Exploring the Potential of Biomass Pyrolysis for Renewable and Sustainable Energy Production: A Comparative Study of Corn Cob, Vine Rod, and Sunflower
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermogravimetric Analysis (TGA)
2.2. Gas Chromatographic (GC) Analysis
2.3. Gas Chromatography-Mass Spectrometry (GS-MS) Analysis
2.4. Fourier Transform–Infrared Spectroscopy
2.5. Multicriteria Analysis of the Biomass Quality
3. Results and Discussions
3.1. Mass Change during Pyrolysis Using TGA
3.2. Gas Analysis Results from Pyrolysis of Corn Cob, Vine Rod and Sunflower
3.3. Analysis of Organic Compounds in Pyrolysis Oils of Corn Cob, Vine Rod, and Sunflower
3.4. FT-IR Spectroscopic Analysis of Raw Biomass and Char Products at 500 °C
3.5. Multicriteria Assessment of the Biomass Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A global assessment: Can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Rizzi, F.; van Eck, N.J.; Frey, M. The production of scientific knowledge on renewable energies: Worldwide trends, dynamics and challenges and implications for management. Renew. Energy 2014, 62, 657–671. [Google Scholar] [CrossRef]
- Kumar, M. Social, economic, and environmental impacts of renewable energy resources. In Wind Solar Hybrid Renewable Energy System; IntechOpen: London, UK, 2020; Volume 1. [Google Scholar]
- Khan, A.A.; Khan, S.U.; Ali, M.A.S.; Safi, A.; Gao, Y.; Luo, J. Identifying impact of international trade and renewable energy consumption on environmental quality improvement and their role in global warming. Environ. Sci. Pollut. Res. 2022, 29, 33935–33944. [Google Scholar] [CrossRef] [PubMed]
- Levenda, A.M.; Behrsin, I.; Disano, F. Renewable energy for whom? A global systematic review of the environmental justice implications of renewable energy technologies. Energy Res. Soc. Sci. 2021, 71, 101837. [Google Scholar] [CrossRef]
- Ang, T.Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A comprehensive study of renewable energy sources: Classifications, challenges and suggestions. Energy Strategy Rev. 2022, 43, 100939. [Google Scholar] [CrossRef]
- Anser, M.K.; Shabbir, M.S.; Tabash, M.I.; Shah, S.H.A.; Ahmad, M.; Peng, M.Y.P.; Lopez, L.B. Do renewable energy sources improve clean environmental-economic growth? Empirical investigation from South Asian economies. Energy Explor. Exploit. 2021, 39, 1491–1514. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Chowdhury, C.R.; Yadav, D.; Verma, R.; Dutta, S.; Jaiswal, K.S.; Karuppasamy, K.S.K. Renewable and sustainable clean energy development and impact on social, economic, and environmental health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- International Energy Agency. Renewable Energy Market Update, Outlook for 2022 and 2023; Official Report; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Belyakov, N. Sustainable Power Generation. In Current Status, Future Challenges, and Perspectives; Academic Press: Cambridge, MA, USA, 2019; pp. 417–438. [Google Scholar]
- Pishvaee, M.S.; Mohseni, S.; Bairamzadeh, S. Biomass to Biofuel Supply Chain Design and Planning Under Uncertainty: Concepts and Quantitative Methods; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- International Energy Agency. IEA World Energy Outlook 2022; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Errera, M.R.; Dias, T.A.d.C.; Maya, D.M.Y.; Lora, E.E.S. Global bioenergy potentials projections for 2050. Biomass Bioenergy 2023, 170, 106721. [Google Scholar] [CrossRef]
- Kusumo, F.; Mahlia, T.M.I.; Shamsuddin, A.H.; Ahmad, A.R.; Silitonga, A.S.; Dharma, S.; Mofijur, M.; Ideris, F.; Ong, H.C.; Sebayang, R.; et al. Optimisation of biodiesel production from mixed Sterculia foetida and rice bran oil. Int. J. Ambient. Energy 2022, 43, 4380–4390. [Google Scholar] [CrossRef]
- Atabani, A.E.; Shobana, S.; Mohammed, M.N.; Uğuz, G.; Kumar, G.; Arvindnarayan, S.; Aslam, M.; Ala’a, H. Integrated valorization of waste cooking oil and spent coffee grounds for biodiesel production: Blending with higher alcohols, FT–IR, TGA, DSC and NMR characterizations. Fuel 2019, 244, 419–430. [Google Scholar] [CrossRef]
- Sharmina, M.; McGlade, C.; Gilbert, P.; Larkin, A. Global energy scenarios and their implications for future shipped trade. Mar. Policy 2017, 84, 12–21. [Google Scholar] [CrossRef]
- Aravind, S.; Kumar, P.S.; Kumar, N.S.; Siddarth, N. Conversion of green algal biomass into bioenergy by pyrolysis: A review. Environ. Chem. Lett. 2020, 18, 829–849. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Rizzo, A.M.; Pari, L. Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Appl. Energy 2017, 185, 963–972. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef]
- He, J.; Strezov, V.; Kumar, R.; Weldekidan, H.; Jahan, S.; Dastjerdi, B.H.; Zhou, X.; Kan, T. Pyrolysis of heavy metal contaminated Avicennia marina biomass from phytoremediation: Characterisation of biomass and pyrolysis products. J. Clean. Prod. 2019, 234, 1235–1245. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A.; Antonakou, E.V.; Papazisi, K.M.; Lappas, A.A.; Athanassiou, C. Investigating the potential for energy, fuel, materials and chemicals production from corn residues (cobs and stalks) by non-catalytic and catalytic pyrolysis in two reactor configurations. Renew. Sustain. Energy Rev. 2009, 13, 750–762. [Google Scholar] [CrossRef]
- Zabaniotou, A.A.; Kantarelis, E.K.; Theodoropoulos, D.C. Sunflower shells utilization for energetic purposes in an integrated approach of energy crops: Laboratory study pyrolysis and kinetics. Bioresour. Technol. 2008, 99, 3174–3181. [Google Scholar] [CrossRef]
- Coriolano, A.C.; Alves, A.A.; Araujo, R.A.; Delgado, R.C.; Carvalho, F.R.; Fernandes, V.J.; Araujo, A.S. Thermogravimetry study of the ester interchange of sunflower oil using Mg/Al layered double hydroxides (LDH) impregnated with potassium. J. Therm. Anal. Calorim. 2017, 127, 1863–1867. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, J.Y.; Xiao, Y.; Zhang, Y.N.; Huang, A.C.; Shu, C.M. Thermal analysis of the pyrolysis and oxidation behaviour of 1/3 coking coal. J. Therm. Anal. Calorim. 2017, 129, 1779–1786. [Google Scholar] [CrossRef]
- Liden, A.G.; Berruti, F.; Scott, D.S. A Kinetic Model for the Production of Liquids from the Flash Pyrolysis of Biomass. Chem. Eng. Commun. 2010, 65, 207–221. [Google Scholar] [CrossRef]
- Lo, S.-L.; Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H. Microwave Pyrolysis of Lignocellulosic Biomass. Energy Procedia 2017, 105, 41–46. [Google Scholar] [CrossRef]
- Yong, K.J.; Wu, T.Y. Second-generation bioenergy from oilseed crop residues: Recent technologies, techno-economic assessments and policies. Energy Convers. Manag. 2022, 267, 115869. [Google Scholar] [CrossRef]
- Bijarchiyan, M.; Sahebi, H.; Mirzamohammadi, S. A sustainable biomass network design model for bioenergy production by anaerobic digestion technology: Using agricultural residues and livestock manure. Energy Sustain. Soc. 2020, 10, 19. [Google Scholar] [CrossRef]
- Spigno, G.; Pizzorno, T.; De Faveri, D.M. Cellulose and hemicelluloses recovery from grape stalks. Bioresour. Technol. 2008, 99, 4329–4337. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Ioannidou, O.; Antonakou, E.; Lappas, A. Experimental study of pyrolysis for potential energy, hydrogen and carbon material production from lignocellulosic biomass. Int. J. Hydrogen Energy 2008, 33, 2433–2444. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Okieimen, C.O.; Kusuma, H.S. Biomass pyrolysis technologies for value-added products: A state-of-the-art review. Environ. Dev. Sustain. 2021, 23, 14324–14378. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Production: Crops and Livestock Products; FAO: Rome, Italy, 2021. [Google Scholar]
- Eurostat. 2023. Available online: https://ec.europa.eu/eurostat (accessed on 23 August 2023).
- ASTM D7582; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM International: West Conshohocken, PA, USA, 2015.
- Shariff, A.; Aziz, N.M.; Ismail, N.I.; Abdullah, N. Corn cob as a potential feedstock for slow pyrolysis of biomass. J. Phys. Sci. 2016, 27, 123–137. [Google Scholar] [CrossRef]
- Pütün, A.E.; Koçkar, Ö.M.; Yorgun, S.; Gerçel, H.F.; Andresen, J.; Snape, C.E.; Pütün, E. Fixed-bed pyrolysis and hydropyrolysis of sunflower bagasse: Product yields and compositions. Fuel Process. Technol. 1996, 46, 49–62. [Google Scholar] [CrossRef]
- Weldekidan, H.; Strezov, V.; Li, R.; Kan, T.; Town, G.; Kumar, R.; He, J.; Flamant, G. Distribution of solar pyrolysis products and product gas composition produced from agricultural residues and animal wastes at different operating parameters. Renew. Energy 2020, 151, 1102–1109. [Google Scholar] [CrossRef]
- Staš, M.; Auersvald, M.; Kejla, L.; Vrtiška, D.; Kroufek, J.; Kubička, D. Quantitative analysis of pyrolysis bio-oils: A review. Trends Anal. Chem. 2020, 126, 115857. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Characterization of bio-oils in chemical families. Biomass Bioenergy 2007, 31, 222–242. [Google Scholar] [CrossRef]
- National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 23 August 2023).
- Ceranic, M.; Kosanic, T.; Djuranovic, D.; Kaludjerovic, Z.; Djuric, S.; Gojkovic, P.; Bozickovic, R. Experimental investigation of corn cob pyrolysis. J. Renew. Sustain. Energy 2016, 8, 063102. [Google Scholar] [CrossRef]
- Yorgun, S.; Gülbaran, H.S. Pyrolysis of Sunflower Press Bagasse: Heating Values and Energy Distribution of the Pyrolysis Products. Energy Sources 2003, 25, 809–817. [Google Scholar] [CrossRef]
- He, X.; Yang, L.; Wu, H.; Liu, N.; Zhang, Y.; Zhou, A. Characterization and pyrolysis behaviors of sunflower stalk and its hydrolysis residue, Asia-Pac. J. Chem. Eng. 2016, 11, 803–811. [Google Scholar]
- Suárez, S.; Rosas, J.G.; Sánchez, M.E.; López, R.; Gómez, N.; Cara-Jiménez, J. Parametrization of a Modified Friedman Kinetic Method to Assess Vine Wood Pyrolysis Using Thermogravimetric Analysis. Energies 2019, 12, 2599. [Google Scholar] [CrossRef]
- Rathore, N.S.; Panwar, N.L. Biomass Production and Efficient Utilization for Energy Generation; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Casoni, A.I.; Bidegain, M.; Cubitto, M.A.; Curvetto, N.; Volpe, M.A. Pyrolysis of sunflower seed hulls for obtaining bio-oils. Bioresour. Technol. 2015, 177, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Abatyough, M.T.; Ajibola, V.O.; Agbaji, E.B.; Yashim, Z.I. Properties of upgraded bio-oil from pyrolysis of waste corn cobs. J. Sustain. Environ. Manag. 2022, 1, 120–128. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2000; pp. 10815–10837. [Google Scholar]
- Mulligan, C.J.; Strezov, L.; Strezov, V. Thermal Decomposition of Wheat Straw and Mallee Residue under Pyrolysis Conditions. Energy Fuels 2010, 24, 46–52. [Google Scholar] [CrossRef]
- Strezov, V.; Evans, T.J. Thermal Processing of Paper Sludge and Characterisation of its Pyrolysis Products. Waste Manag. 2009, 29, 1644–1648. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Tung, C.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Fahmy, T.; Fahmy, Y.; Mobarak, F.; El-Sakhaway, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Xin-gang, Z.; Wei, W.; Shuran, H.; Xuan, L. Impacts of Government Policies on the Adoption of Biomass Power: A System Dynamic Perspective. Sustainability 2023, 15, 1723. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Hochenauer, C.; Scharler, R. Bioenergy technologies, uses, market and future trends with Austria as a case study. Renew. Sustain. Energy Rev. 2021, 135, 110237. [Google Scholar] [CrossRef]
- IEA. Net Zero by 2050: A Roadmap for the Global Energy Sector; International Energy Agency: Paris, France, 2021. [Google Scholar]
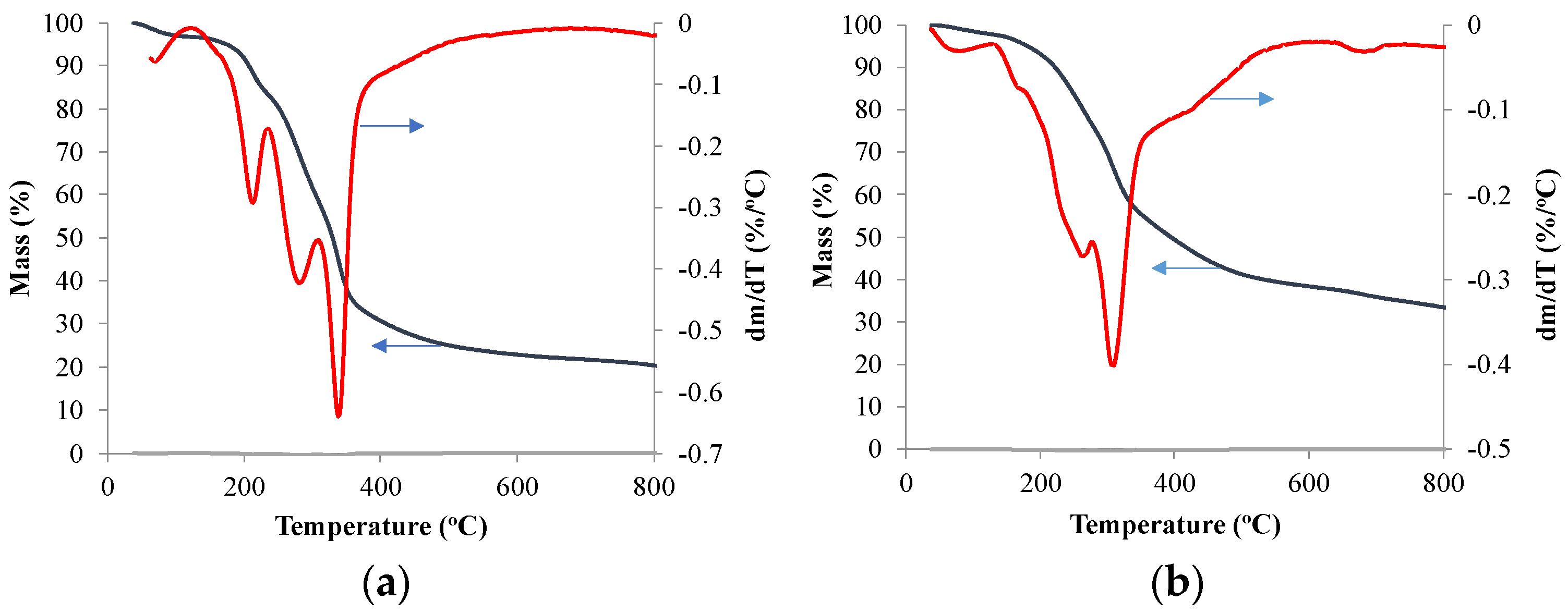
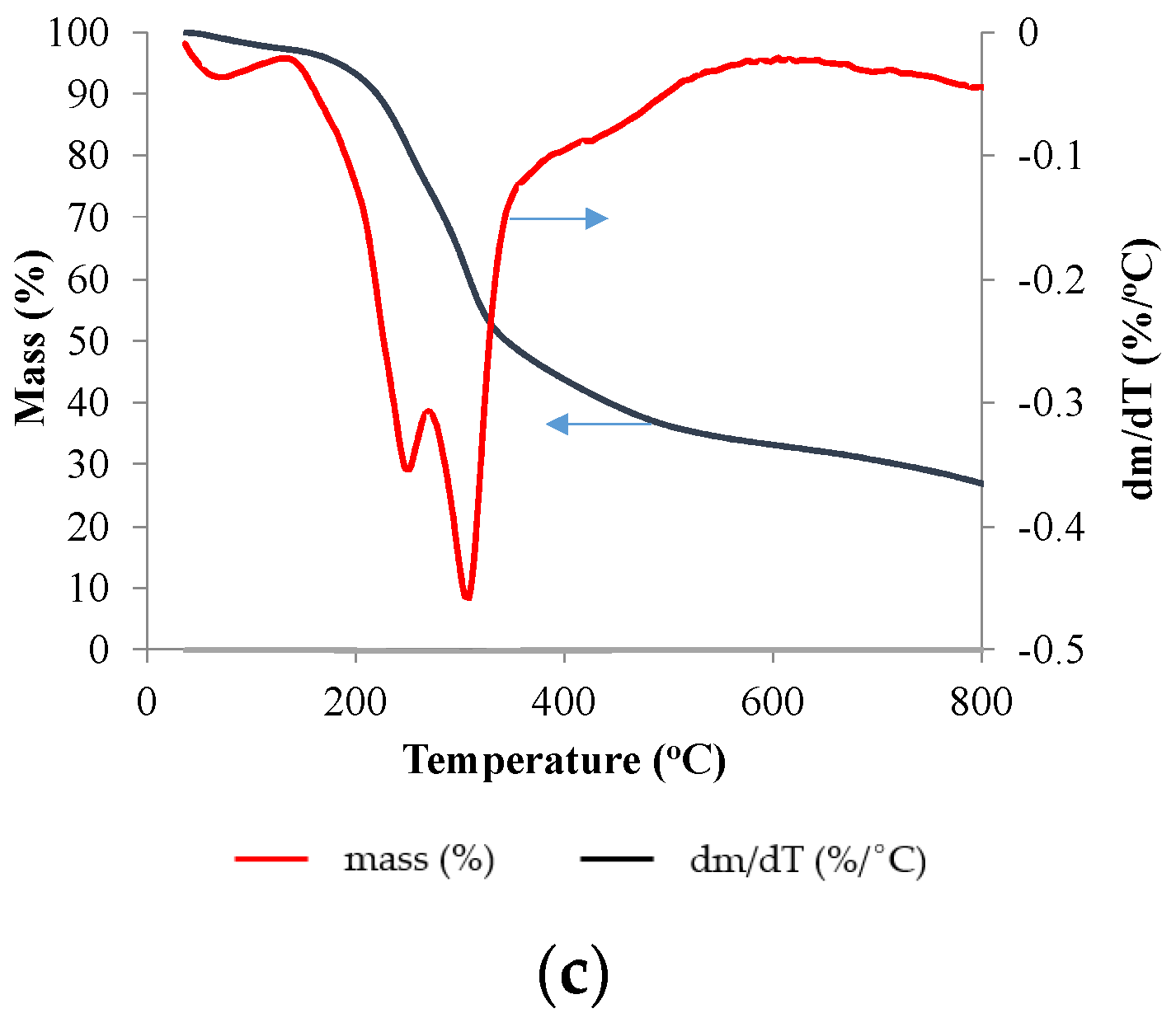
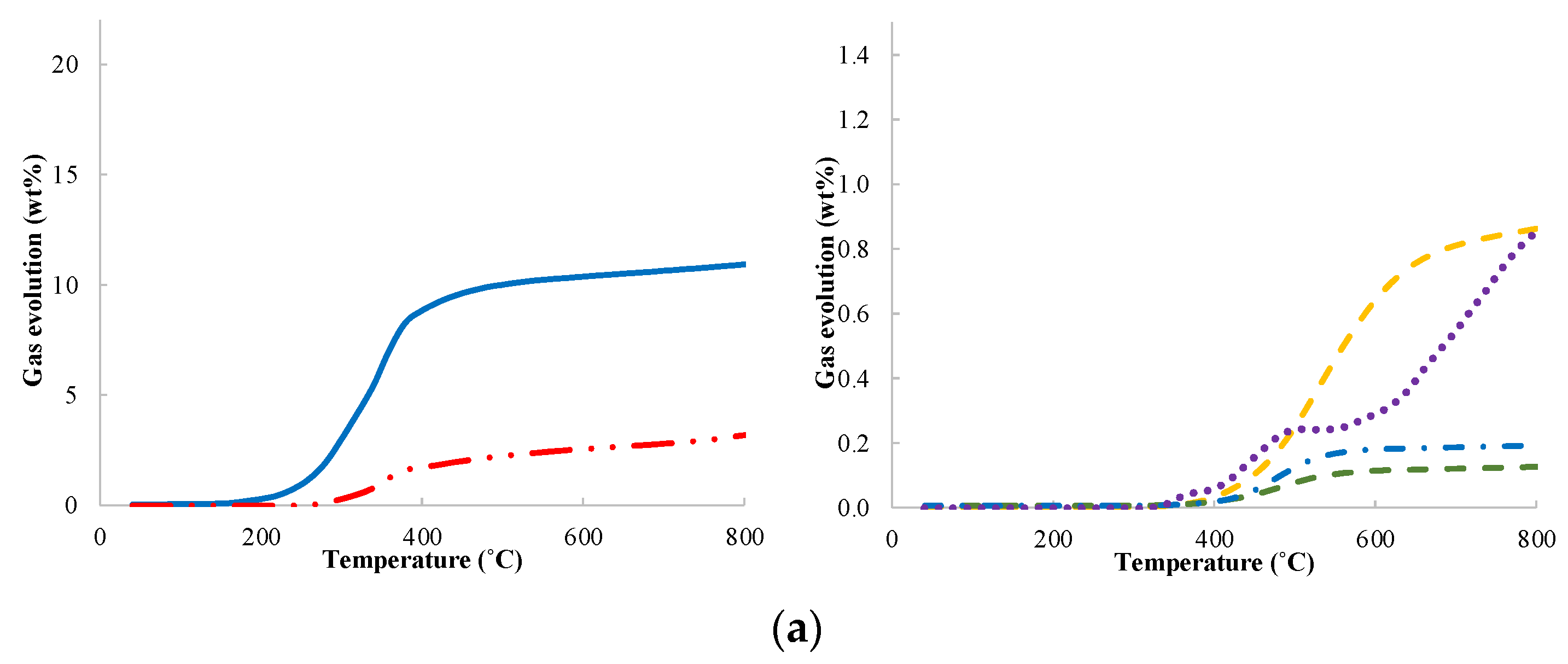
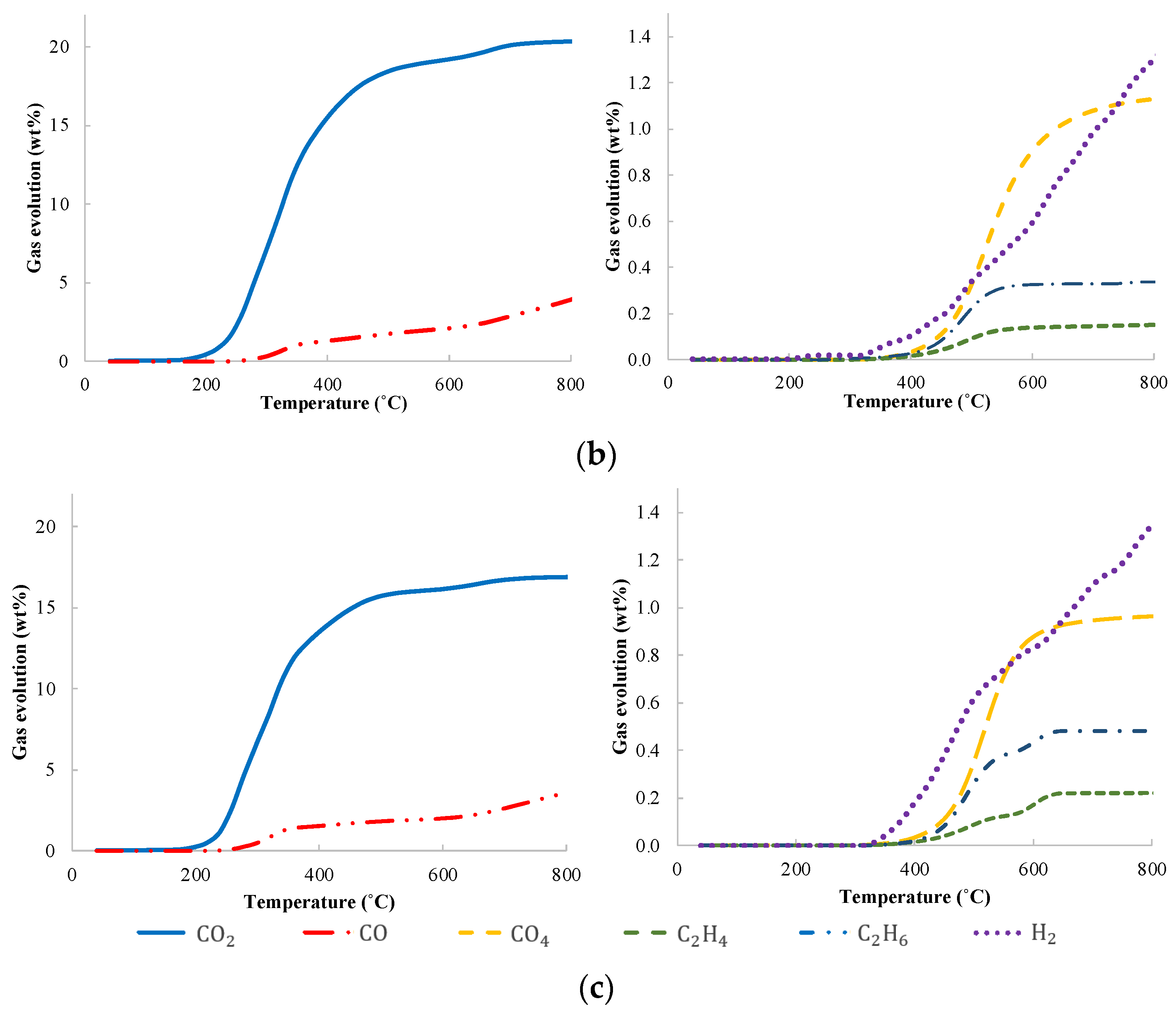
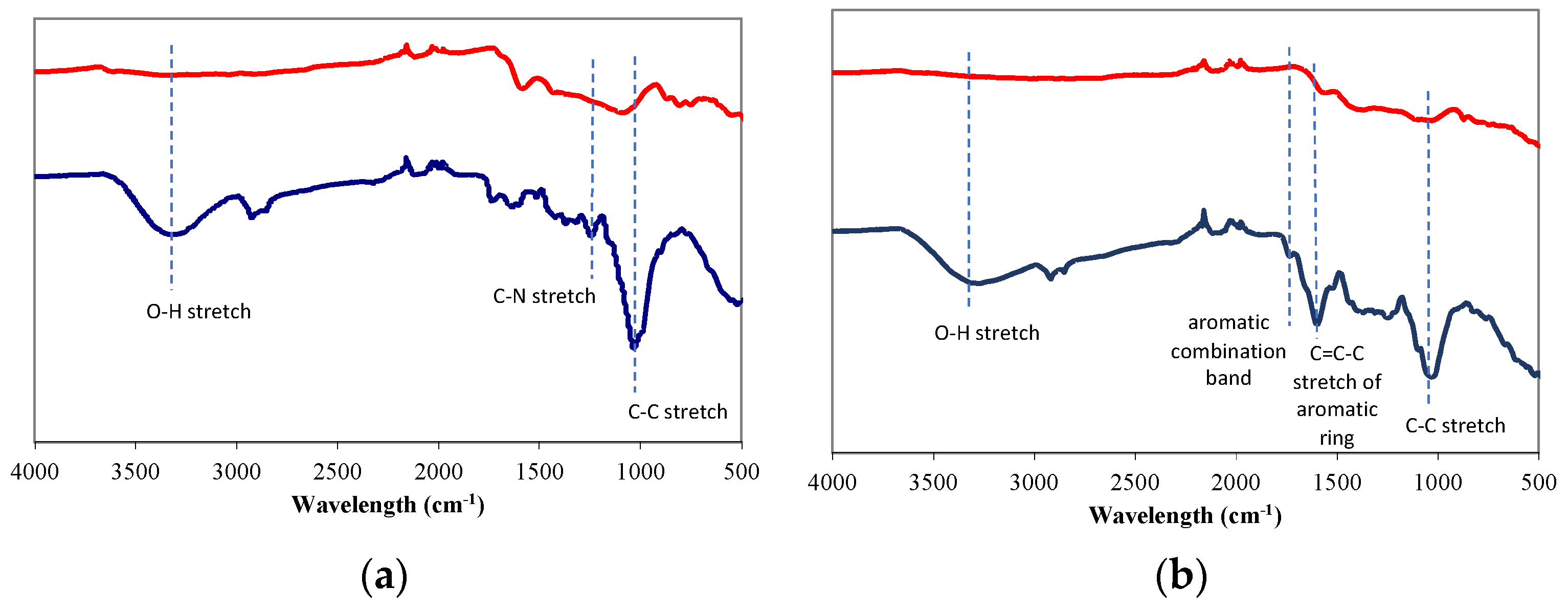
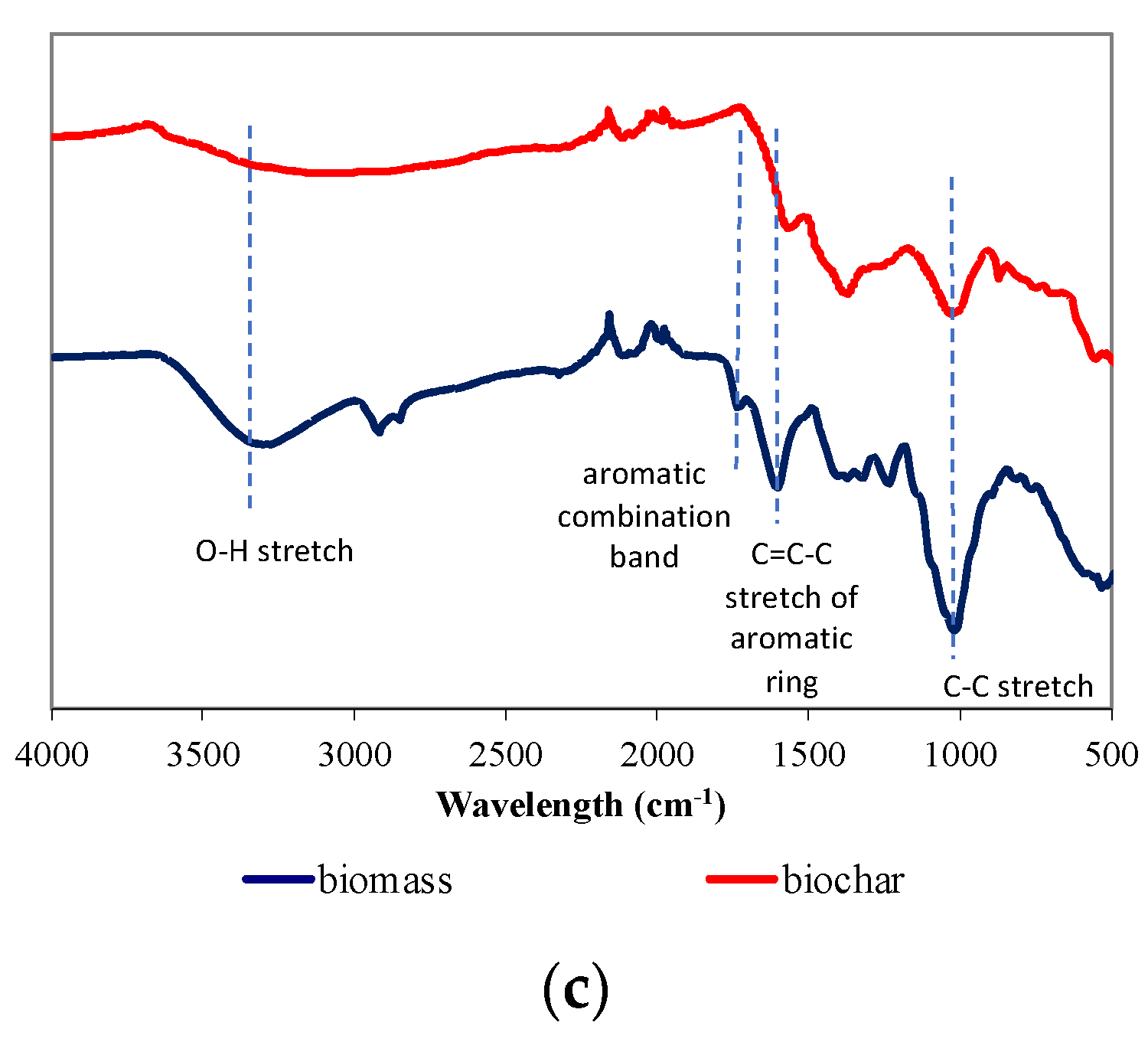
| Moisture (%) | Volatile Matter (%) | Ash (%) | Fixed Carbon (%) | |
|---|---|---|---|---|
| Corn cob | 2.77 | 80.18 | 1.73 | 15.33 |
| Vine rod | 3.90 | 69.33 | 4.06 | 22.71 |
| Sunflower | 2.94 | 72.05 | 8.30 | 16.71 |
| Number | Compound | Area (%) |
|---|---|---|
| 1 | Catechol (C6H6O2) | 4.8 |
| 2 | 9-Octadecenoic acid, (E)- (C18H34O2) | 4.6 |
| 3 | Palmitic acid (C16H32O2) | 4.51 |
| 4 | 2-Hydroxycyclohexane-1-carboxylic acid (C7H12O3) | 3.75 |
| 5 | 9,12-Octadecadienoic acid (Z,Z)- (C18H32O2) | 3.54 |
| 6 | cis-4-Hydroxycyclohexanecarboxylic acid (C7H12O3) | 3.36 |
| 7 | 1,2,3,4-Tetrahydroisoquinolin-6-ol-1-carboxylic acid, 7-Methoxy-1-methyl- (C12H15NO4) | 3.08 |
| 8 | M-cresol (C7H8O) | 2.89 |
| 9 | Pimelic acid (C7H12O4) | 2.72 |
| 10 | 2-hexenoic acid, (E)- (C6H10O2) | 2.65 |
| 11 | Isobutanol (C4H10O) | 2.63 |
| 12 | Furfuryl alcohol (C5H6O2) | 2.59 |
| 13 | Levoglucosan (C6H10O5) | 2.59 |
| 14 | D-(+)-ribono-1,4-lactone (C5H8O5) | 2.55 |
| 15 | Triethylene glycol (C6H14O4) | 2.03 |
| 16 | Phenol (C6H6O) | 1.75 |
| 17 | 2,2-Dimethyl-5-[2-(ethoxymethoxy)-propyl]-[1,3]dioxolane-4-carboxaldehyde (C12H22O5) | 1.71 |
| 18 | Glycerol (C3H8O3) | 1.66 |
| 19 | Syringol (C8H10O3) | 1.5 |
| 20 | Stearic acid (C18H36O2) | 1.39 |
| 21 | 2-Oxooctanoic acid (C8H14O3) | 1.29 |
| 22 | Hydroquinone (C6H6O2) | 1.22 |
| 23 | Oleic acid, (Z)- (C18H34O2) | 1.22 |
| 24 | Sorbic acid (C6H8O2) | 1.21 |
| 25 | Neopentyl alcohol (C5H12O) | 1.05 |
| 26 | Cyclopentene-3-carboxylic acid, 1-hydroxyl- (C7H10O3) | 1.02 |
| Number | Compound | Area (%) |
|---|---|---|
| 1 | Catechol (C6H6O2) | 9.15 |
| 2 | 4-Methylcatechol (C7H8O2) | 5.74 |
| 3 | 3-Pyridinol (C5H8NO8) | 4.13 |
| 4 | Palmitic acid (C16H32O2) | 3.93 |
| 5 | Phenol (C6H6O) | 3.63 |
| 6 | M-cresol (C7H8O8) | 3.16 |
| 7 | 2-Pyrrolidinone (C4H7NO) | 3.04 |
| 8 | Hydroquinone (C6H6O2) | 2.71 |
| 9 | Syringol (C8H10O3) | 2.4 |
| 10 | Triethylene glycol (C6H14O4) | 2.39 |
| 11 | 9,12-Octadecadienoic acid (Z,Z)- (C18H32O2) | 2.33 |
| 12 | Acetamide (C2H5NO) | 2.15 |
| 13 | 2-(3,4-Hydroxyl)ethanamine (C8H11NO2) | 1.88 |
| 14 | Stearic acid (C18H36O2) | 1.87 |
| 15 | .Alpha.-linolenic acid (C18H30O2) | 1.81 |
| 16 | Hexanoic acid (C6H12O2) | 1.68 |
| 17 | 3,5-Dimethylphenol (C8H10O) | 1.55 |
| 18 | P-cresol (C7H8O) | 1.33 |
| 19 | O-cresol (C7H8O8) | 1.13 |
| 20 | Stigmast-5-ene, 3.beta.-(hydroxyl)-, (24S)- (C29H50O) | 1.06 |
| 21 | Hymexazole (C4H5NO2) | 1.01 |
| Number | Compound | Area (%) |
|---|---|---|
| 1 | Isoprimaric acid (C20H20O2) | 9.23 |
| 2 | Abietic acid (C20H30O2) | 4.93 |
| 3 | Palmitic acid (C13H32O2) | 4.86 |
| 4 | Hydroquinone (C6H6O2) | 4.83 |
| 5 | Hexanoic acid, 3-hydroxyl- (C6H12O3) | 4.21 |
| 6 | 9,12-Octadecadienoic acid (Z,Z)- (C18H32O2) | 3.89 |
| 7 | Phenol (C6H6O) | 3.7 |
| 8 | Catechol (C6H6O2) | 3.65 |
| 9 | Alpha-linolenic acid (C18H30O2) | 2.1 |
| 10 | Triethylene glycol (C6H14O4) | 2.02 |
| 11 | Isopimaric acid (C20H30O2) | 2.02 |
| 12 | 3-pyridinol (C5H5NO) | 1.64 |
| 13 | Hexanoic acid (C6H12O2) | 1.53 |
| 14 | Ethylene glycol (C2H6O2) | 1.51 |
| 15 | Glycerol (C3H8O3) | 1.31 |
| 16 | Acetamide (C2H5NO) | 1.18 |
| 17 | M-cresol (C7H8O) | 1.15 |
| 18 | Stearic acid (C18H36O2) | 1.1 |
| 19 | Furfuryl alcohol (C5H6O2) | 1.03 |
| Char Yield at 500 °C (%) | Pyrolytic Gas CV (MJ/kg Biomass) | Bio-Oil Value (AUD/g Biomass) | |
|---|---|---|---|
| Corn cob | 25.06 | 0.786 | 33.23 |
| Vine rod | 41.29 | 0.962 | 7 |
| Sunflower | 36.35 | 1.381 | 18 |
| Criteria 1 (Char) | Criteria 2 (Biogas) | Criteria 3 (Bio-Oil) | Total | |
|---|---|---|---|---|
| Corn cob | 0.61 | 0.57 | 1 | 2.18 |
| Vine rod | 1 | 0.70 | 0.21 | 1.91 |
| Sunflower | 0.88 | 1 | 0.54 | 2.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domazetovska, S.; Strezov, V.; Filkoski, R.V.; Kan, T. Exploring the Potential of Biomass Pyrolysis for Renewable and Sustainable Energy Production: A Comparative Study of Corn Cob, Vine Rod, and Sunflower. Sustainability 2023, 15, 13552. https://doi.org/10.3390/su151813552
Domazetovska S, Strezov V, Filkoski RV, Kan T. Exploring the Potential of Biomass Pyrolysis for Renewable and Sustainable Energy Production: A Comparative Study of Corn Cob, Vine Rod, and Sunflower. Sustainability. 2023; 15(18):13552. https://doi.org/10.3390/su151813552
Chicago/Turabian StyleDomazetovska, Simona, Vladimir Strezov, Risto V. Filkoski, and Tao Kan. 2023. "Exploring the Potential of Biomass Pyrolysis for Renewable and Sustainable Energy Production: A Comparative Study of Corn Cob, Vine Rod, and Sunflower" Sustainability 15, no. 18: 13552. https://doi.org/10.3390/su151813552
APA StyleDomazetovska, S., Strezov, V., Filkoski, R. V., & Kan, T. (2023). Exploring the Potential of Biomass Pyrolysis for Renewable and Sustainable Energy Production: A Comparative Study of Corn Cob, Vine Rod, and Sunflower. Sustainability, 15(18), 13552. https://doi.org/10.3390/su151813552









