview. Int. J. Herb. Med. 2017, 16, 16–40. [Google Scholar]
- Javed, A.; Ahmad, A.; Tahir, A.; Shabbir, U.; Nouman, M.; Hameed, A. Potato peel waste-its nutraceutical, industrial and biotechnological applacations. AIMS Agric. Food 2019, 4, 807–823. [Google Scholar] [CrossRef]
- Torbica, A.; Blažek, K.M.; Belović, M.; Hajnal, E.J. Quality prediction of bread made from composite flours using different parameters of empirical rheology. J. Cereal Sci. 2019, 89, 102812. [Google Scholar] [CrossRef]
- Ohimain, E.I. The prospects and challenges of cassava inclusion in wheat bread policy in Nigeria. Int. J. Sci. Technol. Soc. 2014, 2, 6–17. [Google Scholar] [CrossRef]
- Shittu, T.A.; Raji, A.O.; Sanni, L.O. Bread from composite cassava-wheat flour: I. Effect of baking time and temperature on some physical properties of bread loaf. Food Res. Int. 2007, 40, 280–290. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, M.A.; Alam, A.; Swieca, M.; Skeczyk, L.; Gawlik-Dziki, U.; Dziki, D.; Rekowski, A.; et al. A comparative study on the chemical composition of wild and cuntivated germaplasm of Phaseolus lunatus L. Waste Manag. 2020, 7, 296–305. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, M.A.; Alam, A. Immune response in COVID-19: A review. J. Infect. Public Health 2020, 13, 1619–1629. [Google Scholar] [CrossRef]
- Aman, F.; Masood, S. How Nutrition can help to fight against COVID-19 Pandemic. Pakistan J. Med. Sci. 2020, 36, S121. [Google Scholar] [CrossRef]
- Xiao, H.-W.; Pan, Z.; Deng, L.-Z.; El-Mashad, H.M.; Yang, X.-H.; Mujumdar, A.S.; Gao, Z.-J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- ICMR-NiN Short Report of Nutritional requiremnets of Indians-RDA and Estimated Average Requirements-ICMR-NiN 2020. Available online: https://www.nin.res.in/RDA_short_Report_2020.html (accessed on 28 April 2023).
- Chandra, S.; Singh, S.; Kumari, D. Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. J. Food Sci. Technol. 2015, 52, 3681–3688. [Google Scholar] [CrossRef]
- Paulami, G.; Upadhyay, S. Food Waste Management and Nutrient Recycling in a Sustainable Way—A Review. In International Conference on Advances and Innovations in Recycling Engineering; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 157–164. [Google Scholar]
- Al-Attabi, Z.H.; Merghani, T.M.; Ali, A.; Rahman, M.S. Effect of barley flour addition on the physico-chemical properties of dough and structure of bread. J. Cereal Sci. 2017, 75, 61–68. [Google Scholar] [CrossRef]
- Živančev, D.; Torbica, A.; Tomić, J.; Janić-Hajnal, E. Possibility of utilization alternative cereals (millet and barley) for improvement technological properties of bread gained from flour of poor technological quality. J. Process. Energy Agric. 2016, 20, 165–169. [Google Scholar]
- AAACC, American Association of Cereal Chemists. Approved Methods Committee. In Approved Methods of the American Association of Cereal Chemists; Amer Assn of Cereal Chemists: St Paul, MN, USA, 2000; Volume 1. [Google Scholar]
- Rekowski, A.; Langenkämper, G.; Dier, M.; Wimmer, M.A.; Scherf, K.A.; Zörb, C. Determination of soluble wheat protein fractions using the Bradford assay. Cereal Chem. 2021, 98, 1059–1065. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Kohli, D.; Kumar, A.; Kumar, S.; Upadhyay, S. Waste Utilization of Amla Pomace and Germinated Finger Millets for Value Addition of Biscuits. Curr. Res. Nutr. Food Sci. J. 2019, 7, 272–279. [Google Scholar] [CrossRef]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol--sulphuric acid method assisted by multivariate calibration. Chemom. Intell. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Chandra, P.; Singh, I.S.; Singh, S.B. Biochemical changes during flowering of sugarcane. Sugar Tech. 2005, 7, 160–162. [Google Scholar] [CrossRef]
- Giami, S.Y. Comparison of bread making properties of composite flour from kernels of roasted and boiled African bread fruit (Treculia Africana decne) seeds. J. Mat. Res. 2004, 1, 16–25. [Google Scholar]
- Lim, P.Y.; Sim, Y.Y.; Nyam, K.L. Influence of kenaf (Hibiscus cannabinus L.) leaves powder on the physico-chemical, antioxidant and sensorial properties of wheat bread. J. Food Meas. Charact. 2020, 14, 2425–2432. [Google Scholar] [CrossRef]
- Arya, S.S.; Shakya, N.K. High fiber, low glycaemic index (GI) prebiotic multigrain functional beverage from barnyard, foxtail and kodo millet. LWT 2021, 135, 109991. [Google Scholar] [CrossRef]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruits and Vegetables Products; Tata Me Graw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Usman, M.; Ahmed, S.; Mehmood, A.; Bilal, M.; Patil, P.J.; Akram, K.; Farooq, U. Effect of apple pomace on nutrition, rheology of dough and cookies quality. J. Food Sci. Technol. 2020, 57, 3244–3251. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.; Butt, M.S.; Anjum, F.M.; Sharif, K.; Minhas, R. Effect of moisture on the shelf life of wheat flour. Int. J. Agric. Biol 2003, 5, 458–459. [Google Scholar]
- Daubert, C.R.; Foegeding, E.A. Rheological principles for food analysis. In Food Analysis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 541–554. [Google Scholar]
- Wang, Z.; Li, Y.; Yang, Y.; Liu, X.; Qin, H.; Dong, Z.; Zheng, S.; Zhang, K. New insight into the function of wheat glutenin proteins as investigated with two series of genetic mutants. Sci. Rep. 2017, 7, 3428. [Google Scholar] [CrossRef] [PubMed]
- Ubbor, S.C.; Akobundu, E.N.T. Quality characteristics of cookies from composite flours of watermelon seed, cassava and wheat. Pakistan J. Nutr. 2009, 8, 1097–1102. [Google Scholar] [CrossRef]
- Udomkun, P.; Tirawattanawanich, C.; Ilukor, J.; Sridonpai, P.; Njukwe, E.; Nimbona, P.; Vanlauwe, B. Promoting the use of locally produced crops in making cereal-legume-based composite flours: An assessment of nutrient, antinutrient, mineral molar ratios, and aflatoxin content. Food Chem. 2019, 286, 651–658. [Google Scholar] [CrossRef]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Swieca, M.; Skeczyk, L.; Gawlik-Dziki, U.; Dziki, D. Bread enriched with quinoa leaves-the influence of protein-phenolics interactions on the nutritional and antioxidant quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef]
- Kumar, S.; Krishali, V.; Purohit, P.; Saini, I.; Kumar, V.; Singh, S.; Upadhyay, S.; Joshi, H.C.; Wilson, I.; Singh Tomar, M. Physicochemical properties, nutritional and sensory quality of low-fat Ashwagandha and Giloy-fortified sponge cakes during storage. J. Food Process. Preserv. 2022, 46, e16280. [Google Scholar] [CrossRef]



| Flour Mix | Apple Pomace Powder | Indian Gooseberry Pomace Powder | Potato Peel Powder | Bottle Gourd Peel Powder | Whole Wheat Flour |
|---|---|---|---|---|---|
| PPWF1 | 10% | 5% | 5% | 4% | 76% |
| PPWF2 | 15% | 10% | 10% | 8% | 57% |
| PPWF3 | 20% | 15% | 15% | 12% | 38% |
| PPWF4 | 25% | 20% | 20% | 16% | 19% |
| Ingredients | G1 (20–80%) | G2 (30–70%) | Control 100% |
|---|---|---|---|
| Refined wheat flour | 80 g | 70 g | 100 g |
| PPWF3 | 20 g | 30 g | 0 g |
| Lukewarm Water (43 °C) | 60 mL | 60 mL | 60 mL |
| Salt | 2 g | 2 g | 2 g |
| Baker’s yeast | 6 g | 6 g | 6 g |
| Sugar | 4 g | 4 g | 4 g |
| SoybeanCookingOil | 2 mL | 2 mL | 2 mL |
| Attributes | Apple Pomace Powder | Indian Gooseberry Pomace Powder | Potato Peels Powder | Bottle Gourd Peels Powders | Whole Wheat Flour |
|---|---|---|---|---|---|
| Moisture (%) | 5.5 ± 0.28 | 9.31 ± 0.16 | 11.44 ± 0.04 | 9.40 ± 0.04 | 12.3 ± 0.28 |
| Ash (%) | 1.89 ± 0.10 | 0.86 ± 0.09 | 2.92 ± 0.04 | 3.92 ± 0.01 | 1.5 ± 0.07 |
| Fat (%) | 4.15 ± 0.19 | 6.14 ± 0.20 | 2.42 ± 0.03 | 2.43 ± 0.12 | 1.7 ± 0.36 |
| Fiber (%) | 10.15 ± 1.19 | 13.15 ± 0.29 | 8.15 ± 0.22 | 7.24 ± 0.13 | 0.3 ± 0.11 |
| Pectin (%) | 10.2 ± 0.21 | 4.27 ± 0.28 | 0.64 ± 0.42 | 1.17 ± 0.21 | Non Detectable |
| Vitamin C (mg/g) | 10.54 ± 0.17 | 272.71 ± 0.06 | 2.14 ± 0.07 | 13.53 ± 0.05 | 1.48 ± 0.06 |
| Protein (%) | 1.53 ± 0.05 | 1.77 ± 0.11 | 2.17 ± 0.07 | 2.74 ± 0.07 | 11.79 ± 0.10 |
| WAC (%) a | 418.66 ± 3.53 | 831 ± 33.94 | 367.33 ± 8.48 | 316 ± 12.72 | 129 ± 16.26 |
| OAC (%) b | 132 ± 5.65 | 454 ± 5.65 | 168.66 ± 13.43 | 152 ± 10.60 | 169 ± 16.97 |
| TPC (mg/GAE/g) c | 10.447 ± 0.06 | 45.754 ± 0.08 | 2.144 ± 0.03 | 6.467 ± 0.03 | 2.23 ± 0.05 |
| TS (mg/g) d | 121.35 ± 0.12 | 72.54 ± 0.20 | 41.40 ± 0.24 | 95.33 ± 0.18 | 25.14 ± 0.10 |
| RS (mg/g) e | 16.19 ± 0.30 | 34.71 ± 0.32 | 23.69 ± 0.06 | 3.37 ± 0.12 | 11.41 ± 0.13 |
| NRS (mg/g) f | 99.79 ± 0.16 | 35.75 ± 0.05 | 17.08 ± 0.002 | 87.33 ± 0.08 | 13 ± 0.02 |
| Parameters | PPWF1 | PPWF2 | PPWF3 | PPWF4 | Control |
|---|---|---|---|---|---|
| Moisture (%) | 12.246 ± 0.30 | 9.5 ± 0.28 | 9.5 ± 0.07 a | 9.38 ± 0.37 | 12.2 ± 0.28 b |
| Ash (%) | 3.30 ± 0.23 | 3.5 ± 0.28 | 2.58 ± 0.23 a | 4.67 ± 0.14 | 0.50 ± 0.07 a |
| Fat (%) | 1.5 ± 0.28 | 5.17 ± 0.05 | 3.3 ± 0.28 a | 3.15 ± 0.03 | 1.74 ± 0.36 a |
| Fiber (%) | 6.26 ± 0.11 | 7.28 ± 0.01 | 8.16 ± 0.17 a | 8.28 ± 0.01 | 3.16 ± 0.11 b |
| Protein (%) | 2.57 ± 0.14 | 2.82 ± 0.04 | 3.18 ± 0.06 a | 3.16 ± 0.09 | 10.79 ± 0.10 b |
| Vitamin C (mg/100g) | 7.16 ± 0.11 | 8.28 ± 0.01 | 13.64 ± 0.09 a | 4.4 ± 0.49 | 1.48 ± 0.06 b |
| WAC (%) | 424.66 ± 2.12 | 424.4 ± 13.43 | 431.4± 25.45 a | 460.4 ± 8.48 | 129.4 ± 16.26 a |
| OAC (%) | 255.66 ± 8.48 | 231.66 ± 24.78 | 253 ± 2.82 a | 235.66 ± 13.43 | 169 ± 16.97 a |
| TPC (mg/GAE/g) | 13.34 ± 0.06 | 11.89 ± 0.08 | 14.48 ± 0.11 a | 13.27 ± 0.09 | 2.23 ± 0.05 b |
| TS (mg/g) | 93.56 ± 0.31 | 105.67 ± 0.26 | 78.66 ± 0.29 a | 59.31 ± 0.12 | 25.14 ± 0.10 b |
| RS (mg/g) | 47.48 ± 0.03 | 66.54 ± 0.10 | 38.05 ± 3.26 a | 41.55 ± 0.15 | 11.41 ± 0.13 b |
| NRS (mg/g) | 43.77 ± 0.33 | 37.17 ± 0.35 | 41.14 ± 0.55 a | 16.87 ± 0.03 | 13 ± 0.02 b |
| Parameters | 100% | G1 (20–80)% | G2 (30–70)% |
|---|---|---|---|
| Moisture Content (%) | 20.31 ± 0.40 a | 30.5 ± 0.28 b | 27.9 ± 0.09 b |
| Ash Content (%) | 4.7 ± 0.25 a | 4.8 ± 0.11 a | 5.4 ± 0.01 b |
| Fat (%) | 3.44 ± 0.28 a | 3.26 ± 0.14 a | 8.24 ± 0.35 b |
| Protein (%) | 2.3 ± 0.19 a | 2.42 ± 0.05 a | 2.44 ± 0.42 a |
| Fibre (%) | 2.57 ± 0.06 a | 5.1 ± 0.35 b | 4.95 ± 0.14 b |
| Carbohydrates (%) | 65.41 ± 0.26 a | 54.32 ± 0.57 b | 70.36 ± 0.15 ab |
| Energy (Kcal/100 gm) | 303.04 ± 0.04 a | 255.2 ± 0.21 b | 365.46 ± 0.52 ab |
| Vitamin C (mg/100g) | 2.18 ± 0.07 a | 2.11 ± 0.01 a | 2.75 ± 0.008 b |
| TPC (mg/GAE/g) | 7.46 ± 0.08 a | 9.58 ± 0.06 b | 9.26 ± 0.10 b |
| TS (mg/g) | 121 ± 0.14 a | 72 ± 60 b | 48.5 ± 0.05 ab |
| RS (mg/g) | 28.41 ± 0.15 a | 22.35 ± 0.13 b | 34.6 ± 0.32 ab |
| NRS (mg/g) | 88.26 ± 0.28 a | 47.74 ± 0.01 b | 13.20 ± 0.25 ab |
| Attributes | Apple Pomace Powder | Amla Pomace Powder | Potato Peels Powder | Bottle Gourd Peels |
|---|---|---|---|---|
| Swelling capacity(mL) | 17.60 ± 1.85 | 15.40 ± 1.85 | 19.00 ± 0.71 | 17.40 ± 1.85 |
| Emulsion capacity (%) | 36.33 ± 3.05 | 38.4 ± 3.22 | 41.66 ± 3.77 | 43.88 ± 4.12 |
| Foaming capacity (%) | 16.9 ± 4.00 | 17.2 ± 5.3 | 15.22 ± 4.04 | 17.33 ± 3.23 |
| Parameters | PPWF1 | PPWF2 | PPWF3 | PPWF4 | Control |
|---|---|---|---|---|---|
| Swelling capacity(mL) | 17.30 ± 1.85 | 18.20 ± 0.81 | 20.18 ± 0.71 | 19.45 ± 0.56 | 11.77 ± 0.51 |
| Emulsion capacity (%) | 47.88 ± 5.12 | 48.88 ± 4.12 | 41.88 ± 3.52 | 33.88 ± 5.12 | 23.88 ± 4.12 |
| Foaming capacity (%) | 20.72 ± 5.03 | 21.12 ± 4.7 | 24.66 ± 5.5 | 25.36 ± 5.77 | 12.42 ± 5.3 |
| Parameters | 100% | G1 (20–80)% | G2 (30–70)% |
|---|---|---|---|
| Loaf weight (g) | 93.8 ± 1.68 a | 96.2 ± 3.7 a | 96.17 ± 0.88 a |
| Loaf Volume (cm3) | 349 ± 2.6 a | 302 ± 24.1 a | 247 ± 12.4 b |
| Specific Volume (cm3/g) | 3.68 ± 0.07 a | 3.60 ± 0.07 a | 3.96 ± 0.06 b |
| Color L* | 74.9 ± 0.08 a | 55.7 ± 1.6 b | 42.9 ± 1.25 c |
| a* | 1.59 ± 0.17 b | 2.39 ± 0.70 b | 3.84 ± 0.46 a |
| b* | 21.6 ± 0.17 b | 36.0 ± 2.98 a | 37.0 ± 1.97 a |
| Hardness | 317 ± 62.6 c | 421 ± 71.2 a | 464 ± 76.4 b |
| Cohesiveness | 0.89 ± 0.04 a | 0.84 ± 0.02 a | 0.82 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, S.; Tiwari, R.; Kumar, S.; Gupta, S.M.; Kumar, V.; Rautela, I.; Kohli, D.; Rawat, B.S.; Kaushik, R. Utilization of Food Waste for the Development of Composite Bread. Sustainability 2023, 15, 13079. https://doi.org/10.3390/su151713079
Upadhyay S, Tiwari R, Kumar S, Gupta SM, Kumar V, Rautela I, Kohli D, Rawat BS, Kaushik R. Utilization of Food Waste for the Development of Composite Bread. Sustainability. 2023; 15(17):13079. https://doi.org/10.3390/su151713079
Chicago/Turabian StyleUpadhyay, Shuchi, Rajeev Tiwari, Sanjay Kumar, Shradhha Manish Gupta, Vinod Kumar, Indra Rautela, Deepika Kohli, Bhupendra S. Rawat, and Ravinder Kaushik. 2023. "Utilization of Food Waste for the Development of Composite Bread" Sustainability 15, no. 17: 13079. https://doi.org/10.3390/su151713079
APA StyleUpadhyay, S., Tiwari, R., Kumar, S., Gupta, S. M., Kumar, V., Rautela, I., Kohli, D., Rawat, B. S., & Kaushik, R. (2023). Utilization of Food Waste for the Development of Composite Bread. Sustainability, 15(17), 13079. https://doi.org/10.3390/su151713079









