The Role in the Human Diet of Bioaccumulation of Selenium, Copper, Zinc, Manganese and Iron in Edible Mushrooms in Various Habitat Conditions of NW Poland—A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Characteristics of the Soil
2.3. Fungal and Soil Materials
2.4. Analytical Procedures
2.4.1. Soil
2.4.2. Mushrooms
2.5. Statistical Analysis
3. Results and Discussion
3.1. Concentration of Microelements in Soil and Fungi
3.1.1. Selenium
3.1.2. Copper
3.1.3. Zinc
3.1.4. Manganese
3.1.5. Iron
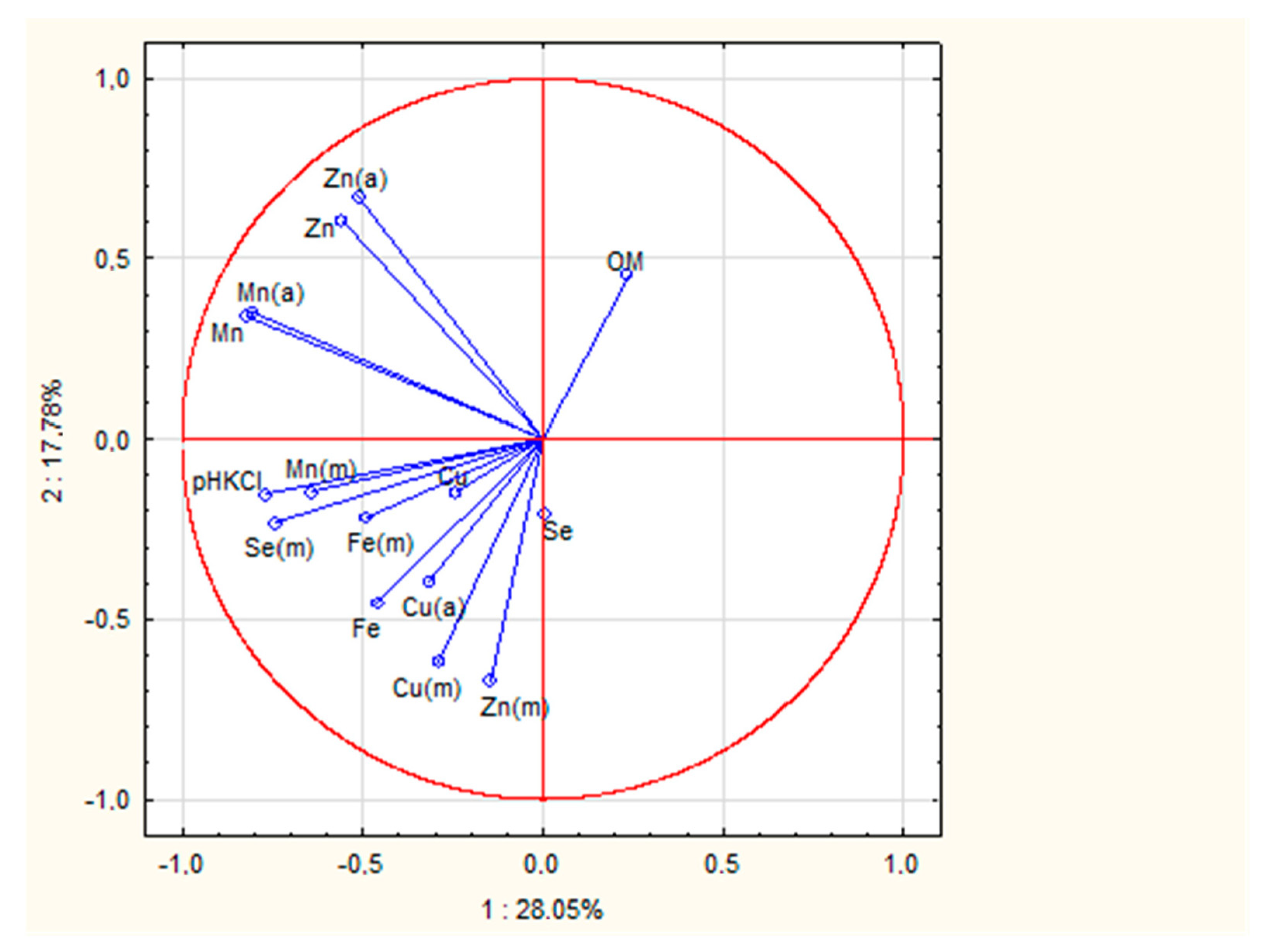
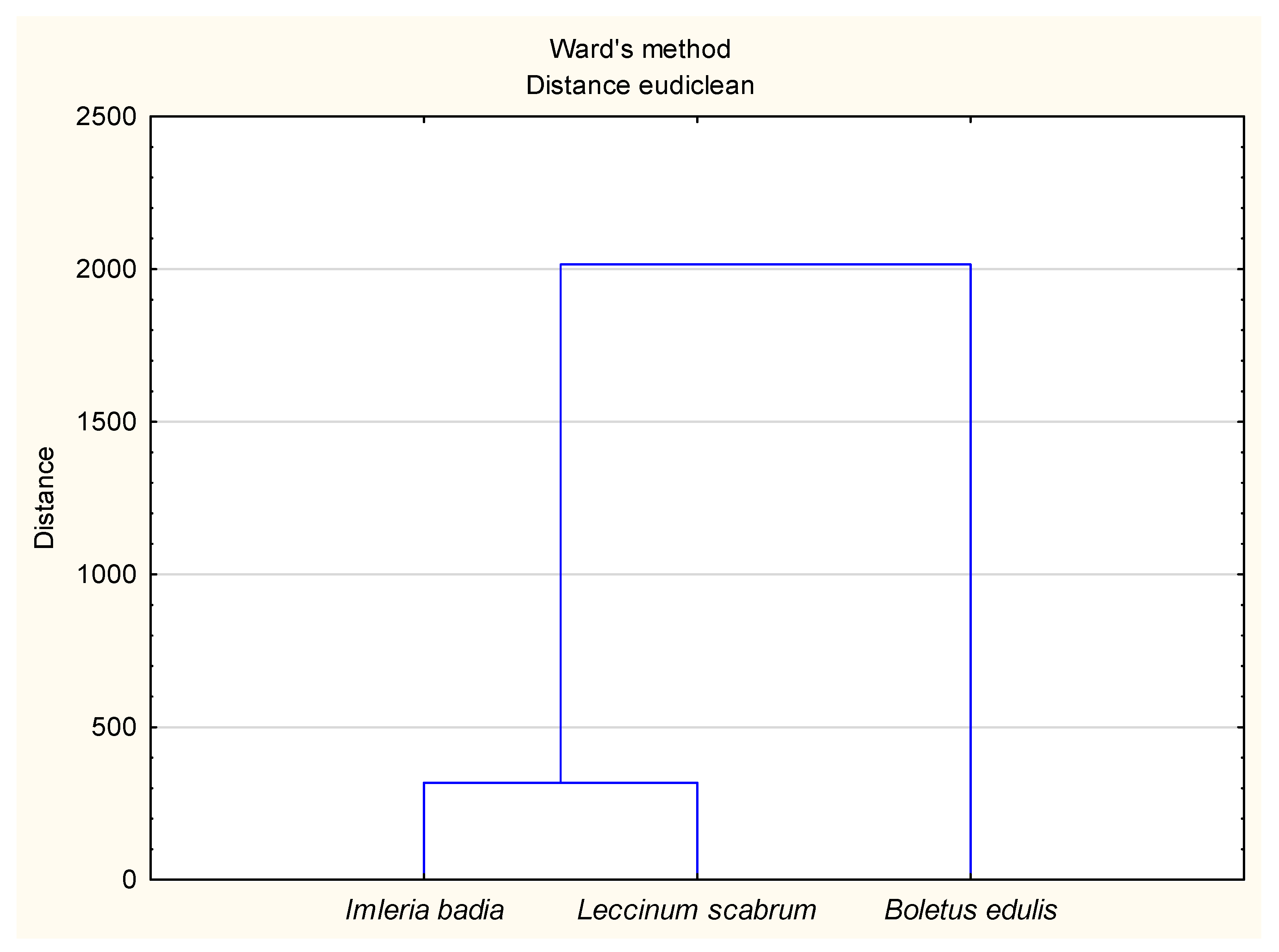
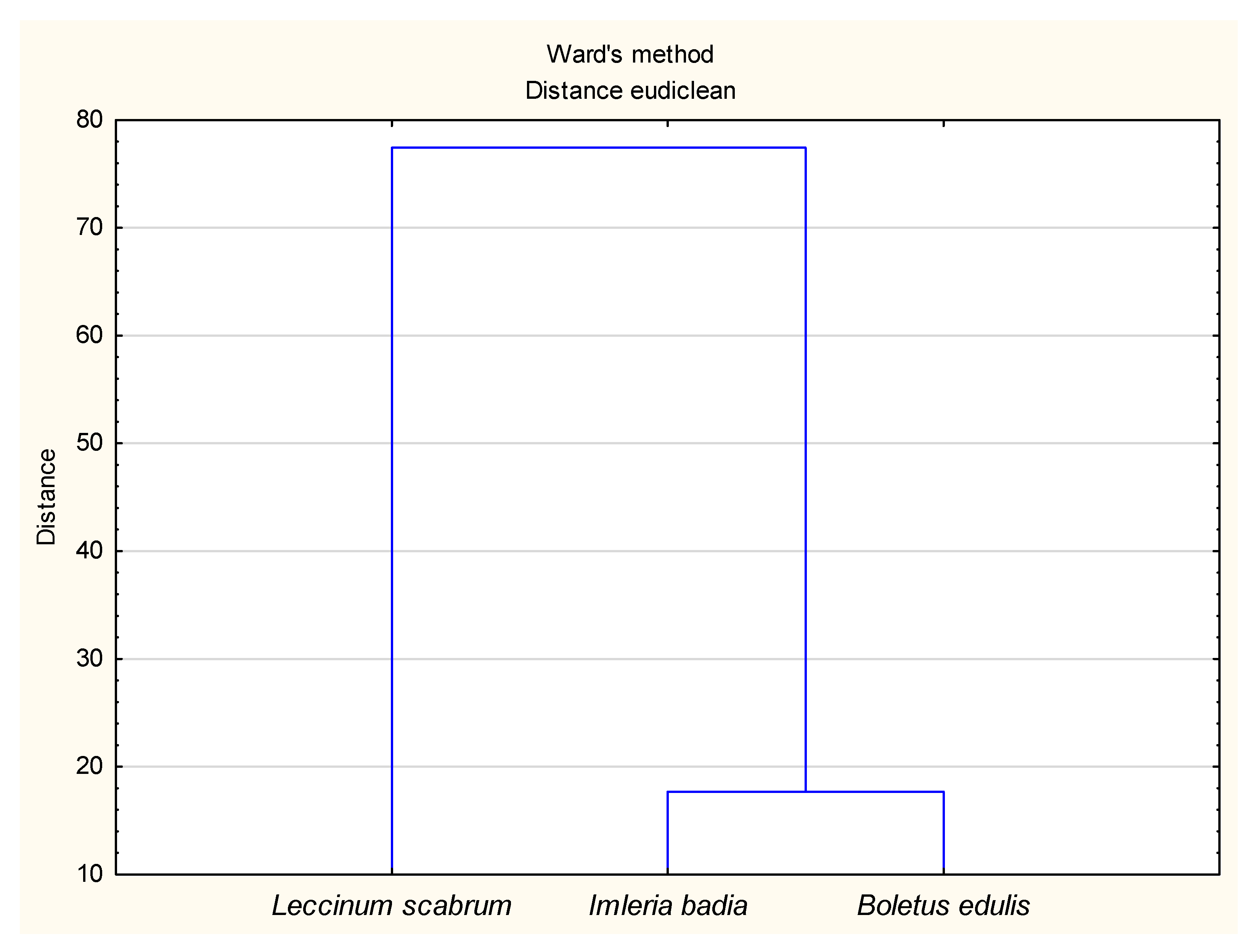
3.2. The Principal Component Analysis (PCA) for Soil and Mushroom Chemical Composition and Ward’s Cluster Analysis for Micronutrient Contents in Soils and Mushrooms
3.3. Accumulation of the Micronutrients in the Studied Fungi
3.4. Potential Impact of Mushroom Consumption on Humans
3.4.1. Selenium
3.4.2. Copper
3.4.3. Zinc
3.4.4. Manganese
3.4.5. Iron
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.G.; Lian, M.X.; Han, Y.; Lv, S.M. Antitumor and immunomodulatory activity of a polysaccharide from fungus Coprinus comatus (Mull.: Fr.) Gray. Int. J. Biol. Macromol. 2013, 58, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; van Griensven, L.J.I.D.; Soković, A.; Ferreira, I.C.F.R. Nutrients and non-nutrients composition and bioactivity of wild and cultivated Coprinus comatus (O.F.Müll.) Pers. Food Chem. Toxicol. 2013, 59, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Falandysz, J. Selenium in Edible Mushrooms. J. Environ. Sci. Health Part C 2008, 26, 256–299. [Google Scholar] [CrossRef]
- Falandysz, J.; Treu, R.; Meloni, D. Distribution and bioconcentration of some elements in the edible mushroom Leccinum scabrum from locations in Poland. J. Environ. Sci. Health Part B 2021, 56, 396–414. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Socha, K.; Zujko, M.E.; Terlikowska, K.M.; Borawska, M.H.; Witkowska, A.M. Copper, manganese, selenium and zinc in wild-growing edible mushrooms from the eastern territory of “Green Lungs of Poland”: Nutritional and toxicological implications. Int. J. Environ. Res. Public Health 2019, 16, 3614. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R. Mineral composition of three popular wild mushrooms from Poland. Molecules 2020, 25, 3588. [Google Scholar] [CrossRef]
- Malinowski, R.; Sotek, Z.; Stasińska, M.; Malinowska, K.; Radke, P.; Malinowska, A. Bioaccumulation of macronutrients in edible mushrooms in various habitat conditions of NW Poland—Role in the human diet. Int. J. Environ. Res. Public Health 2021, 18, 8881. [Google Scholar] [CrossRef]
- Pelkonen, R.; Alfthan, G.; Järvinen, O. Cadmium, Lead, Arsenic and Nickel in Wild Edible Mushrooms; Finnish Environment Institute: Helsinki, Finland, 2006. [Google Scholar]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef]
- García, M.Á.; Alonso, J.; Melgar, M.J. Lead in edible mushrooms: Levels and bioaccumulation factors. J. Hazard. Mater. 2009, 167, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.V.; Vanderlelie, J.J. Multiple micronutrient supplementation and birth outcomes: The potential importance of selenium. Placenta 2016, 48, 61–65. [Google Scholar] [CrossRef]
- Hogan, C.; Perkins, A.V. Selenoproteins in the human placenta: How essential is selenium to a healthy start to life? Nutrients 2022, 14, 628. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium biofortification: Roles, mechanisms, responses and prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef] [PubMed]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. The role of vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Wojtasik, A.; Woźniak, A.; Stoś, K.; Jarosz, M. Składniki mineralne. In Normy Żywienia dla Populacji Polski i Ich Zastosowanie; Jarosz, M., Rychlik, E., Stoś, K., Charzewska, J., Eds.; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warsaw, Poland, 2020; pp. 273–315. (In Polish) [Google Scholar]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. Review: The role of zinc in the endocrine system. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar]
- Vickram, S.; Rohini, K.; Srinivasan, S.; Veenakumari, D.N.; Archana, K.; Anbarasu, K.; Jeyanthi, P.; Thanigaivel, S.; Gulothungan, G.; Rajendiran, N.; et al. Role of zinc (Zn) in human reproduction: A journey from initial spermatogenesis to childbirth. Int. J. Mol. Sci. 2021, 22, 2188. [Google Scholar] [CrossRef]
- Johnson, C.C.; Fordyce, F.M.; Rayman, M.P. Symposium on ‘geographical and geological influences on nutrition’: Factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc. Nutr. Soc. 2010, 69, 119–132. [Google Scholar] [CrossRef]
- Xiong, Y.M.; Mo, X.Y.; Zou, X.Z.; Song, R.X.; Sun, W.Y.; Lu, W.; Chen, Q.; Yu, Y.X.; Zang, W.J. Association study between polymorphisms in selenoprotein genes and susceptibility to Kashin-Beck disease. Osteoarthr. Cartil. 2010, 18, 817–824. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Acute human toxicity and mortality after selenium ingestion: A review. J. Trace Elem. Med. Biol. 2019, 58, 126435. [Google Scholar] [CrossRef]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef]
- Rocourt, C.R.B.; Cheng, W.-H. Selenium supranutrition: Are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance? Nutrients 2013, 5, 1349–1365. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The essential element manganese, oxidative stress, and metabolic diseases: Links and interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Borowska, K.; Koper, J.; Tykwińska, T. Zawartość selenu w wybranych typach gleb mineralnych regionu Kujaw i Pomorza na tle aktywności oksydoreduktaz. Ochr. Sr. Zasobów Nat. 2007, 31, 18–23. [Google Scholar]
- Reimann, C.; Birke, M.; Demetriades, A.; Filzmoser, P.; O’Connor, P. Chemistry of Europe’s Agricultural Soils. In Geologisches Jahrbuch; Reihe, B., Band, B., Eds.; Schweizerbart Science Publishers: Stuttgart, Germany, 2014; Volume 103, p. 352. [Google Scholar]
- Kondracki, J. Geografia Regionalna Polski; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2002. [Google Scholar]
- Borówka, R.K. Geographic environment. In The Natural World of Western Pomerania; Borówka, R., Friedrich, S., Heese, T., Jasnowska, J., Kochanowska, R., Opęchowski, M., Stamecka, E., Zyska, W., et al., Eds.; Oficyna in Plus: Szczecin, Poland, 2007. [Google Scholar]
- Koźmiński, C.; Michalska, B.; Czarnecka, M. Climate of Western Pomeranian Province; Akademia Rolnicza w Szczecinie, Uniwersytet Szczeciński: Szczecin, Poland, 2007. [Google Scholar]
- Knudsen, H.; Vesterholt, J. Funga Nordica: Agaricoid, Boletoid, Cyphelloid and Gastroid Genera; Nordsvamp: Copenhagen, Denmark, 2012. [Google Scholar]
- Grzebuła, S.; Witkowski, P. Oznaczenie śladowych ilości selenu w materiale biologicznym metodą fluorymetryczną. Cz. I. Oznaczenie selenu w tkankach i płynach ustrojowych. Pol. Arch. Weter. 1977, 20, 125–138. [Google Scholar]
- Levesque, M.; Vendette, E. Selenium determination in soil and plant materials. Can. J. Soil Sci. 1971, 51, 85–93. [Google Scholar] [CrossRef]
- Watkinson, J.H. Fluorometric determination of selenium in biological material with 2,3-Diaminonaphthalene. Anal. Chem. 1966, 38, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; PWN: Warsaw, Poland, 1999. (In Polish) [Google Scholar]
- Nikkarinen, M.; Mertanen, E. Impact of geological origin on trace element composition of edible mushrooms. J. Food Compos. Anal. 2004, 17, 301–310. [Google Scholar] [CrossRef]
- Řanda, Z.; Kučera, J. Trace elements in higher fungi (mushrooms) determined by activation analysis. J. Radioanal. Nucl. Chem. 2004, 259, 99–107. [Google Scholar] [CrossRef]
- Domańska, J.; Filipek, T. Kształtowanie się zawartości Cu związanej z frakcjami gleby w zależności od pH i zawartości materii organicznej. Ochr. Sr. Zasobów Nat. 2011, 48, 74–79. [Google Scholar]
- Arias, M.; Pérez-Novo, C.; Osorio, F.; López, E.; Soto, B. Adsorption and desorption of copper and zinc in the surface layer of acid soils. J. Colloid Interface Sci. 2005, 288, 21–29. [Google Scholar] [CrossRef]
- IUNG (Institute of Soil Science and Plant Cultivation). Fertiliser Recommendations Part I. Limits for Estimating Soil Macro- and Microelement Content; Series P. (44); Institute of Soil Science and Plant Cultivation: Puławy, Poland, 1990; pp. 1–26. [Google Scholar]
- IUNG (Institute of Soil Science and Plant Cultivation). Ocena Stopnia Zanieczyszczenia Gleb i Roślin Metalami Ciężkimi i Siarką. Ramowe Wytyczne dla Rolnictwa; Seria P (53); Państwowy Instytut Badawczy w Puławach: Puławy, Poland, 1993; pp. 1–22. [Google Scholar]
- Dz.U. 2016 poz. 1395 (Rozporządzenie Ministra Środowiska z dnia 1 Września 2016 r. w Sprawie Sposobu Prowadzenia Oceny Zanieczyszczenia Powierzchni Ziemi); Min. Środowiska: Warsaw, Poland, 2016.
- Kalač, P.; Svoboda, L. A review of trace element concentrations in edible mushrooms. Food Chem. 2000, 69, 273–281. [Google Scholar] [CrossRef]
- Alonso, J.; García, M.A.; Pérez-López, M.; Melgar, M.J. The concentrations and bioconcentration factors of copper and zinc in edible mushrooms. Arch. Environ. Contam. Toxicol. 2003, 44, 180–188. [Google Scholar] [CrossRef]
- Falandysz, J.; Frankowska, A. Niektóre pierwiastki metaliczne i ich współczynniki biokoncentracji w borowiku szlachetnym (Boletus edulis) z Puszczy Świętokrzyskiej. Bromat. Chem. Toksykol. 2007, 3, 257–260. [Google Scholar]
- Mędyk, M.; Treu, R.; Falandysz, J. Accumulation of minerals by Leccinum scabrum from two large forested areas in Central Europe: Notecka Wilderness and Tuchola Forest (Pinewoods). Chem. Biodivers. 2020, 17, e2000264. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, E.; Szefer, P.; Falandysz, J. Metals bioaccumulation by bay bolete, Xerocomus badius, from selected sites in Poland. Food Chem. 2004, 84, 405–416. [Google Scholar] [CrossRef]
- Kojta, A.K.; Jarzyńska, G.; Falandysz, J. Mineral composition and heavy metal accumulation capacity of Bay Bolete (Xerocomus badius) fruiting bodies collected near a former gold and copper mining area. J. Geochem. Explor. 2012, 121, 76–82. [Google Scholar] [CrossRef]
- Frankowska, A.; Ziółkowska, J.; Bielawski, L.; Falandysz, J. Profile and bioconcentration of minerals by King Bolete (Boletus edulis) from the Płocka Dale in Poland. Food Addit. Contam. Part B 2010, 3, 1–6. [Google Scholar] [CrossRef]
- Gençcelep, H.; Uzun, Y.; Tunçtürk, Y.; Demirel, K. Determination of mineral contents of wild-grown edible mushrooms. Food Chem. 2009, 113, 1033–1036. [Google Scholar] [CrossRef]
- Isildak, Ö.; Turkekul, I.; Elmastas, M.; Tuzen, M. Analysis of heavy metals in some wild-grown edible mushrooms from the middle black sea region, Turkey. Food Chem. 2004, 86, 547–552. [Google Scholar] [CrossRef]
- Vetter, J. Mineral elements in the important cultivated mushrooms Agaricus bisporus and Pleurotus ostreatus. Food Chem. 1994, 50, 277–279. [Google Scholar] [CrossRef]
- Michelot, D.; Siobud, E.; Doré, J.C.; Viel, C.; Poirier, F. Update on metal content profiles in mushrooms—Toxicological implications and tentative approach to the mechanisms of bioaccumulation. Toxicon 1998, 36, 1997–2012. [Google Scholar] [CrossRef] [PubMed]
- Jorhem, L.; Sundström, B. Levels of some trace elements in edible fungi. Z. Lebensm.-Unters. Forsch. 1995, 201, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Muramatsu, Y. Determination of major and trace elements in mushroom, plant and soil samples collected from Japanese forests. Int. J. Environ. Anal. Chem. 1997, 67, 49–58. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhou, D.; Cang, L.; Zhang, H.; Fan, X.H.; Qin, S.W. Soil micronutrient availability to crops as affected by long-term inorganic and organic fertilizer applications. Soil Tillage Res. 2007, 96, 166–173. [Google Scholar] [CrossRef]
- Fan, J.; Ding, W.; Chen, Z.; Ziadi, N. Thirty-year amendment of horse manure and chemical fertilizer on the availability of micronutrients at the aggregate scale in black soil. Environ. Sci. Pollut. Res. 2012, 19, 2745–2754. [Google Scholar] [CrossRef]
- Doĝan, H.H.; Şanda, M.A.; Uyanöz, R.; Öztürk, C.; Çetin, Ü. Contents of metals in some wild mushrooms: Its impact in human health. Biol. Trace Elem. Res. 2006, 110, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Rudawska, M.; Leski, T. Macro-and microelement contents in fruiting bodies of wild mushrooms from the Notecka forest in west-central Poland. Food Chem. 2005, 92, 499–506. [Google Scholar] [CrossRef]
- Kojta, A.K.; Falandysz, J. Metallic elements (Ca, Hg, Fe, K, Mg, Mn, Na, Zn) in the fruiting bodies of Boletus badius. Food Chem. 2016, 200, 206–214. [Google Scholar] [CrossRef]
- Kulczycki, G. Effect of elementary sulphur fertilization on the content of micronutrients in plants and soils. Part II. Manganese and iron. Zesz. Probl. Postep. Nauk. Rol. 2004, 502, 207–213. [Google Scholar]
- Podleśna, A. Uptake of manganese by potato and pea plants in conditions of integrated cultivation with regard to sulfur and manure fertilization. Zesz. Nauk. Akad. Rol. Wrocławiu Rol. 2013, 595, 87–92. [Google Scholar]
- Barcan, V.S.; Kovnatsky, E.F.; Smetannikova, M.S. Absorption of heavy metals in wild berries and edible mushrooms in an area affected by smelter emissions. Water Air Soil Pollut. 1998, 103, 173–195. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Rengel, Z. Aluminum, manganese, and iron tolerance improves performance of wheat genotypes in waterlogged acidic soils. J. Plant Nutr. Soil Sci. 2010, 173, 461–468. [Google Scholar] [CrossRef]
- Tyler, G. Accumulation and exclusion of metals in Collybia peronata and Amanita rubescens. Trans. Br. Mycol. Soc. 1982, 79, 239–245. [Google Scholar] [CrossRef]
- Gast, C.H.; Jansen, E.; Bierling, J.; Haanstra, L. Heavy metals in mushrooms and their relationship with soil characteristics. Chemosphere 1988, 17, 789–799. [Google Scholar] [CrossRef]
- Giannaccini, G.; Betti, L.; Palego, L.; Mascia, G.; Schmid, L.; Lanza, M.; Mela, A.; Fabbrini, L.; Biondi, L.; Lucacchini, A. The trace element content of top-soil and wild edible mushroom samples collected in Tuscany, Italy. Environ. Monit. Assess. 2012, 184, 7579–7595. [Google Scholar] [CrossRef]
- Demirbaş, A. Concentrations of 21 metals in 18 species of mushrooms growing in the East Black Sea region. Food Chem. 2001, 75, 453–457. [Google Scholar] [CrossRef]
- Borovička, J.; Konvalinkova, T.; Žigová, A.; Ďurišová, J.; Gryndler, M.; Hršelová, H.; Kamenik, J.; Leonhardt, T.; Sácký, J. Disentangling the factors of contrasting silver and copper accumulation in sporocarps of the ectomycorrhizal fungus Amanita strobiliformis from two sites. Sci. Total Environ. 2019, 694, 133679. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P.; Stašková, I. Concentrations of lead, cadmium, mercury and copper in mushrooms in the vicinity of a lead smelter. Sci. Total Environ. 1991, 105, 109–119. [Google Scholar] [CrossRef]
- Šíma, J.; Vondruška, J.; Svoboda, L.; Šeda, M.; Rokos, L. The accumulation of risk and essential elements in edible mushrooms Chlorophyllum rhacodes, Suillus grevillei, Imleria badia, and Xerocomellus chrysenteron growing in the Czech Republic. Chem. Biodivers. 2019, 16, e1800478. [Google Scholar] [CrossRef]
- Karmańska, A.; Wędzisz, A. Zawartość wybranych makro i mikroelementów w różnych gatunkach grzybów wielkoowocnikowych z okolic województwa łódzkiego. Bromat. Chem. Toksykol. 2010, 43, 124–129. [Google Scholar]
- Bhatia, P.; Aureli, F.; D’Amato, M.; Prakash, R.; Cameotra, S.S.; Nagaraja, T.P.; Cubadda, F. Selenium bioaccessibility and speciation in biofortified Pleurotus mushrooms grown on selenium-rich agricultural residues. Food Chem. 2013, 140, 225–230. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Stuper-Szablewska, K.; Rissmann, I.; Sobieralski, K.; Goliński, P. Accumulation of elements by edible mushroom species: Part I. Problem of trace element toxicity in mushrooms. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2013, 48, 69–81. [Google Scholar] [CrossRef]
- Romanjek Fajdetić, N.; Popović, B.; Parađiković, N.; Lončarić, Z.; Japundžić Palenkić, B. The influence of substrates having various origins on a nutritive value of champignon mushrooms (Agaricus bisporus Imbach). Poljoprivreda 2019, 25, 12–18. [Google Scholar] [CrossRef]
- Hultberg, M.; Asp, H.; Bergstrand, K.-J.; Golovko, O. Production of oyster mushroom (Pleurotus ostreatus) on sawdust supplemented with anaerobic digestate. Waste Manag. 2023, 155, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tsivileva, O.; Perfileva, A. Selenium compounds biotransformed by mushrooms: Not only dietary sources, but also toxicity mediators. Curr. Nutr. Food Sci. 2017, 13, 82–96. [Google Scholar] [CrossRef]
- IPCS (International Programme on Chemical Safety). Copper—Environmental Health Criteria 200; World Health Organization (WHO): Geneva, Switzerland, 1998. [Google Scholar]
- Commission of the European Communities. Scientific Committee for Food. Reports of the Scientific Committee for Food (Thirty-First Series). Nutrient and Energy Intakes for the European Community; Commission of the European Communities: Luxembourg, 1993. [Google Scholar]
- JECFA. Evaluation of Certain Food Additives and Contaminants; Twenty-sixth Report of the Joint FAO/WHO Expert Committee on Food Additives; Technical Report Series No. 683; World Health Organization (WHO): Geneva, Switzerland, 1982. [Google Scholar]
- WHO; Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Food Additives and Contaminants; Forty-First Report of the WHO Technical Report Series No. 837; World Health Organization (WHO): Geneva, Switzerland, 1993. [Google Scholar]
- Raman, J.; Jang, K.-Y.; Oh, Y.-L.; Oh, M.; Im, J.-H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and nutritional value of prominent Pleurotus spp.: An overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
| Localisation | Available | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Mn | Cu | Zn | Mn | Fe | Se | ||||||||
| mg/kg | |||||||||||||||
| Boletus edulis Soil layer (cm) | |||||||||||||||
| 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–10 | |
| UW | 7.95 a | 2.40 a | 42.73 a | 19.31 a | 76.37 a | 11.66 a | 11.50 a | 8.24 a | 46.22 a | 59.90 a | 86.14 a | 32.44 a | 1858.8 a | 1806.5 a | 0.089 a |
| ±0.76 | ±1.58 | ±18.96 | ±25.69 | ±31.11 | ±5.56 | ±1.00 | ±3.96 | ±17.99 | ±95.82 | ±32.05 | ±9.14 | ±191.6 | ±337.6 | ±0.040 | |
| DP | 7.21 a | 2.90 a | 38.57 a | 4.18 a | 672.37 b | 121.62 b | 40.74 a | 6.67 a | 46.70 a | 14.01 a | 782.10 b | 160.90 b | 3440.8 a | 3533.4 a | 0.164 ab |
| ±3.14 | ±1.74 | ±22.03 | ±0.87 | ±449.9 | ±61.0 | ±61.97 | ±1.03 | ±23.69 | ±1.08 | ±525.0 | ±64.6 | ±1270 | ±1271.4 | ±0.031 | |
| IL | 8.31 a | 2.27 a | 31.32 a | 5.79 a | 109.57 a | 90.16 ab | 11.02 a | 10.02 a | 46.88 a | 20.69 a | 142.16 a | 144.04 ab | 3354.0 a | 7494.1 b | 0.097 a |
| ±1.13 | ±0.54 | ±13.56 | ±4.44 | ±35.93 | ±64.29 | ±1.02 | ±1.44 | ±14.91 | ±6.41 | ±46.24 | ±98.51 | ±1468 | ±4328.4 | ±0.029 | |
| x | 7.76 | 2.52 | 36.88 | 9.76 | 343.65 | 74.48 | 23.86 | 8.31 | 46.66 | 31.53 | 404.41 | 112.46 | 3070.8 | 4278.0 | 0.117 |
| B | A | A | A | A | A | A | A | A | A | A | A | B | A | A | |
| Imleria badia Soil layer (cm) | |||||||||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | |
| UW | 5.33 a | 1.89 a | 31.45 a | 10.28 a | 185.55 a | 45.02 ab | 9.10 a | 6.89 a | 36.89 a | 16.24 a | 204.49 a | 66.18 ab | 1804.7 a | 2423.0 a | 0.096 a |
| ±2.62 | ±0.60 | ±6.92 | ±6.63 | ±65.73 | ±26.03 | ±1.34 | ±1.22 | ±7.42 | ±5.01 | ±62.47 | ±21.34 | ±638.3 | ±385.5 | ±0.048 | |
| DP | 6.10 a | 2.82 a | 25.89 a | 13.48 a | 136.31 ab | 59.55 ab | 11.34 a | 10.36 a | 33.34 a | 19.99 a | 144.71 ab | 71.91 ab | 2005.8 a | 2226.5 a | 0.186 ab |
| ±0.29 | ±0.29 | ±5.92 | ±5.59 | ±56.67 | ±35.98 | ±2.07 | ±0.34 | ±3.18 | ±2.79 | ±57.51 | ±33.04 | ±667.1 | ±264.4 | ±0.014 | |
| IL | 3.71 a | 1.86 a | 24.29 a | 4.59 a | 191.65 a | 44.54 ab | 14.78 a | 41.51 a | 30.13 a | 23.52 a | 206.36 a | 69.79 ab | 1917.8 a | 3890.6 ab | 0.102 a |
| ±2.37 | ±0.046 | ±5.09 | ±2.47 | ±90.85 | ±31.09 | ±10.30 | ±70.49 | ±4.40 | ±18.34 | ±92.16 | ±40.18 | ±936.5 | ±1016.6 | ±0.023 | |
| x | 4.83 | 2.06 | 27.47 | 8.64 | 178.14 | 47.73 | 11.82 | 21.43 | 33.48 | 19.90 | 193.23 | 68.77 | 1890.2 | 2970.7 | 0.116 |
| A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | |
| Leccinum scabrum Soil layer (cm) | |||||||||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | |
| UW | 7.40 a | 3.19 a | 32.53 a | 14.25 a | 111.27 a | 44.77 ab | 12.51 a | 13.04 a | 39.83 a | 19.47 a | 125.44 a | 63.51 ab | 1825.2 a | 1874.0 a | 0.121 a |
| ±4.28 | ±3.57 | ±16.09 | ±11.99 | ±67.01 | ±46.14 | ±8.43 | ±15.04 | ±19.98 | ±13.48 | ±80.29 | ±44.91 | 551.3 | ±205.2 | ±0.084 | |
| DP | 5.18 a | 3.13 a | 39.50 a | 27.41 a | 70.50 a | 50.01 ab | 8.73 a | 11.39 a | 45.42 a | 36.53 a | 74.67 a | 65.87 ab | 1008.8 a | 1385.0 a | 0.280 b |
| ±0.14 | ±0.53 | ±16.17 | ±8.81 | ±15.13 | ±7.86 | ±1.18 | ±0.67 | ±18.12 | ±18.22 | ±17.46 | ±22.42 | ±329.7 | ±169.9 | ±0.079 | |
| IL | 2.36 a | 1.51 a | 39.59 a | 6.72 a | 262.30 ab | 37.82 ab | 9.29 a | 7.98 a | 44.01 a | 17.30 a | 284.31 ab | 62.10 ab | 1600.2 a | 4327.9 ab | 0.118 a |
| ±1.20 | ±0.089 | ±9.22 | ±2.01 | ±156.72 | ±18.49 | ±0.55 | ±0.86 | ±12.57 | ±1.64 | ±173.80 | ±16.83 | ±910.9 | ±100.3 | ±0.013 | |
| x | 5.42 | 2.72 | 36.35 | 15.78 | 141.34 | 44.30 | 10.60 | 11.21 | 42.50 | 23.53 | 154.92 | 63.77 | 1541.2 | 2409.9 | 0.164 |
| AB | A | A | A | A | A | A | A | A | A | A | A | A | A | A | |
| Localisation | Se | Cu | Zn | Mn | Fe |
|---|---|---|---|---|---|
| mg/kg | |||||
| Boletus edulis | |||||
| UW | 12.41 b | 20.36 a | 126.51 a | 7.30 a | 104.27 abc |
| ±2.3 | ±11.3 | ±22.8 | ±3.0 | ±35.5 | |
| DP | 18.34 c | 22.66 a | 103.48 a | 33.62 b | 237.35 bc |
| ±0.6 | ±6.42 | ±11.9 | ±1.4 | ±139.4 | |
| IL | 11.09 b | 24.47 a | 120.17 a | 12.49 ab | 48.20 ab |
| ±2.5 | ±7.9 | ±27.15 | ±9.29 | ±11.46 | |
| x | 14.11 B | 22.38 B | 116.52 A | 18.12 A | 134.75 A |
| Imleria badia | |||||
| UW | 0.24 a | 22.20 a | 115.31 a | 12.70 a | 287.83 c |
| ±0.11 | ±6.10 | ±24.10 | ±2.77 | ±109.24 | |
| DP | 0.09 a | 22.06 a | 128.17 a | 13.05 ab | 32.67 abc |
| ±0.025 | ±4.14 | ±17.77 | ±6.96 | ±18.49 | |
| IL | 0.07 a | 14.86 a | 107.05 a | 8.12 a | 47.23 a |
| ±0.023 | ±14.86 | ±107.05 | ±8.12 | ±47.23 | |
| x | 0.14 A | 19.24 AB | 114.58 A | 10.94 A | 140.56 A |
| Leccinum scabrum | |||||
| UW | 0.79 a | 17.76 a | 114.29 a | 13.43 ab | 175.07 abc |
| ±0.33 | ±20.56 | ±25.54 | ±10.06 | ±147.16 | |
| DP | 0.33 a | 4.90 a | 82.12 ab | 6.74 ab | 32.54 abc |
| ±0.015 | ±1.29 | ±14.72 | ±1.11 | ±7.01 | |
| IL | 0.67 a | 1.42 a | 36.45 b | 2.36 a | 19.50 ab |
| ±0.016 | ±0.01 | ±0.04 | ±0.010 | ±0.010 | |
| x | 0.63 A | 9.80 A | 84.29 B | 8.59 A | 93.77 A |
| Variable | Variable | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Se | Cu | Zn | Mn | Fe | Zn (a) | Cu (a) | Mn (a) | pHKCl | OM | Se (m) | Cu (m) | Zn (m) | Mn (m) | Fe (m) | |
| Se | 1.000000 | 0.082182 | −0.069493 | −0.046534 | 0.271980 | −0.184278 | −0.027666 | −0.057618 | −0.032520 | −0.005728 | 0.138979 | −0.044314 | 0.043395 | 0.108352 | −0.144287 |
| Cu | 0.082182 | 1.000000 | 0.040028 | 0.113133 | 0.299438 | 0.028682 | 0.063737 | 0.096690 | 0.193418 | 0.075501 | 0.298546 | 0.114400 | 0.064753 | 0.028619 | −0.013194 |
| Zn | −0.069493 | 0.040028 | 1.000000 | 0.505989 | −0.037556 | 0.920096 | 0.125103 | 0.488741 | 0.290700 | 0.117241 | 0.259200 | −0.105949 | −0.142405 | 0.186128 | 0.049862 |
| Mn | −0.046534 | 0.113133 | 0.505989 | 1.000000 | 0.264900 | 0.499564 | 0.045153 | 0.996619 | 0.499501 | −0.018532 | 0.494885 | 0.068756 | −0.097055 | 0.412441 | 0.205725 |
| Fe | 0.271980 | 0.299438 | −0.037556 | 0.264900 | 1.000000 | −0.201561 | 0.102066 | 0.252325 | 0.290073 | −0.330230 | 0.555407 | 0.188215 | 0.160825 | 0.271893 | 0.238873 |
| Zn (a) | −0.184278 | 0.028682 | 0.920096 | 0.499564 | −0.201561 | 1.000000 | 0.113419 | 0.500324 | 0.276009 | 0.201810 | 0.179189 | −0.078998 | −0.167734 | 0.148859 | 0.047713 |
| Cu (a) | −0.027666 | 0.063737 | 0.125103 | 0.045153 | 0.102066 | 0.113419 | 1.000000 | 0.040313 | 0.178141 | −0.066058 | 0.361624 | 0.570826 | 0.399150 | 0.112805 | 0.066622 |
| Mn (a) | −0.057618 | 0.096690 | 0.488741 | 0.996619 | 0.252325 | 0.500324 | 0.040313 | 1.000000 | 0.492279 | 0.001773 | 0.468649 | 0.063954 | −0.110045 | 0.388348 | 0.206862 |
| pHKCl | −0.032520 | 0.193418 | 0.290700 | 0.499501 | 0.290073 | 0.276009 | 0.178141 | 0.492279 | 1.000000 | −0.521747 | 0.496290 | 0.088086 | 0.205917 | 0.434563 | 0.595848 |
| OM | −0.005728 | 0.075501 | 0.117241 | −0.018532 | −0.330230 | 0.201810 | −0.066058 | 0.001773 | −0.521747 | 1.000000 | −0.174564 | −0.092481 | −0.210024 | −0.041891 | −0.285780 |
| Se (m) | 0.138979 | 0.298546 | 0.259200 | 0.494885 | 0.555407 | 0.179189 | 0.361624 | 0.468649 | 0.496290 | −0.174564 | 1.000000 | 0.305398 | 0.118524 | 0.485819 | 0.148545 |
| Cu (m) | −0.044314 | 0.114400 | −0.105949 | 0.068756 | 0.188215 | −0.078998 | 0.570826 | 0.063954 | 0.088086 | −0.092481 | 0.305398 | 1.000000 | 0.670788 | 0.220563 | 0.156730 |
| Zn (m) | 0.043395 | 0.064753 | −0.142405 | −0.097055 | 0.160825 | −0.167734 | 0.399150 | −0.110045 | 0.205917 | −0.210024 | 0.118524 | 0.670788 | 1.000000 | 0.112606 | 0.044379 |
| Mn (m) | 0.108352 | 0.028619 | 0.186128 | 0.412441 | 0.271893 | 0.148859 | 0.112805 | 0.388348 | 0.434563 | −0.041891 | 0.485819 | 0.220563 | 0.112606 | 1.000000 | 0.577788 |
| Fe (m) | −0.144287 | −0.013194 | 0.049862 | 0.205725 | 0.238873 | 0.047713 | 0.066622 | 0.206862 | 0.595848 | −0.285780 | 0.148545 | 0.156730 | 0.044379 | 0.577788 | 1.000000 |
| Variable | Factor | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Se | 0.002951 | −0.205177 | 0.235761 | −0.586817 | −0.204097 | −0.564724 | 0.290086 |
| Cu | −0.240400 | −0.145403 | −0.009029 | −0.527631 | 0.286370 | 0.627371 | 0.322553 |
| Zn | −0.557810 | 0.605460 | −0.293769 | 0.004491 | 0.160971 | −0.205486 | 0.288892 |
| Mn | −0.818969 | 0.339229 | 0.050707 | −0.112884 | −0.048946 | 0.008960 | −0.396418 |
| Fe | −0.461197 | −0.454585 | 0.354049 | −0.403455 | 0.091123 | −0.003288 | −0.089340 |
| Zn (a) | −0.512067 | 0.671738 | −0.370259 | 0.094378 | 0.128312 | −0.099349 | 0.246350 |
| Cu (a) | −0.314388 | −0.396654 | −0.637538 | 0.027195 | 0.055612 | −0.120550 | 0.104320 |
| Mn (a) | −0.802699 | 0.351863 | 0.048681 | −0.101932 | −0.054879 | 0.016809 | −0.414412 |
| pHKCl | −0.767104 | −0.153923 | 0.243697 | 0.294706 | 0.271788 | −0.007243 | 0.165737 |
| OM | 0.234952 | 0.452708 | −0.385559 | −0.347947 | −0.545469 | 0.284759 | 0.063806 |
| Se (m) | −0.739441 | −0.230821 | −0.009431 | −0.347294 | 0.023002 | −0.021954 | −0.014752 |
| Cu (m) | −0.290933 | −0.619352 | −0.572337 | 0.044807 | −0.158691 | 0.074581 | −0.161906 |
| Zn (m) | −0.145337 | −0.669553 | −0.450238 | 0.110132 | 0.050461 | −0.100504 | −0.039094 |
| Mn (m) | −0.643156 | −0.145148 | 0.167201 | 0.119372 | −0.597761 | 0.018068 | 0.189491 |
| Fe (m) | −0.490297 | −0.218819 | 0.340062 | 0.549129 | −0.266540 | 0.207179 | 0.268007 |
| Mushroom Species | Cu | Zn | Mn | Fe | Se | ||||
|---|---|---|---|---|---|---|---|---|---|
| Soil layer (cm) | |||||||||
| 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–10 | |
| Boletus edulis | 0.9 | 2.7 | 2.5 | 3.7 | 0.04 | 0.16 | 0.04 | 0.03 | 120.6 |
| Soil layer (cm) | |||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | |
| Imleria badia | 1.6 | 0.9 | 3.4 | 5.8 | 0.06 | 0.16 | 0.07 | 0.05 | 1.2 |
| Soil layer (cm) | |||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | |
| Leccinum scabrum | 0.9 | 0.9 | 2.0 | 3.6 | 0.06 | 0.13 | 0.06 | 0.04 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotek, Z.; Stasińska, M.; Malinowski, R.; Pilarczyk, B.; Pilarczyk, R.; Bąkowska, M.; Malinowska, K.; Radke, P.; Kubus, M.; Malinowska, A.; et al. The Role in the Human Diet of Bioaccumulation of Selenium, Copper, Zinc, Manganese and Iron in Edible Mushrooms in Various Habitat Conditions of NW Poland—A Case Study. Sustainability 2023, 15, 13334. https://doi.org/10.3390/su151813334
Sotek Z, Stasińska M, Malinowski R, Pilarczyk B, Pilarczyk R, Bąkowska M, Malinowska K, Radke P, Kubus M, Malinowska A, et al. The Role in the Human Diet of Bioaccumulation of Selenium, Copper, Zinc, Manganese and Iron in Edible Mushrooms in Various Habitat Conditions of NW Poland—A Case Study. Sustainability. 2023; 15(18):13334. https://doi.org/10.3390/su151813334
Chicago/Turabian StyleSotek, Zofia, Małgorzata Stasińska, Ryszard Malinowski, Bogumiła Pilarczyk, Renata Pilarczyk, Małgorzata Bąkowska, Katarzyna Malinowska, Patrycja Radke, Marcin Kubus, Alicja Malinowska, and et al. 2023. "The Role in the Human Diet of Bioaccumulation of Selenium, Copper, Zinc, Manganese and Iron in Edible Mushrooms in Various Habitat Conditions of NW Poland—A Case Study" Sustainability 15, no. 18: 13334. https://doi.org/10.3390/su151813334
APA StyleSotek, Z., Stasińska, M., Malinowski, R., Pilarczyk, B., Pilarczyk, R., Bąkowska, M., Malinowska, K., Radke, P., Kubus, M., Malinowska, A., & Bukowska, A. (2023). The Role in the Human Diet of Bioaccumulation of Selenium, Copper, Zinc, Manganese and Iron in Edible Mushrooms in Various Habitat Conditions of NW Poland—A Case Study. Sustainability, 15(18), 13334. https://doi.org/10.3390/su151813334







