Numerical Simulation and Optimization of a Phase-Change Energy Storage Box in a Modular Mobile Thermal Energy Supply System
Abstract
:1. Introduction
2. Validation of Model Validity
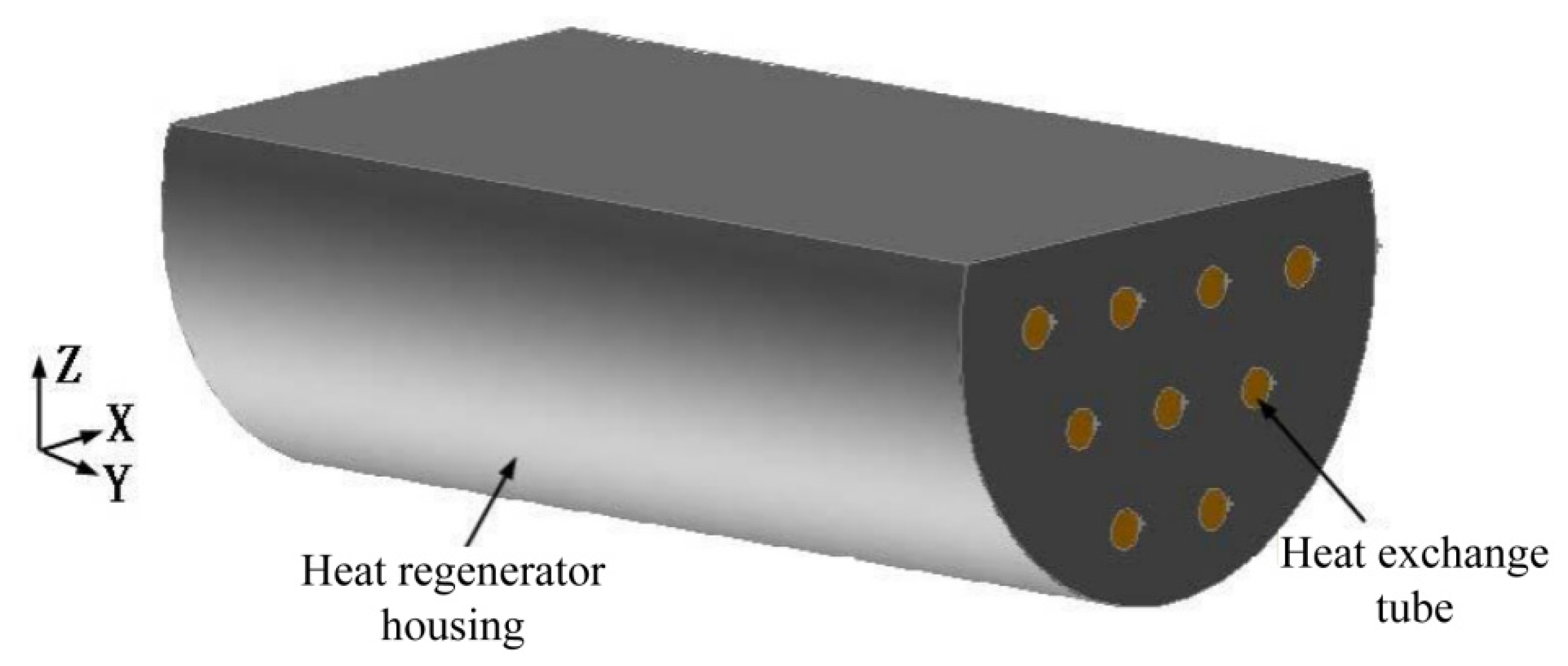
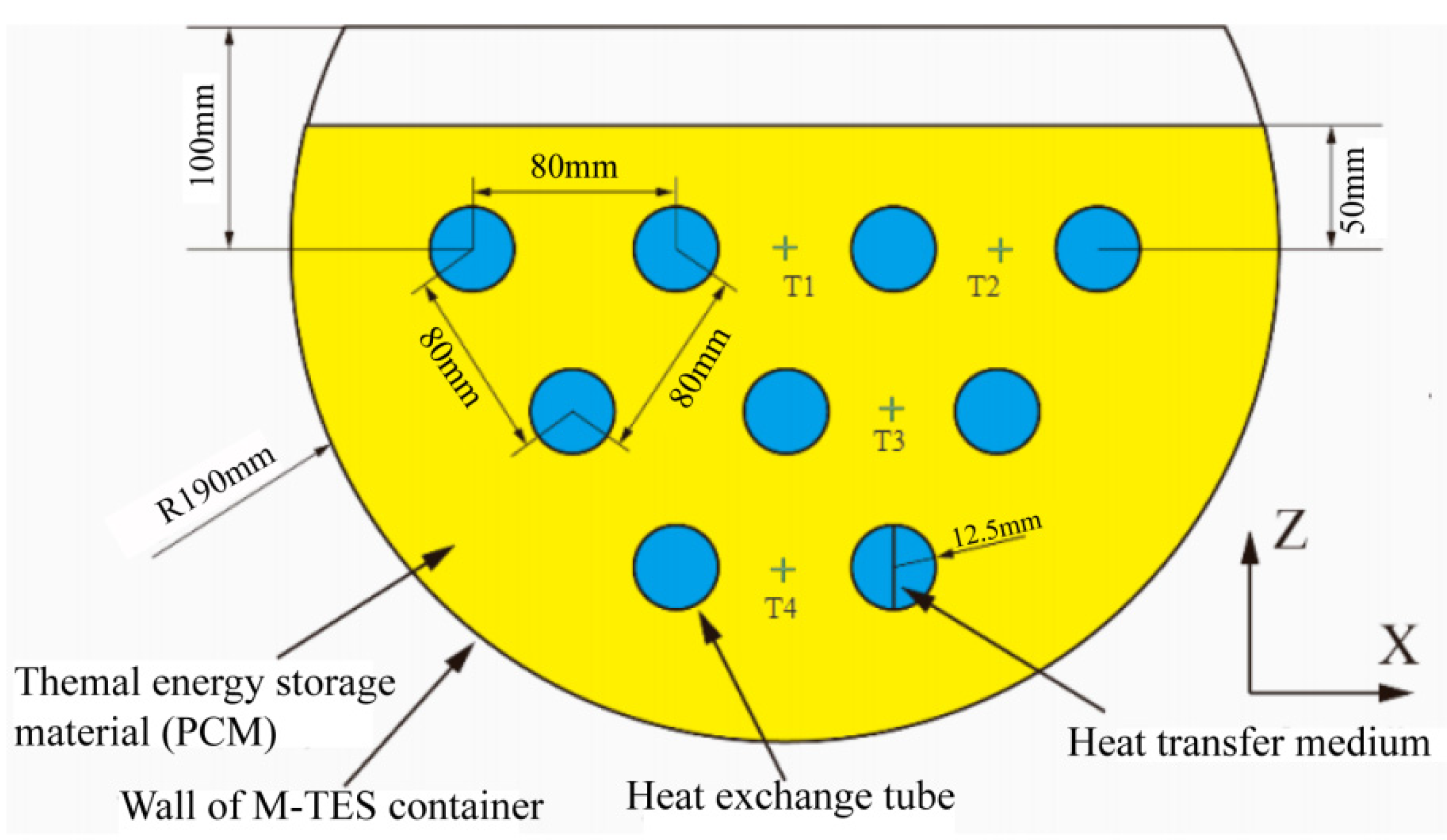
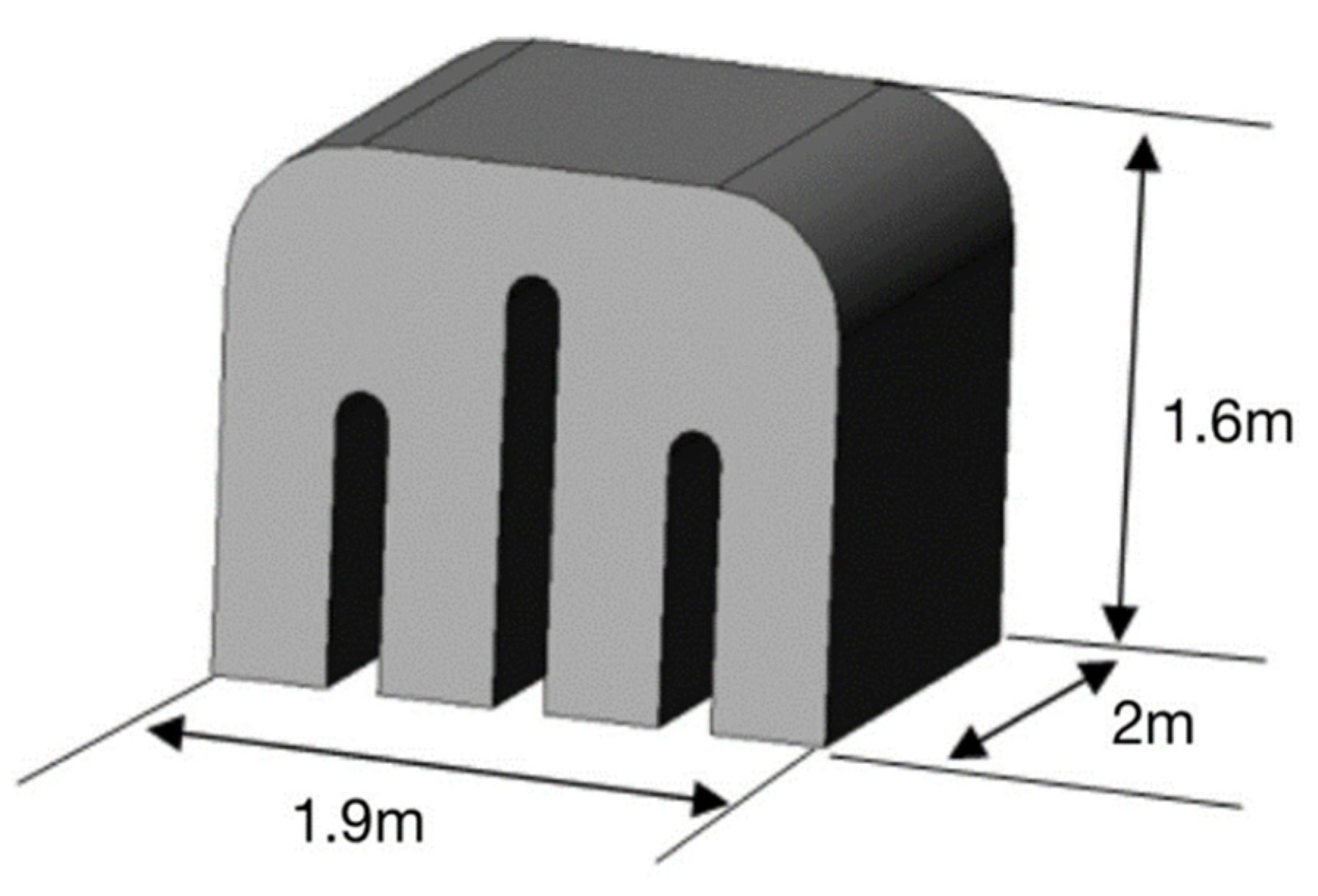
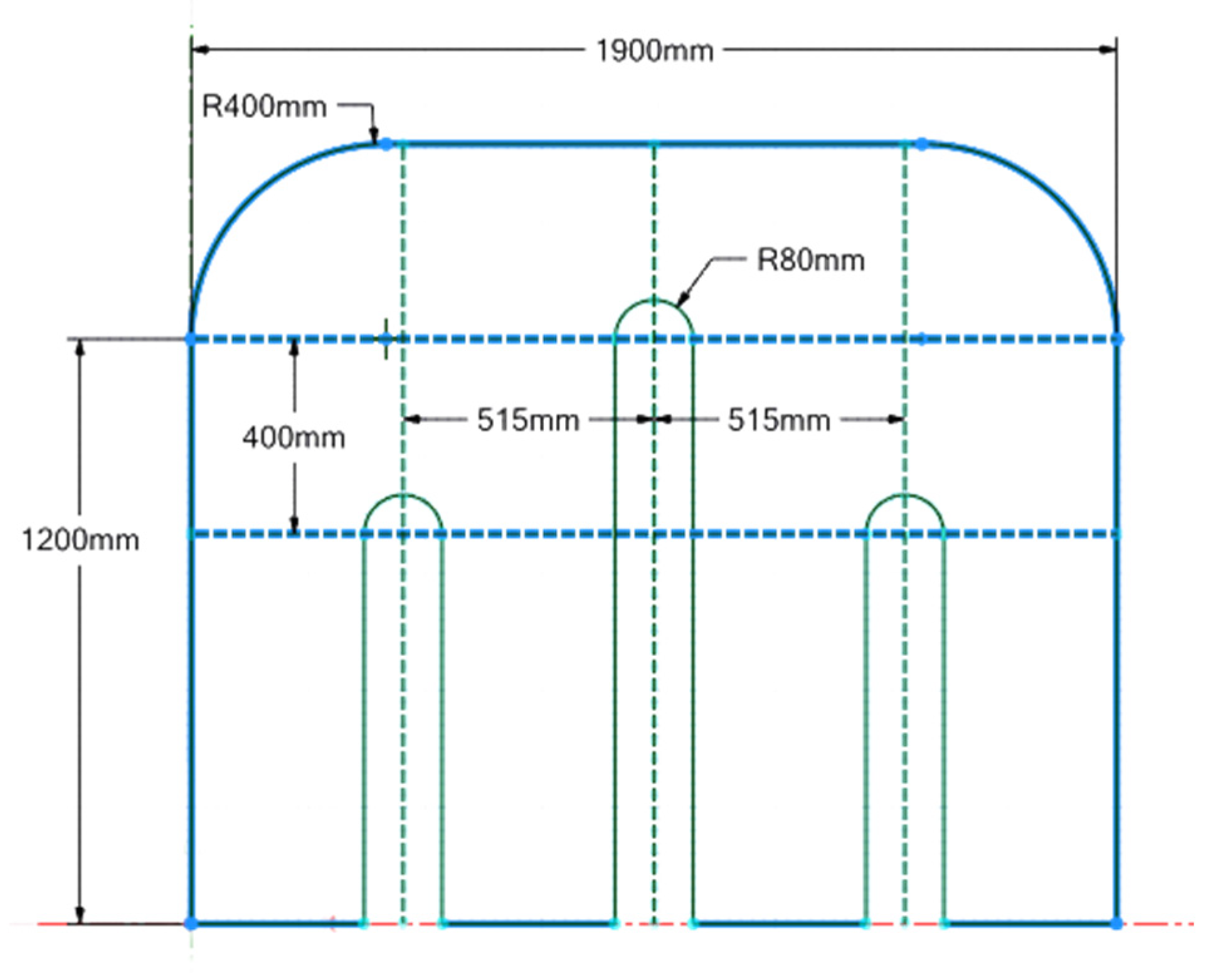
2.1. Physical Model
2.2. Phase-Change Heat Transfer Mathematical Model
2.3. Governing Equation
2.4. Melting/Solidification Modelling
2.5. Model-Related Assumptions
- (1)
- The heat storage material is isotropic;
- (2)
- The heat loss of the heat storage box is neglected;
- (3)
- Compared with the model size, the thickness of the copper tubes inside the heat accumulator is negligible and has little effect on heat transfer, so the tube wall thickness is ignored in the calculations;
- (4)
- The density of the heat storage material is considered to vary with temperature only in the buoyancy force, while the other parameters are used to vary linearly, i.e., the Boussinesq assumption is used;
- (5)
- The radiative heat exchange of the heat storage material is ignored and only the heat exchange due to convection and conduction is calculated.
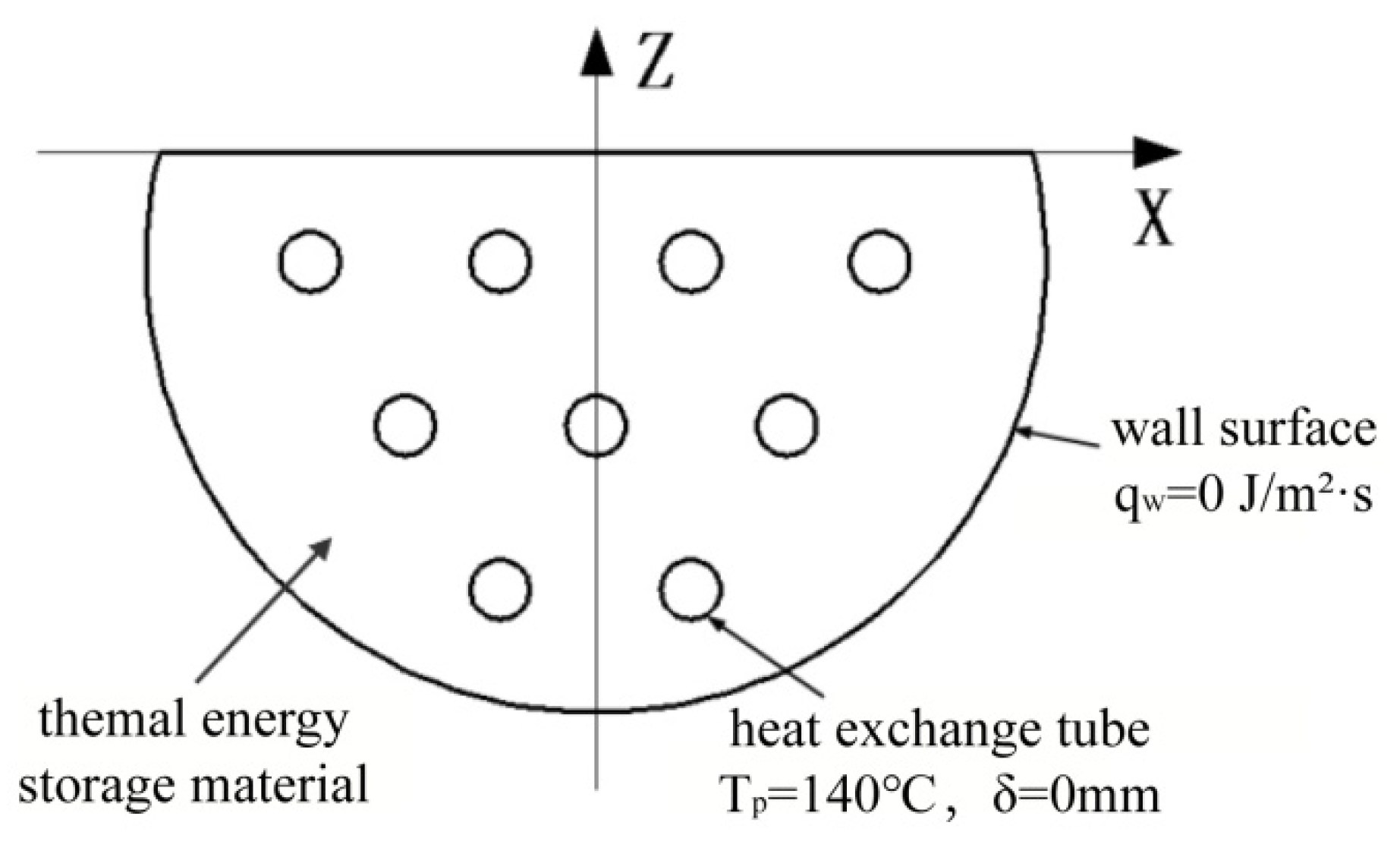
2.6. Calculation Conditions and Calculation Methods
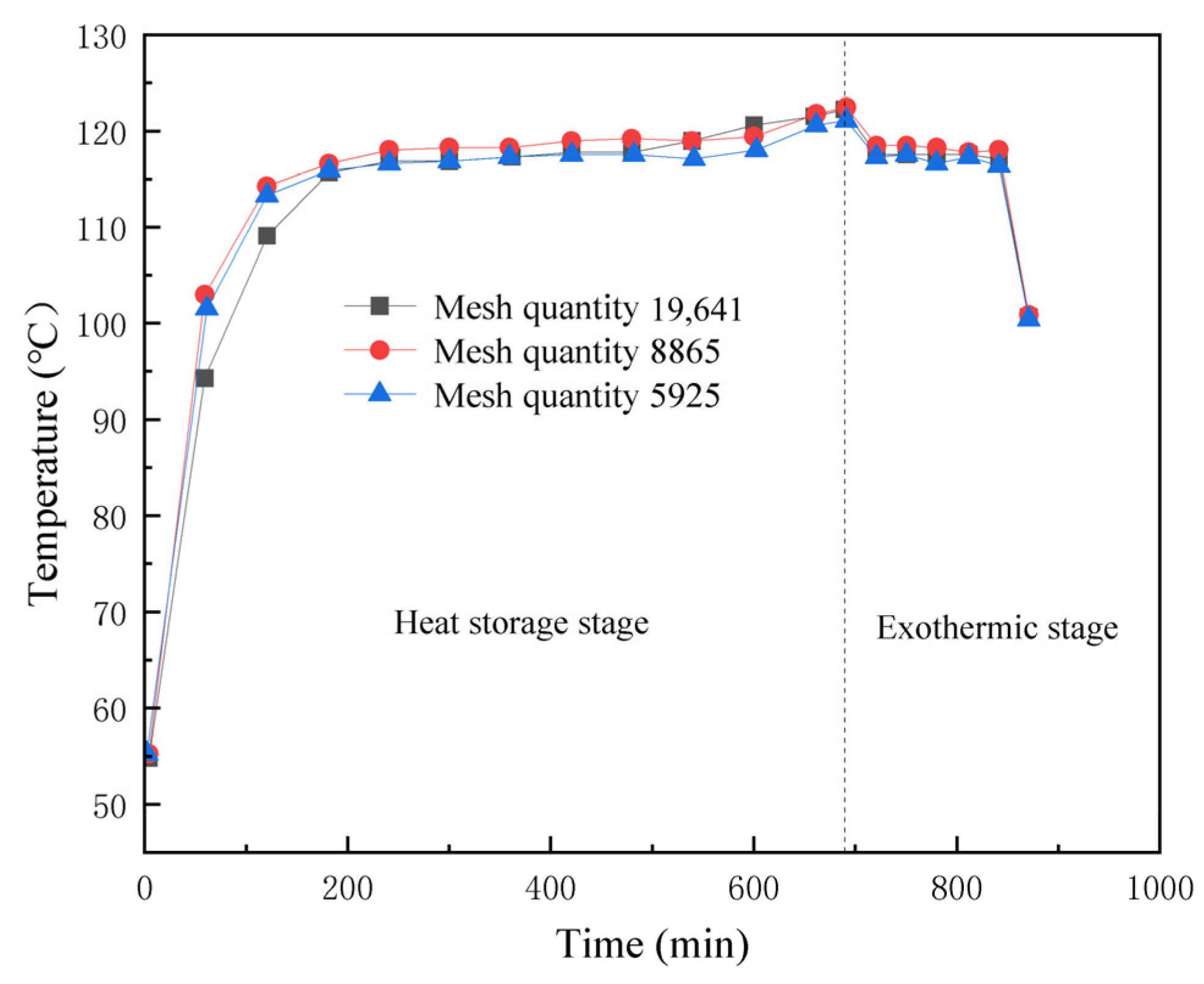
2.7. Mesh Segmentation and Irrelevance Verification
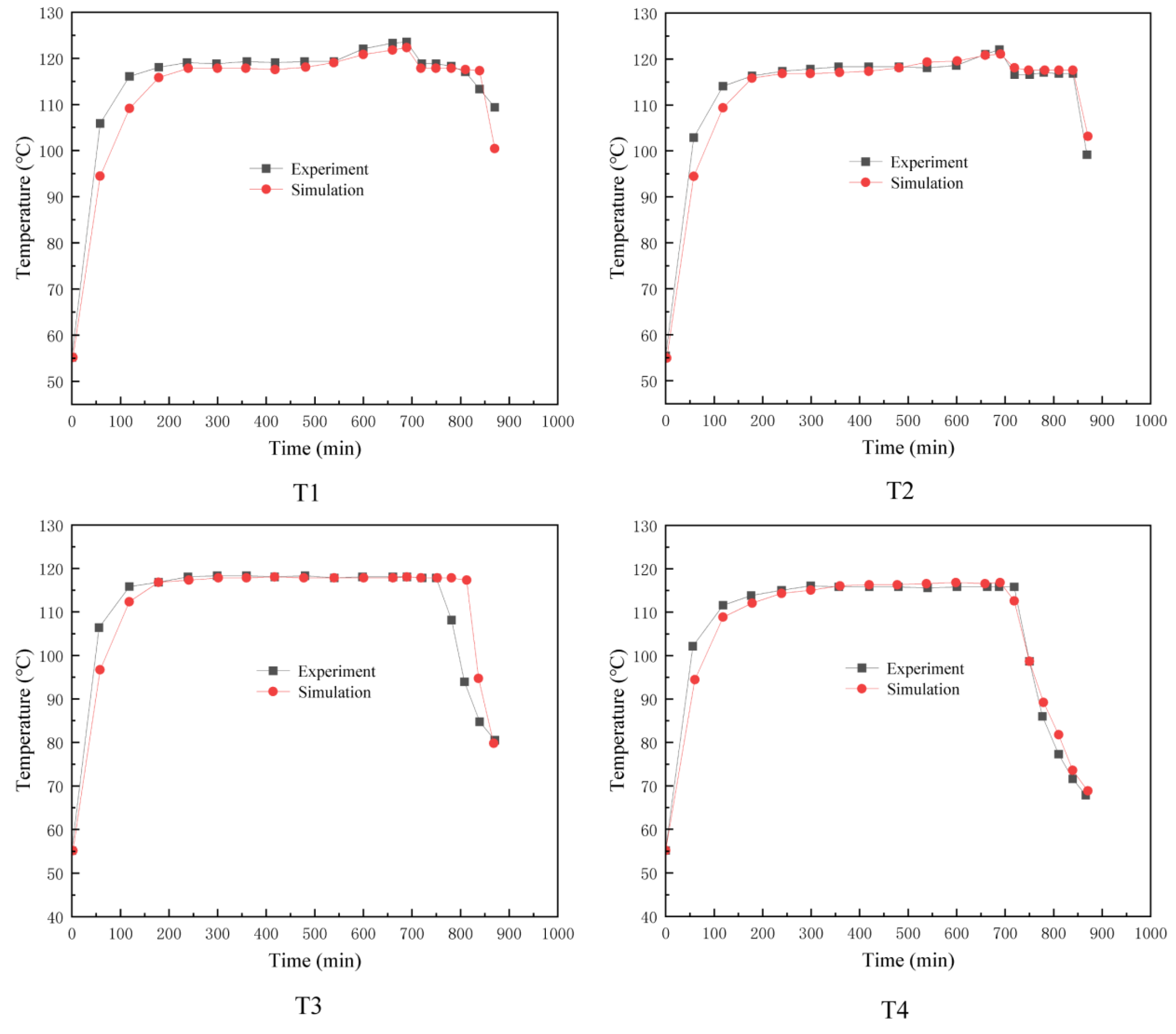
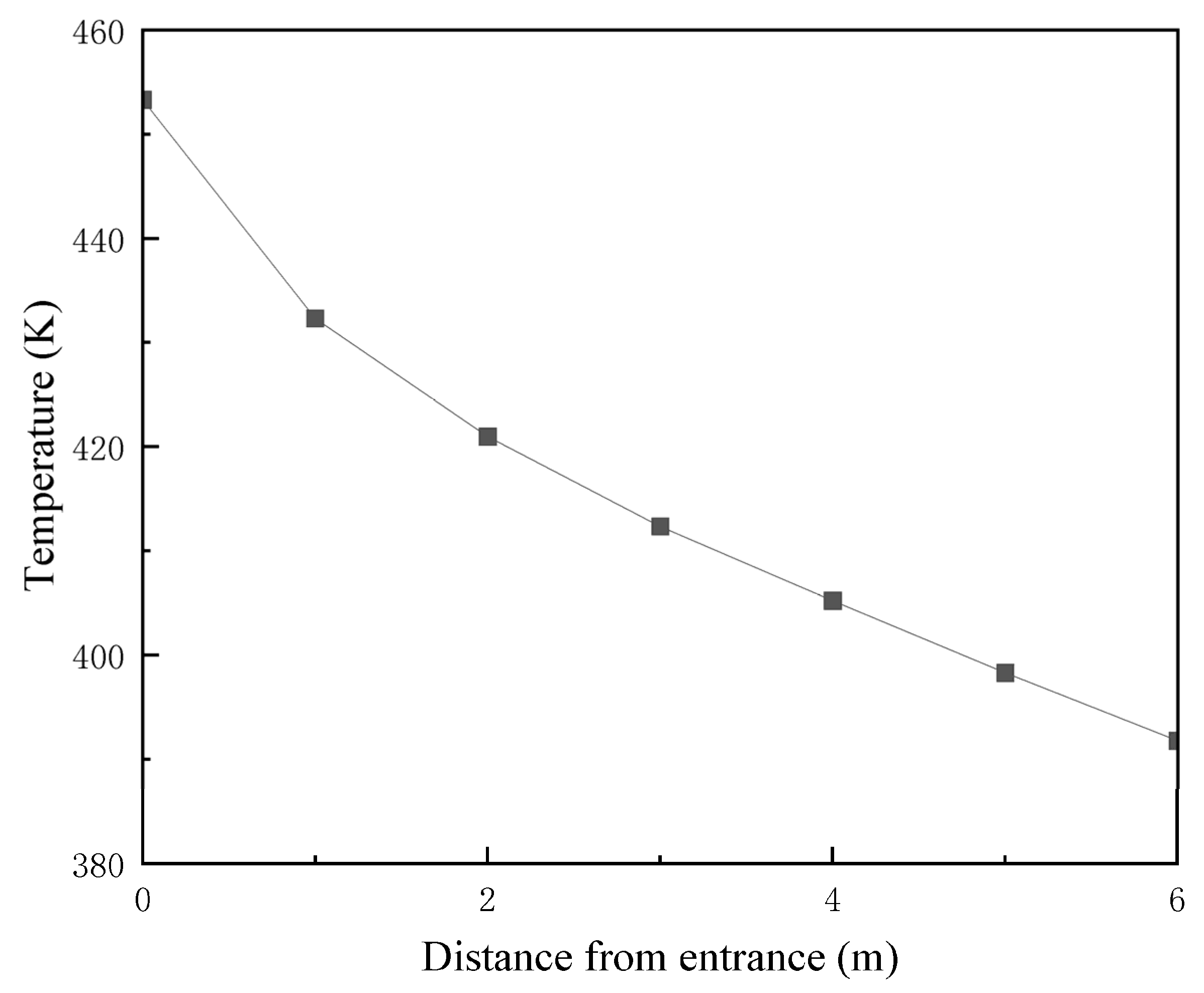
2.8. Model Validation
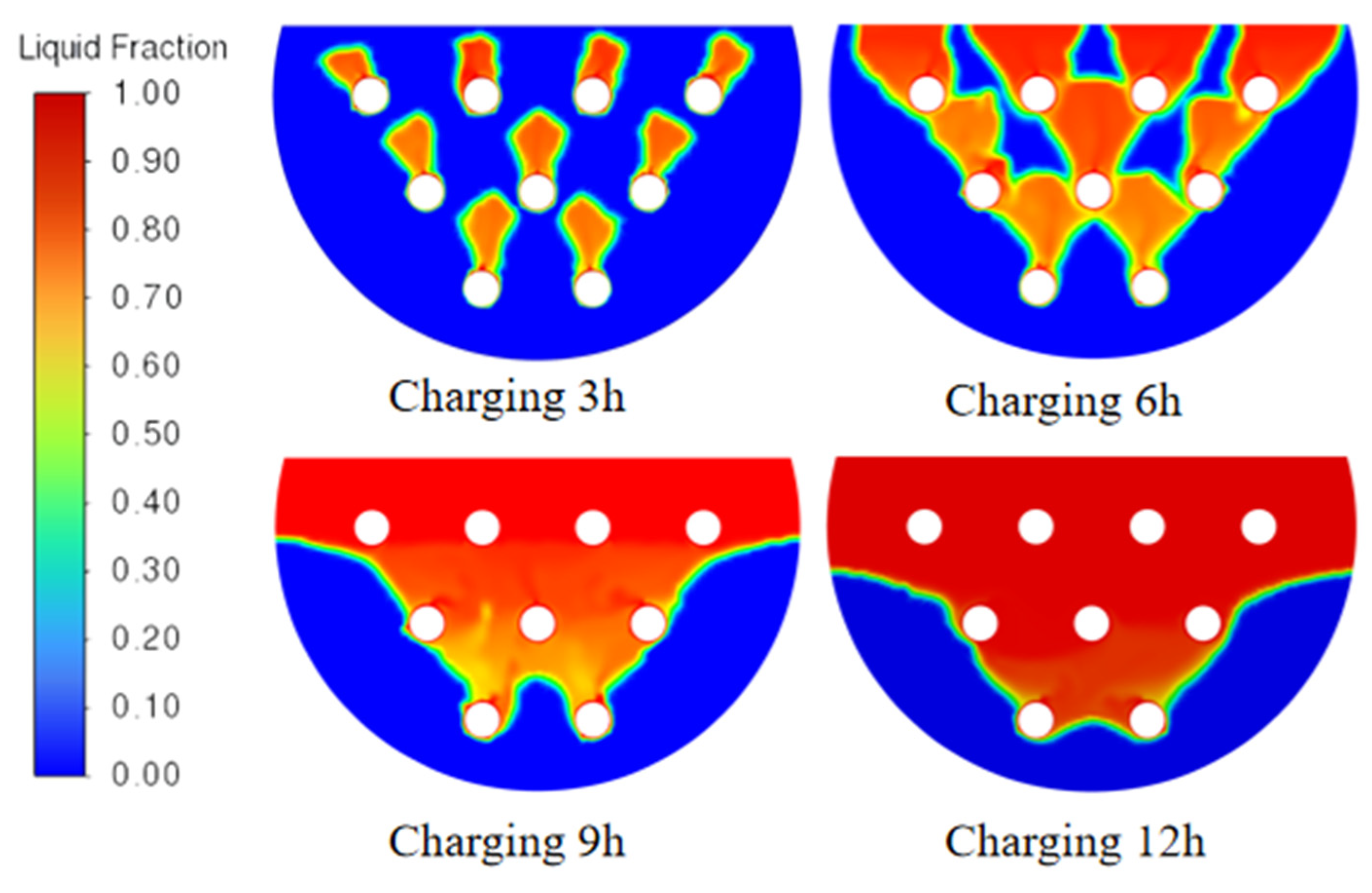
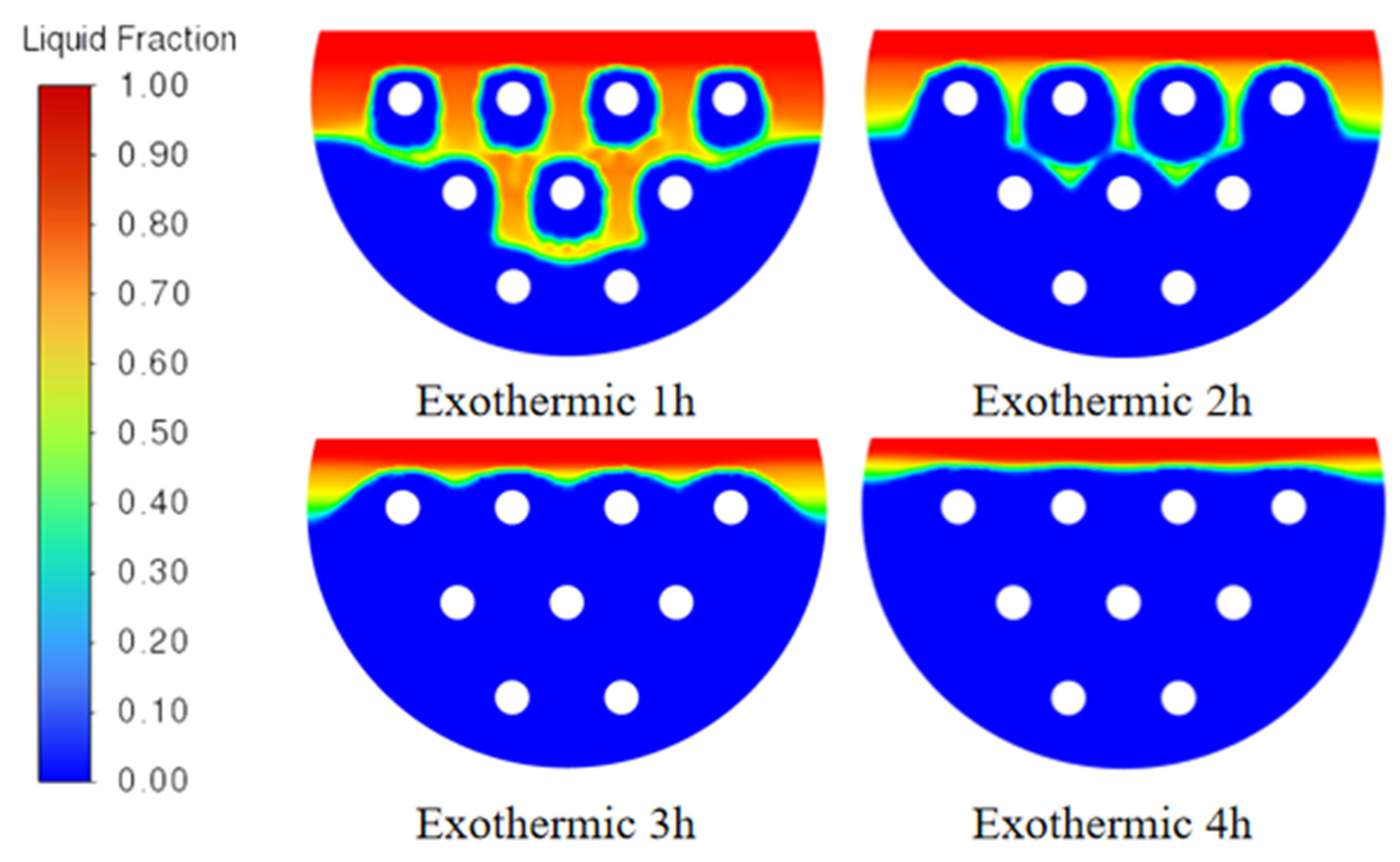
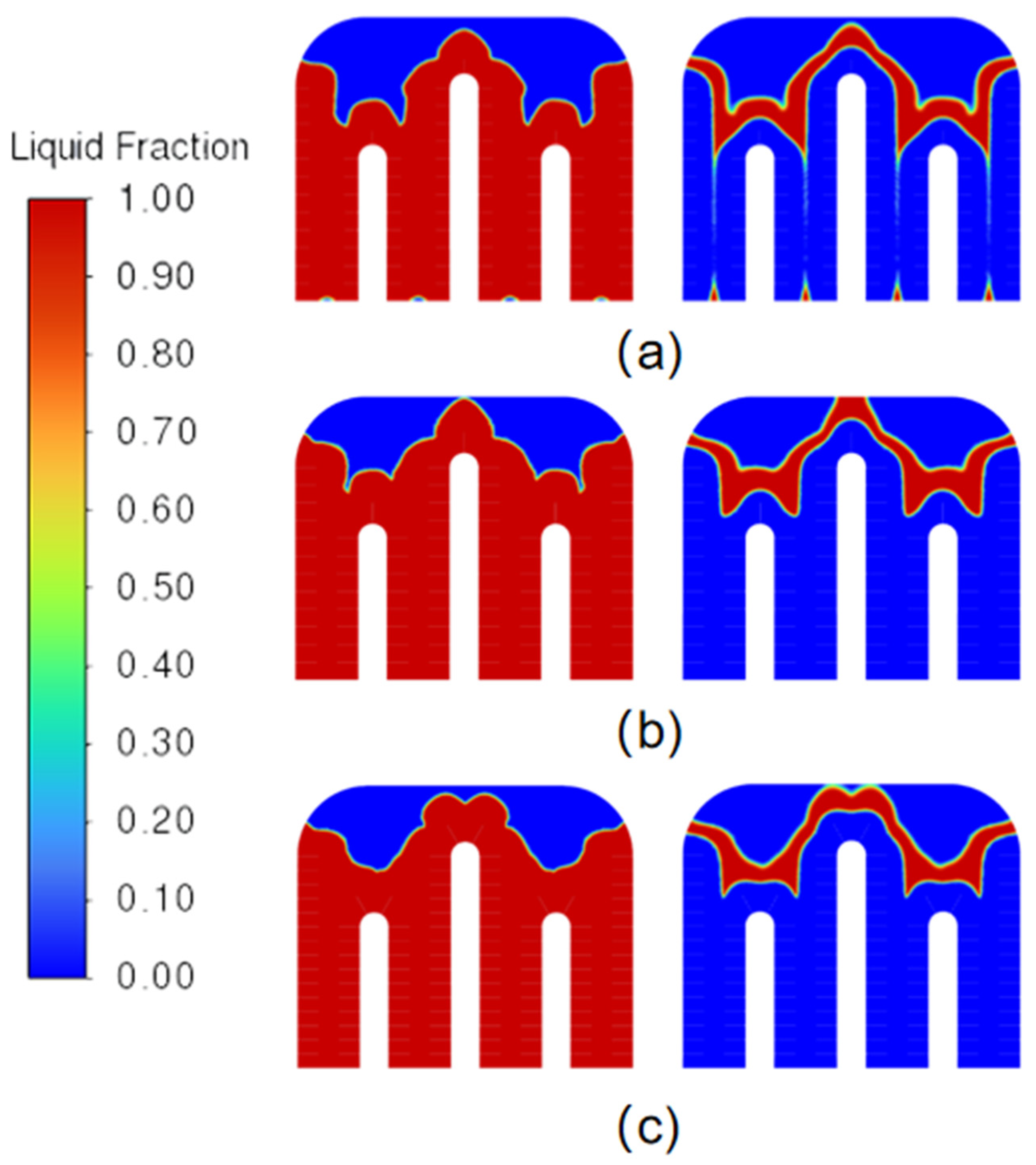
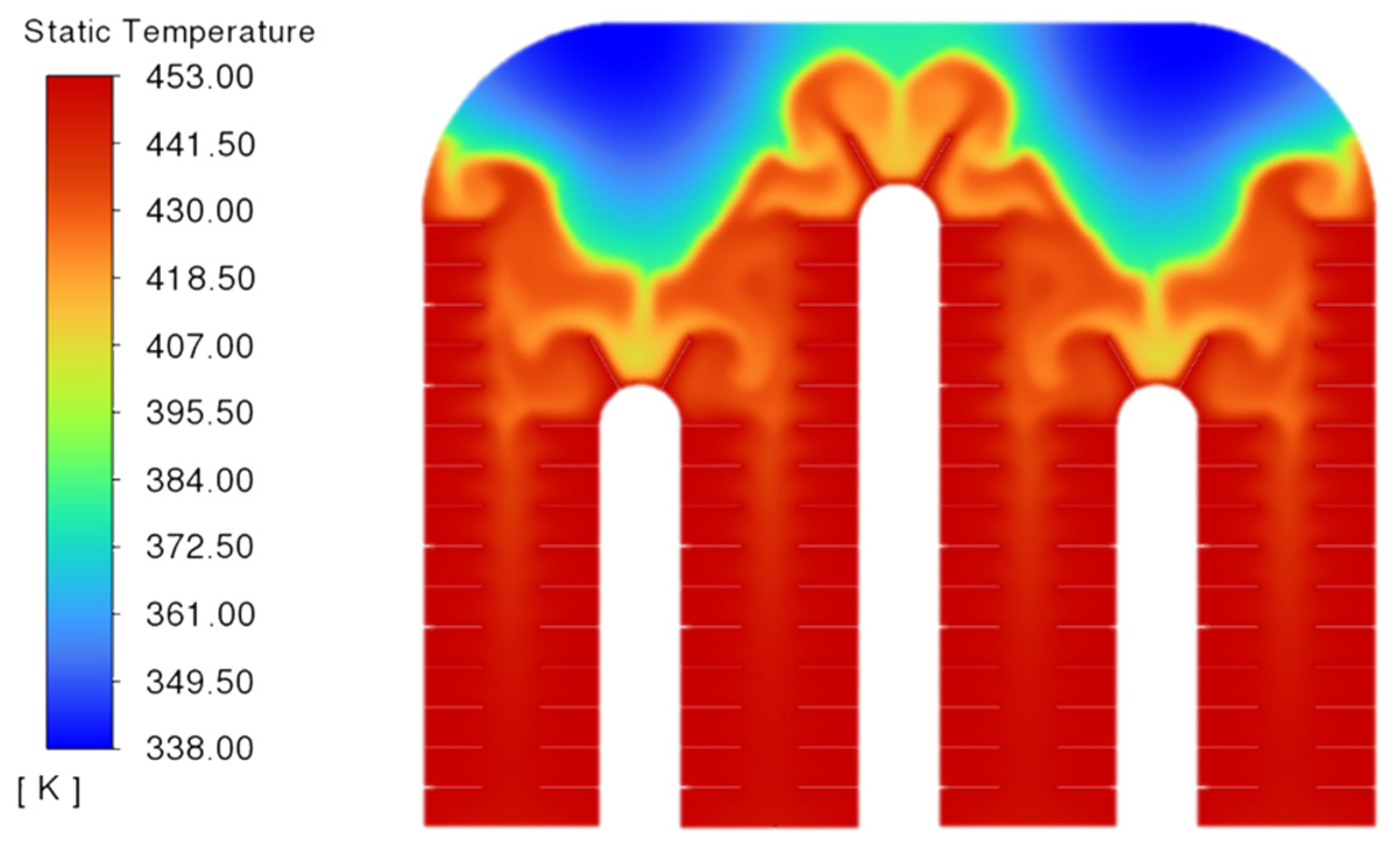
2.9. Analysis of Phase-Transition Processes
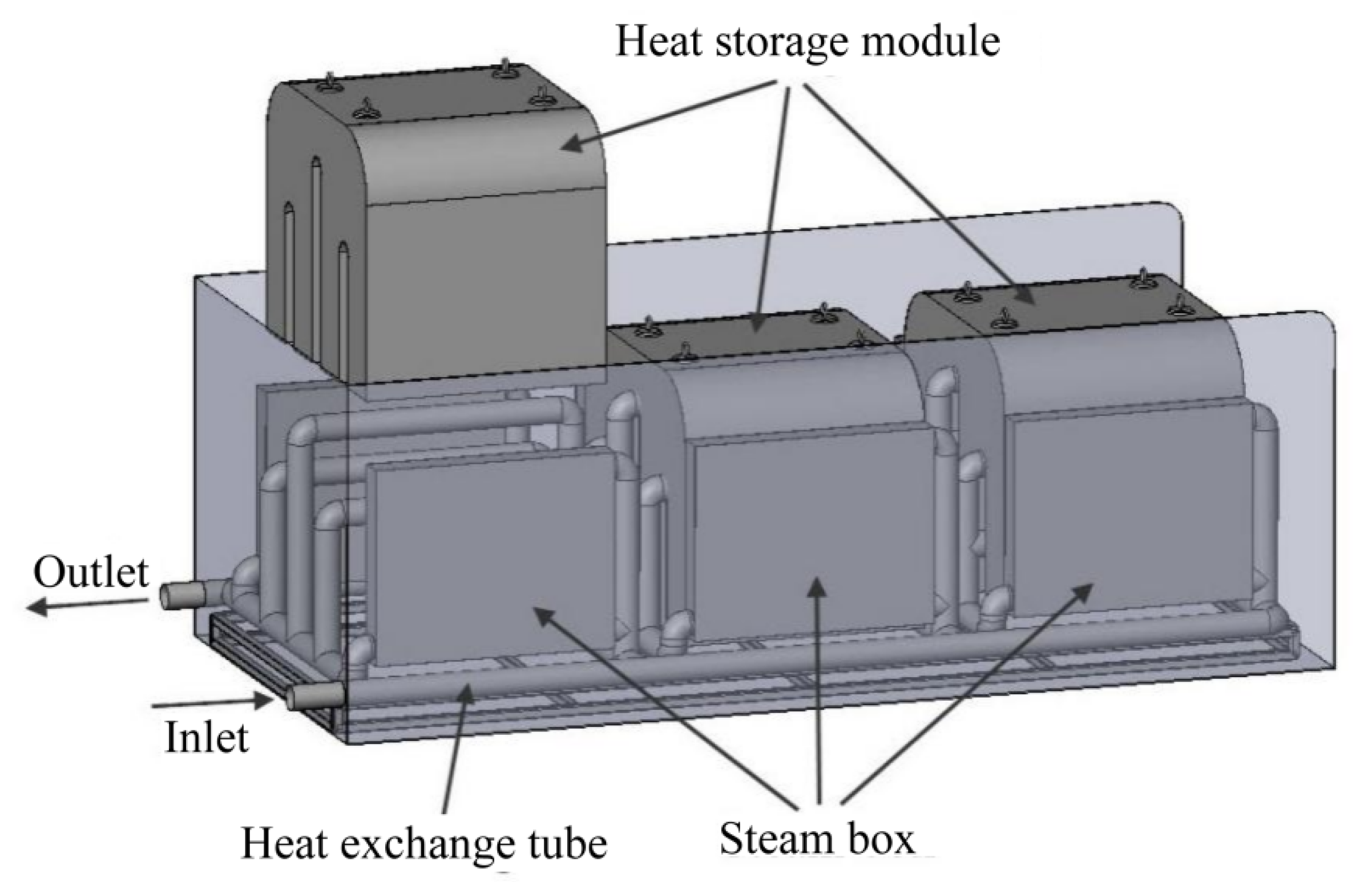
3. Modular Design
4. Modeling and Calculation Methods
4.1. Model Building
4.2. Calculation Method
5. Simulation Results and Discussion
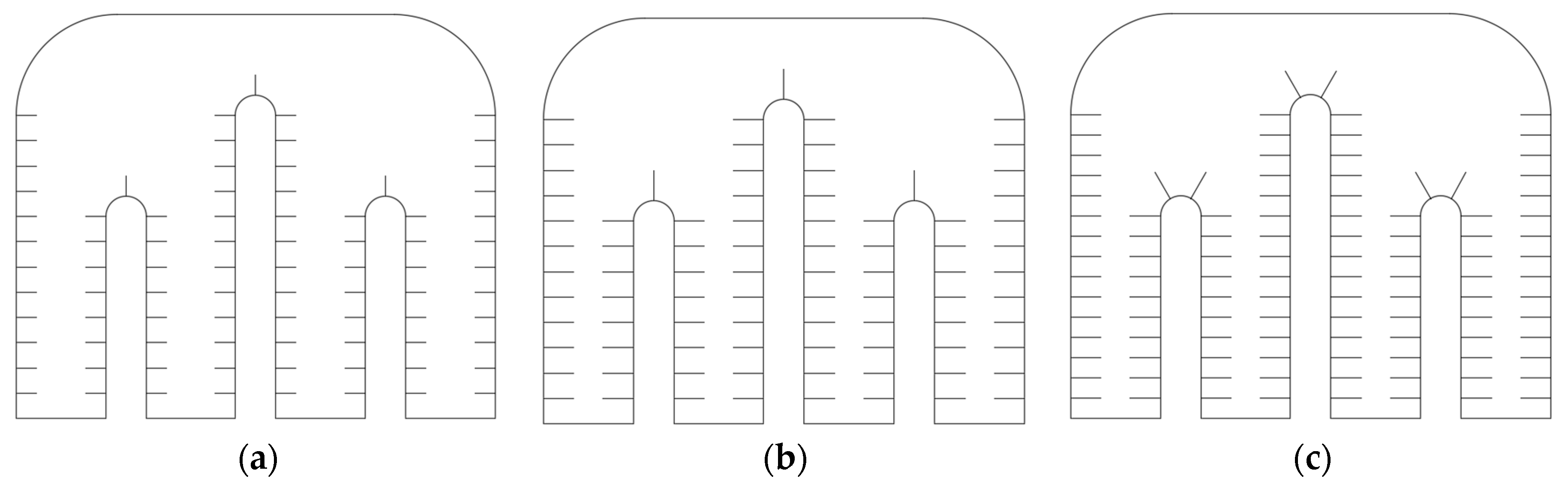
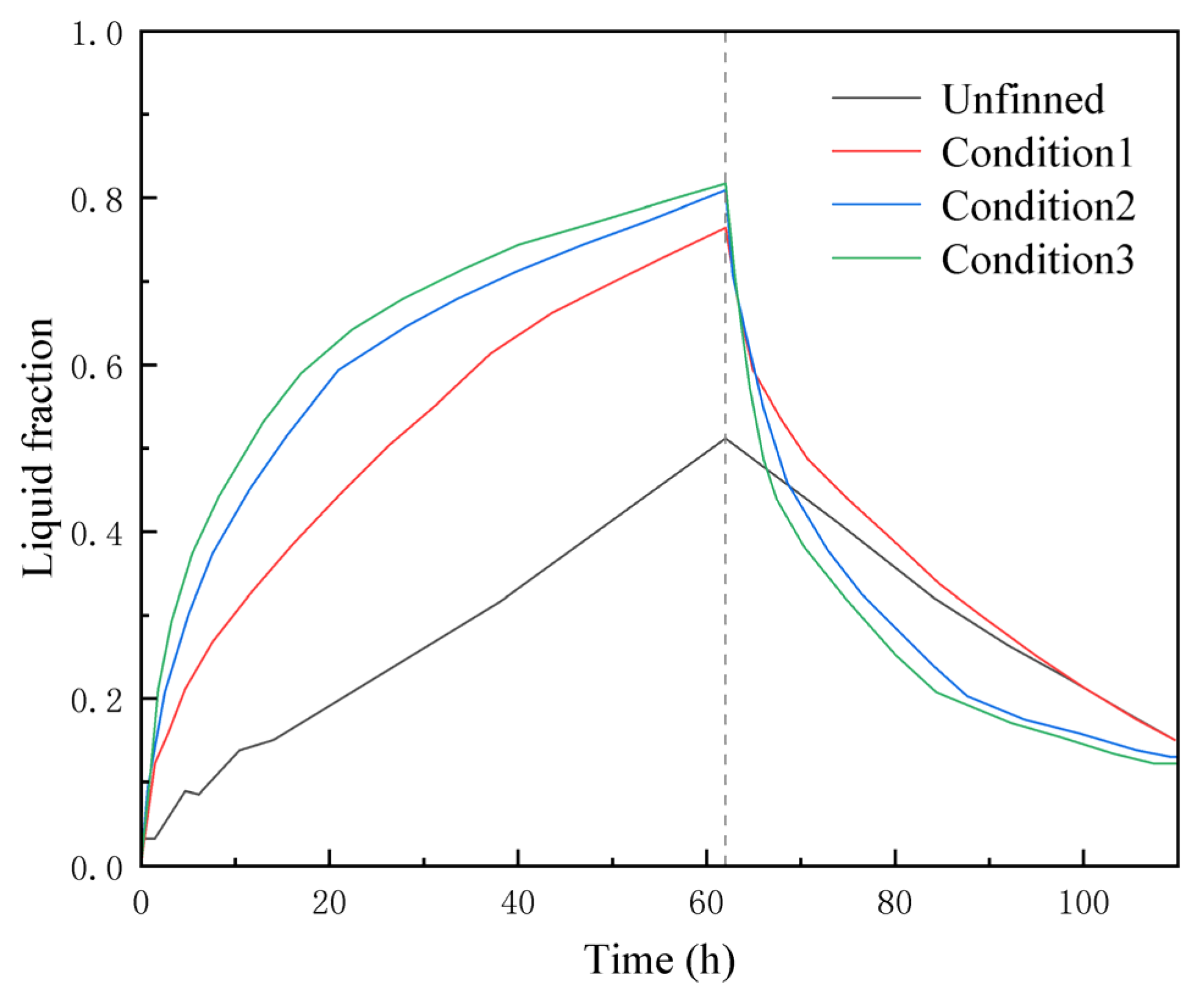
5.1. Simulation Analysis of Different Finning Conditions
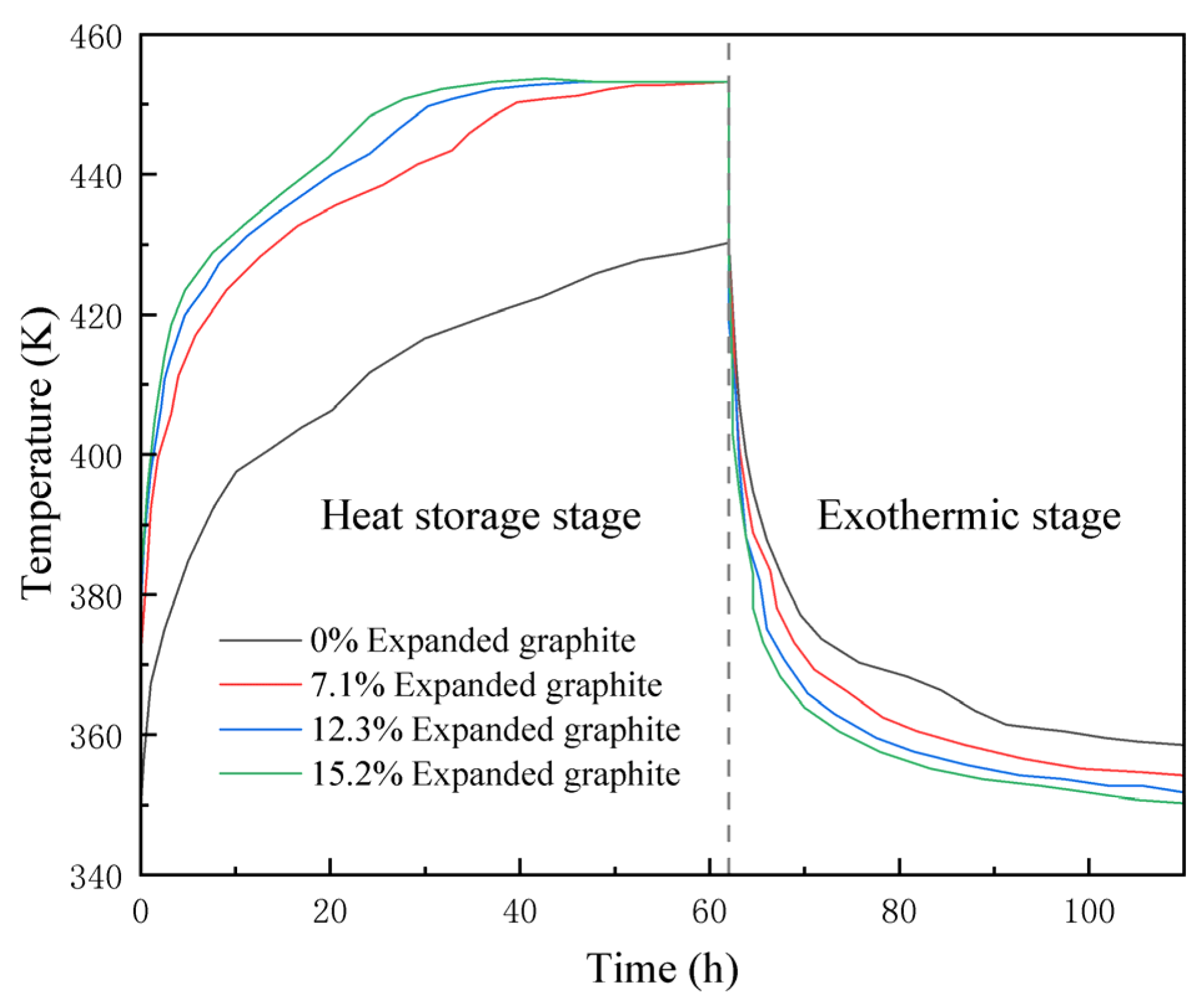
5.2. Comparative Analysis of Composite Materials
6. Conclusions
- (1)
- The proposed new mobile heating system thermal storage box addresses the issue of uneven temperature distribution in traditional thermal storage boxes. The modular design optimizes the arrangement of heat accumulators, reducing the problem of uncoordinated heat storage in the length direction. The modular thermal storage box can be easily installed and uninstalled using a crane, making heat distribution more flexible and efficient.
- (2)
- The original model was optimized by introducing fins and subjected to numerical simulations to obtain model parameters under various operating conditions. The optimal solution resulted in a 30.7% increase in heat storage over 62 h and an 11.2% reduction in exothermic heat release over 48 h when compared to the no-fin condition. In contrast to the significant improvement observed between condition 1 and condition 2, the enhancement seen in condition 3 over condition 2 is less pronounced, accounting for only about half of the former’s improvement. It is noteworthy that continuing to increase the number of fins or their height further diminishes the heat transfer enhancement. This effect is compounded by the fact that excessive fins complicate the design, disrupt the natural flow of the melted phase-change material, increase the nonproductive weight of the container, and reduce the overall efficiency of the box. All these factors contribute to increased operational costs.
- (3)
- The combination of expanded graphite and erythritol in different proportions as a composite heat storage material shows promising results. By adding 12.3% expanded graphite, the complete heat storage time is reduced from 62 h to 26 h, achieving a 50% improvement in heat charging efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, S.; Liu, Q.; Zhao, J.; Jin, G.; Wu, W.; Yan, J.; Li, H.; Jin, H. Mobilized thermal energy storage: Materials, containers and economic evaluation. Energy Convers. Manag. 2018, 177, 315–329. [Google Scholar] [CrossRef]
- Diarce, G.; Gandarias, I.; Campos-Celador, A.; García-Romero, A.; Griesser, U. Eutectic mixtures of sugar alcohols for thermal energy storage in the 50–90 C temperature range. Sol. Energy Mater. Sol. Cells 2015, 134, 215–226. [Google Scholar] [CrossRef]
- Höhlein, S.; König-Haagen, A.; Brüggemann, D. Thermophysical characterization of MgCl2· 6H2O, xylitol and erythritol as phase change materials (PCM) for latent heat thermal energy storage (LHTES). Materials 2017, 10, 444. [Google Scholar] [CrossRef]
- Peiró, G.; Gasia, J.; Miró, L.; Cabeza, L.F. Experimental evaluation at pilot plant scale of multiple PCMs (cascaded) vs. single PCM configuration for thermal energy storage. Renew. Energy 2015, 83, 729–736. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Effect of time on a hierarchical corn skeleton-like composite of CoO@ ZnO as capacitive electrode material for high specific performance supercapacitors. Energies 2018, 11, 3285. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Anil Kumar, Y.; Pallavolu, M.R.; Arbi, H.M.; Alzahmi, S.; Obaidat, I.M. Two-dimensional core-shell structure of cobalt-doped@ MnO2 nanosheets grown on nickel foam as a binder-free battery-type electrode for supercapacitor application. Nanomaterials 2022, 12, 3187. [Google Scholar] [CrossRef]
- Kaizawa, A.; Kamano, H.; Kawai, A.; Jozuka, T.; Senda, T.; Maruoka, N.; Akiyama, T. Thermal and flow behaviors in heat transportation container using phase change material. Energy Convers. Manag. 2008, 49, 698–706. [Google Scholar] [CrossRef]
- Wang, W.; Guo, S.; Li, H.; Yan, J.; Zhao, J.; Li, X.; Ding, J. Experimental study on the direct/indirect contact energy storage container in mobilized thermal energy system (M-TES). Appl. Energy 2014, 119, 181–189. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Guo, S.; He, S.; Ding, J.; Yan, J.; Yang, J. Numerical simulation study on discharging process of the direct-contact phase change energy storage system. Appl. Energy 2015, 150, 61–68. [Google Scholar] [CrossRef]
- Kang, Z.; Zhou, W.; Qiu, K.; Wang, C.; Qin, Z.; Zhang, B.; Yao, Q. Numerical Simulation of an Indirect Contact Mobilized Thermal Energy Storage Container with Different Tube Bundle Layout and Fin Structure. Sustainability 2023, 15, 5511. [Google Scholar] [CrossRef]
- Li, H.; Wang, W.; Yan, J.; Dahlquist, E. Economic assessment of the mobilized thermal energy storage (M-TES) system for distributed heat supply. Appl. Energy 2013, 104, 178–186. [Google Scholar] [CrossRef]
- Krönauer, A.; Lävemann, E.; Brückner, S.; Hauer, A. Mobile sorption heat storage in industrial waste heat recovery. Energy Procedia 2015, 73, 272–280. [Google Scholar] [CrossRef]
- Kuta, M. Mobilized thermal energy storage (M-TES) system design for cooperation with geothermal energy sources. Appl. Energy 2023, 332, 120567. [Google Scholar] [CrossRef]
- Nekoonam, S.; Roshandel, R. Modeling and optimization of a multiple (cascading) phase change material solar storage system. Therm. Sci. Eng. Prog. 2021, 23, 100873. [Google Scholar] [CrossRef]
- Elfeky, K.; Li, X.; Ahmed, N.; Lu, L.; Wang, Q. Optimization of thermal performance in thermocline tank thermal energy storage system with the multilayered PCM (s) for CSP tower plants. Appl. Energy 2019, 243, 175–190. [Google Scholar] [CrossRef]
- Han, D.; Lougou, B.G.; Xu, Y.; Shuai, Y.; Huang, X. Thermal properties characterization of chloride salts/nanoparticles composite phase change material for high-temperature thermal energy storage. Appl. Energy 2020, 264, 114674. [Google Scholar] [CrossRef]
- Fragnito, A.; Bianco, N.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Experimental and numerical analysis of a phase change material-based shell-and-tube heat exchanger for cold thermal energy storage. J. Energy Storage 2022, 56, 105975. [Google Scholar] [CrossRef]
- Bianco, N.; Caliano, M.; Fragnito, A.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Thermal analysis of micro-encapsulated phase change material (MEPCM)-based units integrated into a commercial water tank for cold thermal energy storage. Energy 2023, 266, 126479. [Google Scholar] [CrossRef]
- Pourhemmati, S.; Hossainpour, S. Thermal improvement of the vertical plate-fin heat sink by variable fin thickness pattern and utilizing phase change material: A numerical investigation. J. Energy Storage 2023, 59, 106480. [Google Scholar] [CrossRef]
- Kheirabadi, A.C.; Groulx, D. Simulating phase change heat transfer using comsol and fluent: Effect of the mushy-zone constant. Comput. Therm. Sci. Int. J. 2015, 7, 427–440. [Google Scholar] [CrossRef]
- Shamsundar, N.; Sparrow, E. Analysis of multidimensional conduction phase change via the enthalpy model. J. Heat Trans. Aug. 1975, 97, 333–340. [Google Scholar] [CrossRef]
- Huang, R.; Wu, H.; Cheng, P. A new lattice Boltzmann model for solid–liquid phase change. Int. J. Heat Mass Transf. 2013, 59, 295–301. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Kleijn, C.R.; Richardson, I.M. Sensitivity of numerical predictions to the permeability coefficient in simulations of melting and solidification using the enthalpy-porosity method. Energies 2019, 12, 4360. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, J.; Li, X.; Wang, W.; Yan, J. Experimental study on waste heat recovery with an indirect mobilized thermal energy storage system. In Proceedings of the International Conference on Applied Energy, Perugia, Italy, 16–18 May 2011. [Google Scholar]
- Guo, S.; Zhao, J.; Wang, W.; Yan, J.; Jin, G.; Zhang, Z.; Gu, J.; Niu, Y. Numerical study of the improvement of an indirect contact mobilized thermal energy storage container. Appl. Energy 2016, 161, 476–486. [Google Scholar] [CrossRef]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Oya, T.; Nomura, T.; Okinaka, N.; Akiyama, T. Phase change composite based on porous nickel and erythritol. Appl. Therm. Eng. 2012, 40, 373–377. [Google Scholar] [CrossRef]
- Oya, T.; Nomura, T.; Tsubota, M.; Okinaka, N.; Akiyama, T. Thermal conductivity enhancement of erythritol as PCM by using graphite and nickel particles. Appl. Therm. Eng. 2013, 61, 825–828. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, J.; An, Q.; Zhao, D.; Meng, F.; Liu, X. Experiments on thermal performance of erythritol/expanded graphite in a direct contact thermal energy storage container. Appl. Therm. Eng. 2017, 113, 858–866. [Google Scholar] [CrossRef]

| Thermophysical Property Parameters | Numerical Value |
|---|---|
| Density | 1480 (20 °C), 1300 (140 °C) |
| Specific heat | 1.35 (20 °C), 2.74 (140 °C) |
| Latent heat | 339 |
| Phase-transition temperature | 117.7 |
| Viscosity | 0.02895 (20 °C), 0.01602 (140 °C) |
| Thermal conductivity | 0.732 (20 °C), 0.326 (140 °C) |
| Condition | Height of Fins (cm) | Distance between Fins (cm) | Number of Fins |
|---|---|---|---|
| 1 | 8 | 10 | 83 |
| 2 | 12 | 10 | 83 |
| 3 | 12 | 8 | 106 |
| PCM | Melting Enthalpy (kJ/kg) | Phase-Transition Temperature (°C) | Thermal Conductivity (W/m·K) | Density (kg/m3) | Specific Heat (KJ/kg) |
|---|---|---|---|---|---|
| Erythritol | 339 | 117.7 | 0.326 | 120 | 2.71 [29] |
| Expanded Graphite Volume Share | Heat Storage 62 h Liquid Phase Ratio | Complete Heat Storage Time | 48-h Liquid Phase Ratio | Liquid Phase Ratio Reduced by 75% of the Time |
|---|---|---|---|---|
| 7.1 | 1 | 33.5 | 14.2 | 21.25 |
| 12.3 | 1 | 26 | 8.2 | 14.5 |
| 15.1 | 1 | 22 | 4.6 | 11.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Z.; Tan, R.; Zhou, W.; Qin, Z.; Liu, S. Numerical Simulation and Optimization of a Phase-Change Energy Storage Box in a Modular Mobile Thermal Energy Supply System. Sustainability 2023, 15, 13886. https://doi.org/10.3390/su151813886
Kang Z, Tan R, Zhou W, Qin Z, Liu S. Numerical Simulation and Optimization of a Phase-Change Energy Storage Box in a Modular Mobile Thermal Energy Supply System. Sustainability. 2023; 15(18):13886. https://doi.org/10.3390/su151813886
Chicago/Turabian StyleKang, Zhangyang, Rufei Tan, Wu Zhou, Zhaolong Qin, and Sen Liu. 2023. "Numerical Simulation and Optimization of a Phase-Change Energy Storage Box in a Modular Mobile Thermal Energy Supply System" Sustainability 15, no. 18: 13886. https://doi.org/10.3390/su151813886





