Leaf Biochemical and Kernel Metabolite Profiles as Potential Biomarkers of Water Deficit in Walnut (Juglans regia L.) cv. Chandler
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Leaf Material Sampling
2.3. Leaf Malondialdehyde Concentrations
2.4. Leaf Proline Concentrations
2.5. Leaf Total Soluble Sugar Concentration
2.6. Leaf Total Phenol and Flavonoid Concentration
2.7. Walnut Kernel Metabolite Extraction, Derivatization, and Profiling
2.8. Statistical Analysis
3. Results and Discussion
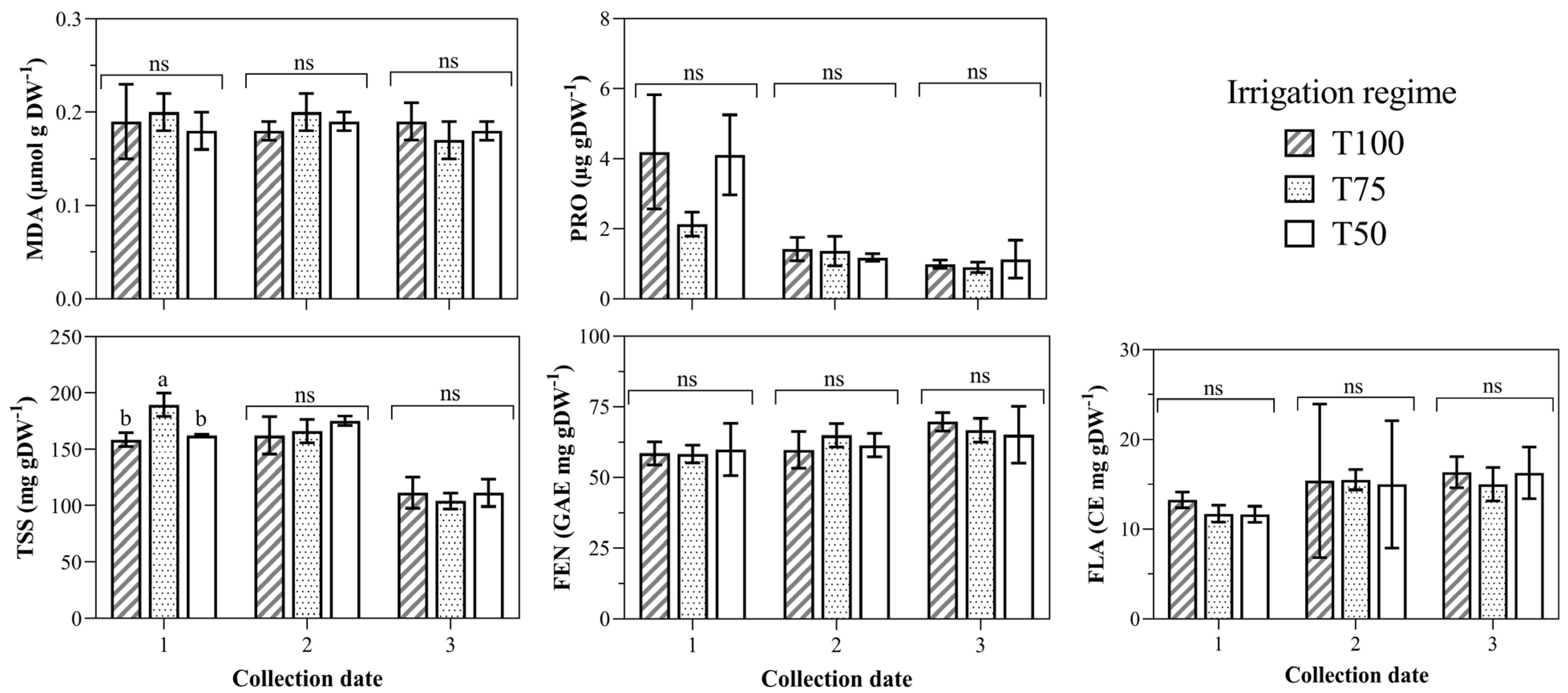
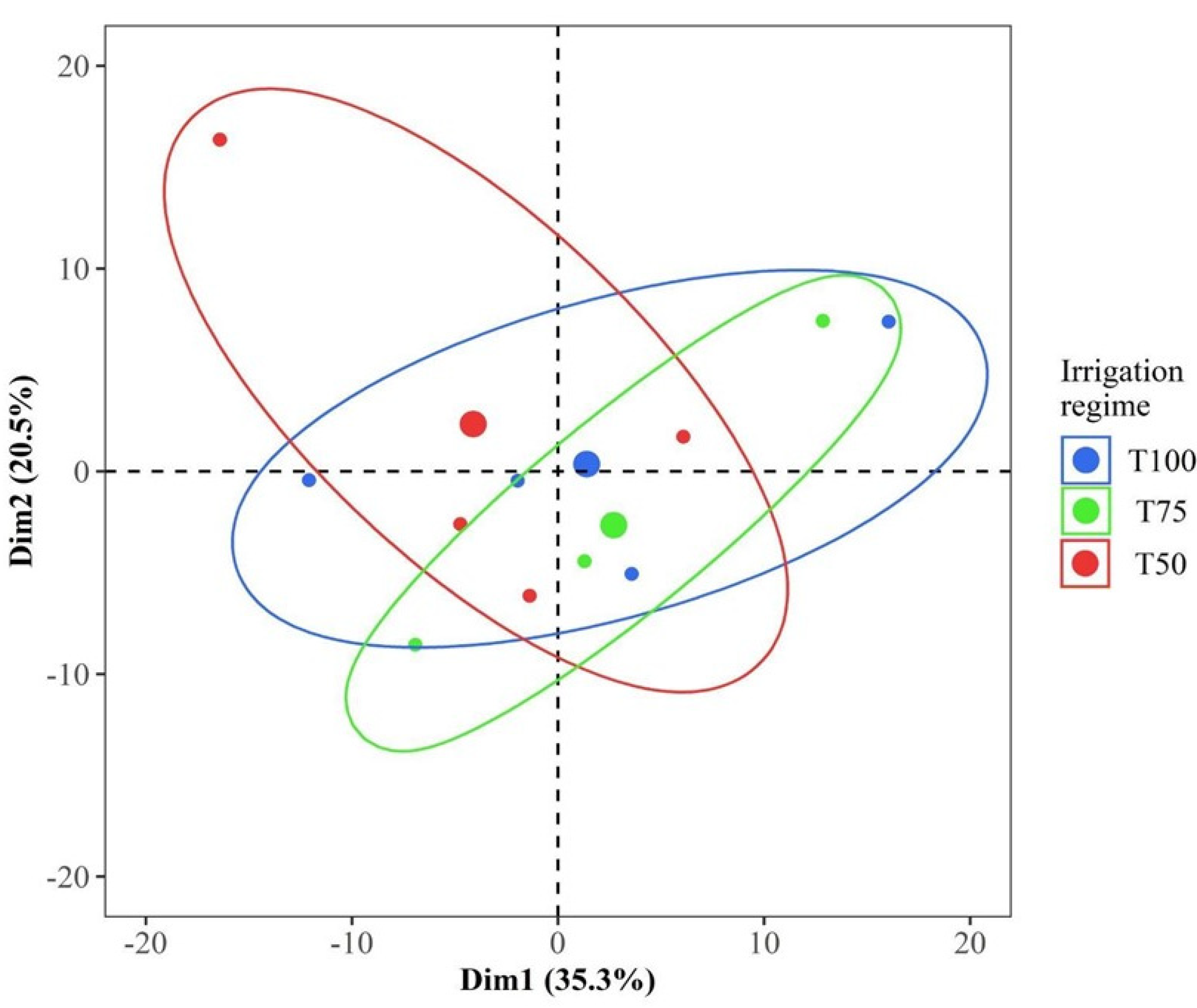
3.1. Biomarkers of Water Deficit in Leaf Tissue
3.2. Kernel Polar Metabolite Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT: Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 June 2023).
- Rubí Bianchi, A.; Cravero, S.A.C. Atlas Climático Digital de la República Argentina, 1st ed.; Rubí Bianchi, A., Ed.; Ediciones Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 2010.
- Goldhamer, D.A. Irrigation scheduling for walnut orchards. In Walnut Production Manual, 1st ed.; Ramos, D., Ed.; University of California Division of Agriculture and Natural Resources: Oakland, CA, USA, 1998. [Google Scholar]
- Searles, P.S.; Alcarás, M.A.; Rousseaux, M.C. Water use by olive orchards (Olea europaea L.) in the Northwest of Argentina: A comparison with the Mediterranean Basin. Ecol. Austral 2011, 21, 15–28. Available online: http://hdl.handle.net/20.500.12110/ecologiaaustral_v021_n01_p015 (accessed on 4 September 2023).
- Rivera, J.A.; Otta, S.; Lauro, C.; Zazulie, N. A Decade of Hydrological Drought in Central-Western Argentina. Front. Water 2021, 3, 640544. [Google Scholar] [CrossRef]
- Yang, B.; Fu, P.; Lu, J.; Ma, F.; Sun, X.; Fang, Y. Regulated deficit irrigation: An effective way to solve the shortage of agricultural water for horticulture. Stress Biol. 2022, 2, 2–28. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.E.; Silvente, S.T.; Trentacoste, E.R. A mini review of the impacts of deficit irrigation strategies for walnut (Juglans regia L.) production in semiarid conditions. Irrig. Sci. 2023, 41, 501–509. [Google Scholar] [CrossRef]
- Lofti, N.; Vahdati, K.; Kholdebarin, B.; Amiri, R. Soluble sugars and proline accumulation play a role as effective indices for drought tolerance screening in Persian walnut (Juglans regia L.) during germination. Fruits 2009, 65, 97–112. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Pei, D.; Yu, L. Combined effects of water stress and salinity on growth, physiological, and biochemical traits in two walnut genotypes. Physiol. Plant. 2020, 177, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Karami, H.; Vahdati, K.; Mokhtassi-Bidgoli, A. Antioxidative responses to short-term salinity stress induce drought tolerance in walnut. Sci. Hortic. 2020, 267, 109322. [Google Scholar] [CrossRef]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef]
- Martínez, M.L.; Maestri, D.M. Oil chemical variation in walnut (Juglans regia L.) genotypes grown in Argentina. Eur. J. Lipid Sci. Tech. 2008, 110, 1183–1189. [Google Scholar] [CrossRef]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3–21. [Google Scholar] [CrossRef]
- Rao, G.; Sui, J.; Zhang, J. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Kalagiouri, N.P.; Manousi, N.; Rosenberg, E.; Zachariadis, G.A.; Paraskevopoulou, A.; Samanidou, V. Exploring the volatile metabolome of conventional and organic walnut oils by solid-phase microextraction and analysis by GC-MS combined with chemometrics. Food Chem. 2021, 363, 130331. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.N.; Suh, J.H. Metabolomics as a tool to evaluate nut quality and safety. Trends Food Sci. 2022, 129, 528–543. [Google Scholar] [CrossRef]
- Holdes, R.L.; DeLong, J.D.; Forney, C.F.; Pranger, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar]
- Carillo, P.; Mastrolonardo, G.; Nacca, F.; Parisi, D.; Verlotta, A.; Fuggi, A. Nitrogen metabolism in durum wheat under salinity: Accumulation of proline and glycine betaine. Func. Plant Biol. 2008, 35, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, J.J.; Emerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Karadeniz, F.; Burdurlu, H.S.; Koca, N.; Soyer, Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk. J. Agric. For. 2005, 29, 297–303. [Google Scholar]
- Roessner, U.; Beckles, D.M. Metabolomics for Salinity Research. In Plant Salt Tolerance; Shabala, S., Cuin, T., Eds.; Humana Press: Totowa, NJ, USA, 2012. [Google Scholar]
- Calvo, F.E.; Trentacoste, E.R.; Silvente, S.T. Vegetative growth, yield, and crop water productivity response to different irrigation regimes in high density walnut orchards (Juglans regia L.) in a semi-arid environment in Argentina. Agric. Water Manag. 2022, 274, 107969. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Zhang, C.F.; Pan, C.D.; Chen, H. The long-term response of photosynthesis in walnut (Juglans regia L.) leaf to a leaf-to-fruit ratio. Photosynthetica 2019, 57, 762–771. [Google Scholar] [CrossRef]
- Améglio, T.; Bodet, C.; Lacointe, A.; Cochard, H. Winter embolism, mechanisms of xylem hydraulic conductivity recovery and springtime growth patterns in walnut and peach trees. Tree Physiol. 2002, 22, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Wichtl, M.; Anton, R. Plantes Thérapeutiques, 2nd ed.; Tec & Doc: Paris, France, 1999; pp. 291–293. [Google Scholar]
- Fernandes, C.; Albuquerque, P.; Mariz-Ponte, N.; Cruz, L.; Tovares, F. Comprehensive diversity assessment of walnut-associated xanthomonads reveal the occurrence of distinct Xanthomonas arboricola lineages and of a new species (Xanthomonas euroxanthea) within the same tree. Plant Pathol. 2020, 70, 943–958. [Google Scholar] [CrossRef]
- Ashrafi, M.; Azimi-Moqadam, M.R.; Mohseni Fard, E.; Shekari, F.; Jafary, H.; Moradi, P.; Pucci, M.; Abate, G.; Mastinu, A. Physiological and molecular aspects of two Thymus species differently sensitive to drought stress. BioTech 2022, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Y.; Zeng, W.H.; LI, Z.; Peng, Y. Mannose regulates water balance, leaf senescence, and genes related to stress tolerance in white clover under osmotic stress. Biol. Plant. 2020, 64, 406–416. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdellatif, Y.M.R. Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef]
- Lee, B.-R.; Jin, Y.-L.; Jung, W.-J.; Avice, J.C.; Morvan-Bertrand, A.; Curry, A.; Park, C.-W.; Kim, W.-A. Water-deficit accumulates sugars by starch degradation –not by de novo synthesis– in white clover leaves (Trifolium repens). Physiol. Plant. 2008, 134, 403–411. [Google Scholar] [CrossRef]
- Phillips, G.O.; Moody, G.J. Radiation chemistry of carbohydrates. Part V. The effect of ultraviolet light on aqueous solutions of D-glucose in oxygen. J. Chem. Soc. 1960, 3398–3404. [Google Scholar] [CrossRef]
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS ONE 2012, 7, e38554. [Google Scholar] [CrossRef]
- Ünal, N.; Okatan, V. Effects of drought stress treatment on phytochemical contents of strawberry varieties. Sci. Horti. 2023, 316, 112013. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A.; Murthy, P.P.N. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Simkin, A.J.; Lopez-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Gates, D.M. Leaf Temperature and Transpiration. Agron. J. 1964, 56, 273. [Google Scholar] [CrossRef]
- Gauthier, M.-M.; Jacobs, D.F. Walnut (Juglans spp.) ecophysiology in response to environmental stresses and potential acclimation to climate change. Ann. For. Sci. 2011, 68, 1277–1290. [Google Scholar] [CrossRef]
- García-Inza, G.P.; Castro, D.N.; Hall, A.J.; Rousseaux, M.C. Opposite oleic acid responses to temperature in oils from the seed and mesocarp of the olive fruit. Eur. J. Agron. 2016, 76, 138–147. [Google Scholar] [CrossRef]
- Jin, F.; Zhou, Y.; Zhang, P.; Hyung, R.; Fan, W.; Li, B.; Li, G.; Song, X.; Pei, D. Identification of Key Lipogenesis Stages and Proteins Involved in Walnut Kernel Development. J. Agric. Food Chem. 2023, 71, 4306–4318. [Google Scholar] [CrossRef] [PubMed]



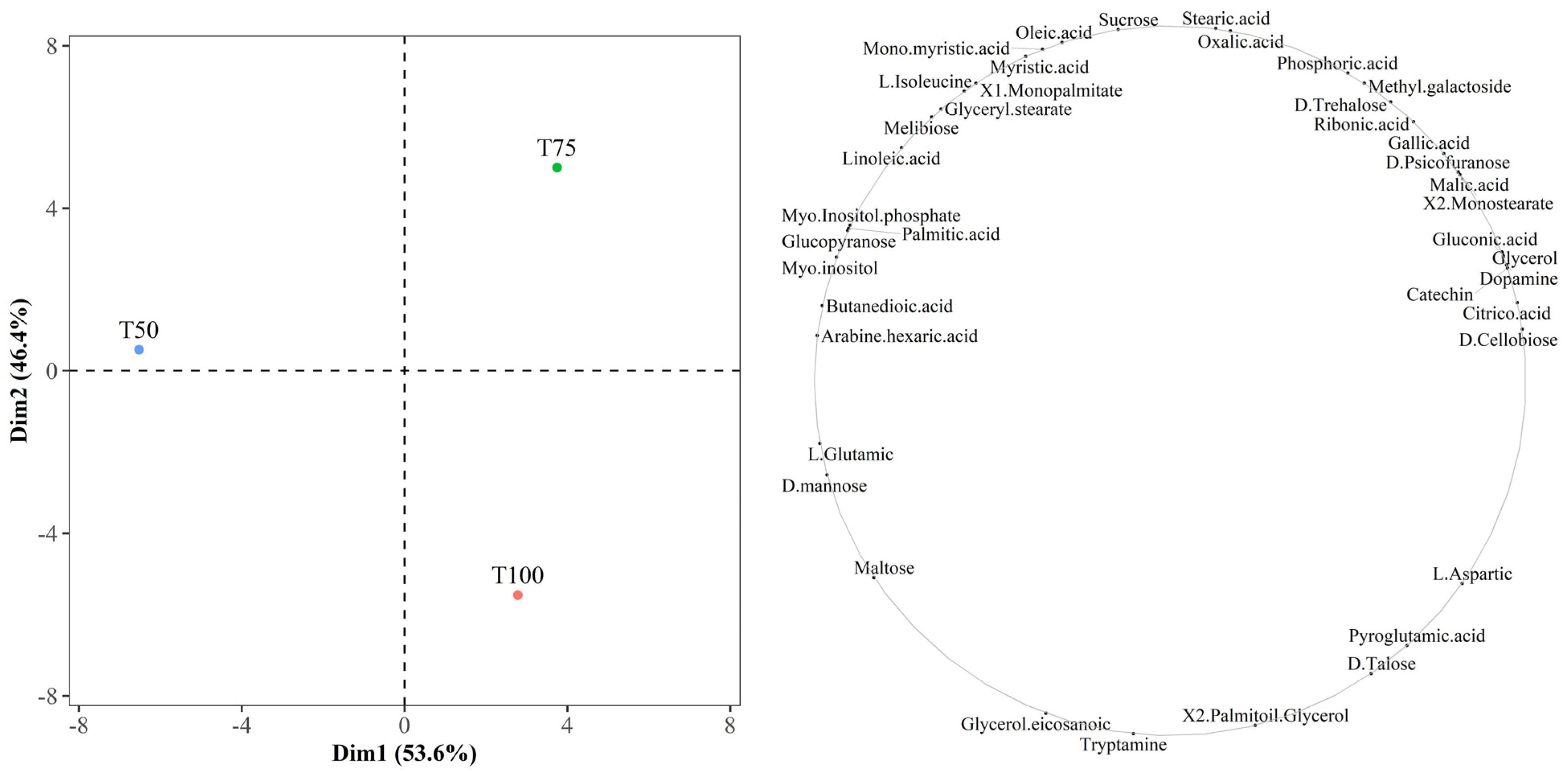
| Rt (s) | Metabolite | Group | T100 (mg gDW−1) | T75 (mg gDW−1) | T50 (mg gDW−1) | p-Value |
|---|---|---|---|---|---|---|
| 695.8 | Oxalic acid | Organic acid | 1.41 ± 0.74 | 1.90 ± 0.64 | 1.62 ± 0.28 | >0.05 |
| 825.0 | Glycerol | Saturated alcohol | 1.24 ± 0.73 | 1.51 ± 0.43 | 0.90 ± 0.18 | >0.05 |
| 836.6 | L-Isoleucine | Amino acid | 0.52 ± 0.22 | 0.62 ± 0.20 | 0.64 ± 0.41 | >0.05 |
| 861.4 | Butanedioic acid | Organic acid | 0.61 ± 0.31 | 0.63 ± 0.47 | 0.78 ± 0.40 | >0.05 |
| 994.9 | Malic acid | Organic acid | 8.44 ± 3.10 | 11.49 ± 6.85 | 6.85 ± 1.93 | >0.05 |
| 1012.6 | Pyroglutamic acid | Organic acid | 0.93 ± 0.32 | 0.82 ± 0.25 | 0.77 ± 0.35 | >0.05 |
| 1017.7 | L-Aspartic | Amino acid | 0.80 ± 0.54 | 0.64 ± 0.56 | 0.49 ± 0.36 | >0.05 |
| 1086.1 | L-Glutamic | Amino acid | 2.69 ± 0.83 | 2.31 ± 1.60 | 3.71 ± 1.17 | >0.05 |
| 1187.3 | Phosphoric acid | Organic acid | 1.32 ± 0.42 | 1.53 ± 0.51 | 1.34 ± 0.70 | >0.05 |
| 1193.3 | Ribonic acid | Organic acid | 0.68 ± 0.38 | 0.91 ± 0.54 | 0.63 ± 0.17 | >0.05 |
| 1197.4 | D-Psicofuranose | Carbohydrate | 0.91 ± 0.46 | 1.13 ± 0.22 | 0.80 ± 0.27 | >0.05 |
| 1221.9 | Citric acid | Organic acid | 2.73 ± 1.00 | 3.01 ± 1.98 | 2.11 ± 0.59 | >0.05 |
| 1224.0 | Myristic acid | Organic acid | 4.77 ± 1.01 | 5.37 ± 0.40 | 5.35 ± 0.49 | >0.05 |
| 1227.1 | Methyl galactoside | Carbohydrate | 1.24 ± 0.28 | 1.55 ± 0.38 | 1.25 ± 0.13 | >0.05 |
| 1276.1 | D-mannose | Carbohydrate | 1.88 ± 0.63 | 1.50 ± 0.55 | 2.57 ± 1.78 | >0.05 |
| 1286.1 | D-Talose | Carbohydrate | 0.98 ± 1.06 | 0.67 ± 0.46 | 0.61 ± 0.27 | >0.05 |
| 1300.7 | Gallic acid | Organic acid | 2.34 ± 1.29 | 2.09 ± 1.74 | 2.12 ± 0.50 | >0.05 |
| 1323.9 | Glucopyranose | Carbohydrate | 0.57 ± 0.22 | 0.94 ± 0.28 | 1.63 ± 2.04 | >0.05 |
| 1338.6 | Palmitic acid | Fatty acid | 1.66 ± 0.70 | 1.75 ± 0.43 | 1.92 ± 0.56 | >0.05 |
| 1344.9 | Gluconic acid | Organic acid | 6.31 ± 3.70 | 6.9 ± 2.86 | 5.52 ± 0.95 | >0.05 |
| 1383.0 | Myo-inositol | Vitamin | 3.70 ± 1.22 | 3.89 ± 0.57 | 4.39 ± 1.04 | >0.05 |
| 1398.1 | Arabine-hexaric acid | Organic acid | 0.84 ± 0.46 | 0.85 ± 0.21 | 1.02 ± 0.44 | >0.05 |
| 1427.7 | Linoleic acid | Fatty acid | 1.48 ± 0.46 | 1.76 ± 0.35 | 1.95 ± 0.99 | >0.05 |
| 1430.7 | Oleic acid | Fatty acid | 1.09 ± 0.45 | 1.54 ± 0.45 | 1.48 ± 0.57 | >0.05 |
| 1439.1 | Tryptamine | Amino acid | 0.45 ± 0.41 | 0.36 ± 0.26 | 0.31 ± 0.53 | >0.05 |
| 1444.1 | Stearic acid | Fatty acid | 0.93 ± 0.18 | 1.17 ± 0.43 | 1.04 ± 0.43 | >0.05 |
| 1526.3 | Mono myristic acid | Organic acid | 2.66 ± 0.74 | 3.07 ± 0.16 | 3.04 ± 0.56 | >0.05 |
| 1533.7 | Myo-Inositol-phosphate | Phospholipid | 0.81 ± 0.29 | 0.89 ± 0.25 | 1.03 ± 0.35 | >0.05 |
| 1561.2 | Dopamine | Amine | 1.45 ± 0.97 | 1.55 ± 0.35 | 1.29 ± 1.28 | >0.05 |
| 1600.9 | 2-Palmitoil Glicerol | Carbohydrate | 1.57 ± 0.63 | 1.43 ± 0.17 | 1.46 ± 0.42 | >0.05 |
| 1617.4 | 1-Monopalmitate | Fatty acid | 36.67 ± 8.95 | 41.78 ± 2.05 | 42.67 ± 5.89 | >0.05 |
| 1665.8 | D-Trehalose | Carbohydrate | 54.67 ± 12.71 | 66.42 ± 9.23 | 53.83 ± 5.02 | >0.05 |
| 1684.9 | 2-Monostearate | Fatty acid | 0.94 ± 0.27 | 1.07 ± 0.16 | 0.88 ± 0.29 | >0.05 |
| 1700.6 | Glyceryl-stearate | Fatty acid | 26.52 ± 6.40 | 30.29 ± 1.81 | 31.66 ± 5.21 | >0.05 |
| 1707.8 | Maltose | Carbohydrate | 0.74 ± 0.13 | 0.52 ± 0.17 | 0.87 ± 1.01 | >0.05 |
| 1751.8 | Catechin | Flavonoid | 1.27 ± 0.77 | 1.46 ± 0.31 | 0.99 ± 0.38 | >0.05 |
| 1762.1 | D-Cellobiose | Carbohydrate | 1.17 ± 0.38 | 1.23 ± 1.08 | 0.95 ± 0.72 | >0.05 |
| 1780.2 | Glycerol-eicosanoic | Carbohydrate | 0.69 ± 0.63 | 0.43 ± 0.10 | 0.63 ± 0.21 | >0.05 |
| 1811.5 | Melibiose | Carbohydrate | 3.68 ± 2.60 | 3.26 ± 1.24 | 2.28 ± 0.40 | >0.05 |
| 2036.4 | Sucrose | Carbohydrate | 18.5 ± 5.02 | 23.91 ± 4.49 | 22.31 ± 5.20 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, F.E.; Silvente, S.T.; Trentacoste, E.R. Leaf Biochemical and Kernel Metabolite Profiles as Potential Biomarkers of Water Deficit in Walnut (Juglans regia L.) cv. Chandler. Sustainability 2023, 15, 13472. https://doi.org/10.3390/su151813472
Calvo FE, Silvente ST, Trentacoste ER. Leaf Biochemical and Kernel Metabolite Profiles as Potential Biomarkers of Water Deficit in Walnut (Juglans regia L.) cv. Chandler. Sustainability. 2023; 15(18):13472. https://doi.org/10.3390/su151813472
Chicago/Turabian StyleCalvo, Franco E., Sonia T. Silvente, and Eduardo R. Trentacoste. 2023. "Leaf Biochemical and Kernel Metabolite Profiles as Potential Biomarkers of Water Deficit in Walnut (Juglans regia L.) cv. Chandler" Sustainability 15, no. 18: 13472. https://doi.org/10.3390/su151813472
APA StyleCalvo, F. E., Silvente, S. T., & Trentacoste, E. R. (2023). Leaf Biochemical and Kernel Metabolite Profiles as Potential Biomarkers of Water Deficit in Walnut (Juglans regia L.) cv. Chandler. Sustainability, 15(18), 13472. https://doi.org/10.3390/su151813472







