Present Situation and Research Progress of Comprehensive Utilization of Antimony Tailings and Smelting Slag
Abstract
:1. Introduction
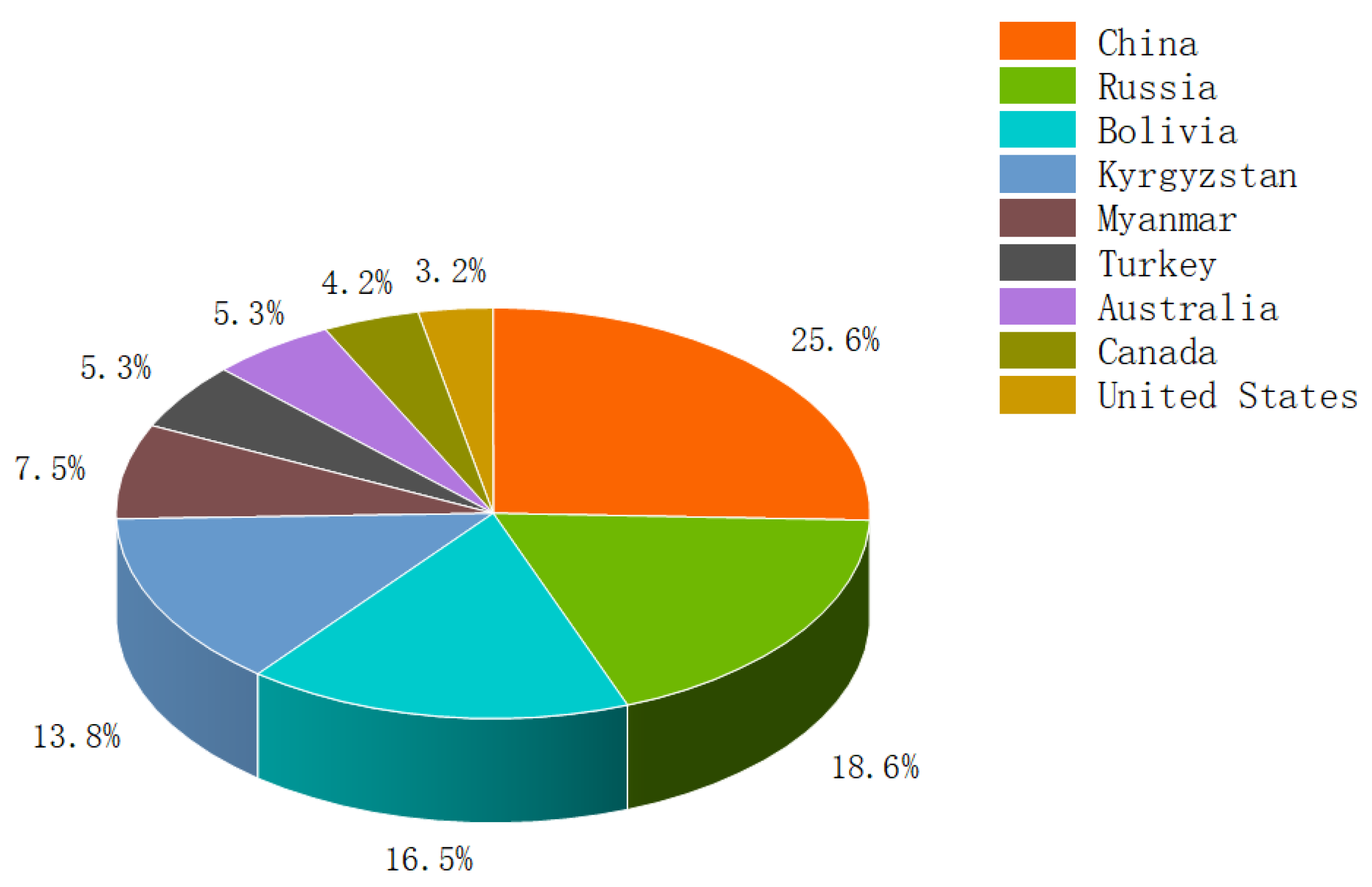
2. Pollution Status and Problems
3. Comprehensive Utilization Status of Antimony Tailings
3.1. Recovery of Antimony Tailings Resources
3.2. Using Antimony Tailings to Prepare Building Materials and Underground Filling Materials
3.2.1. Using Antimony Tailings to Prepare Raw Materials for Building Materials
3.2.2. Using Antimony Tailings as Underground Filling Materials
4. Comprehensive Utilization Status of Smelting Slag
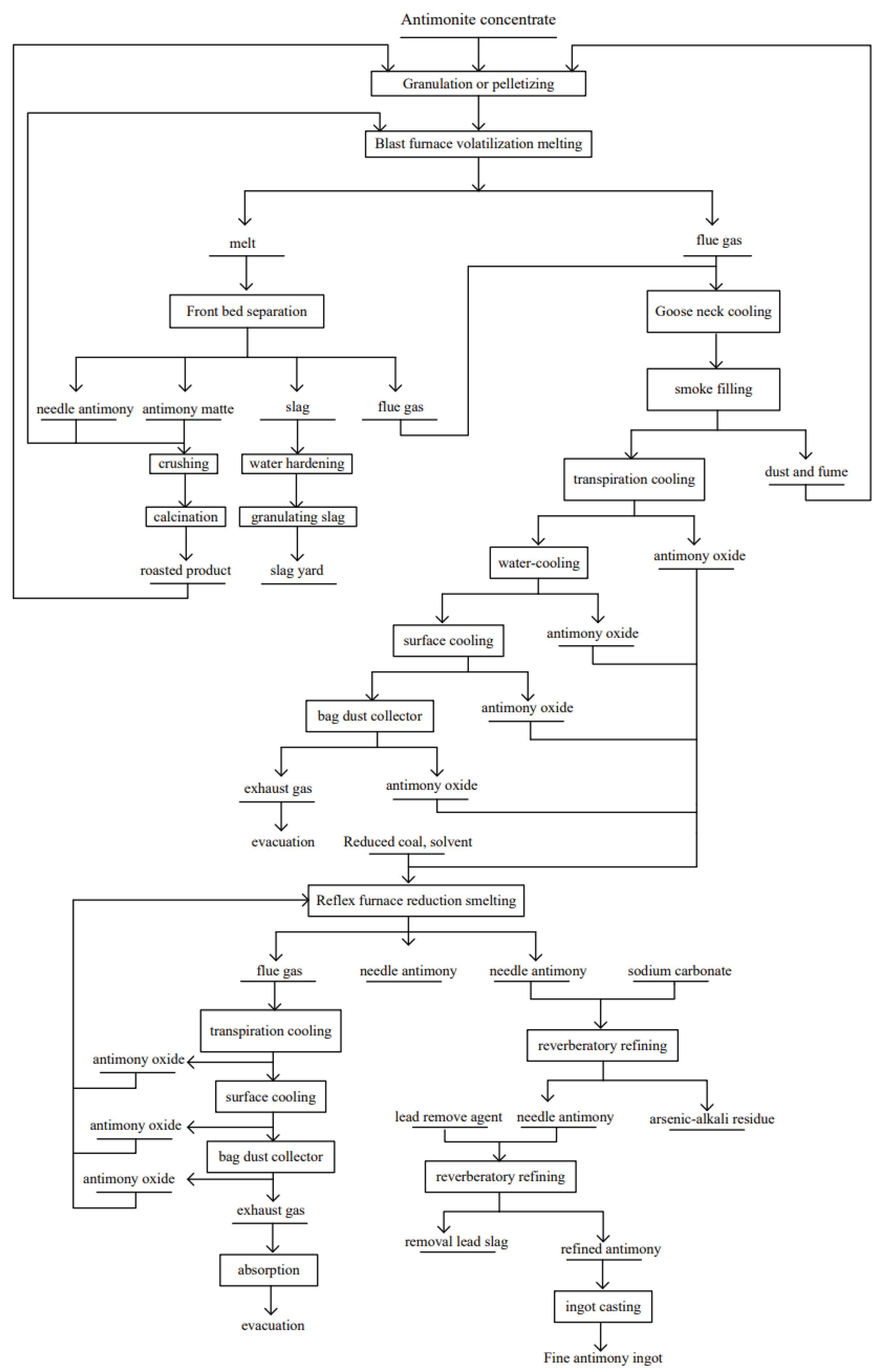
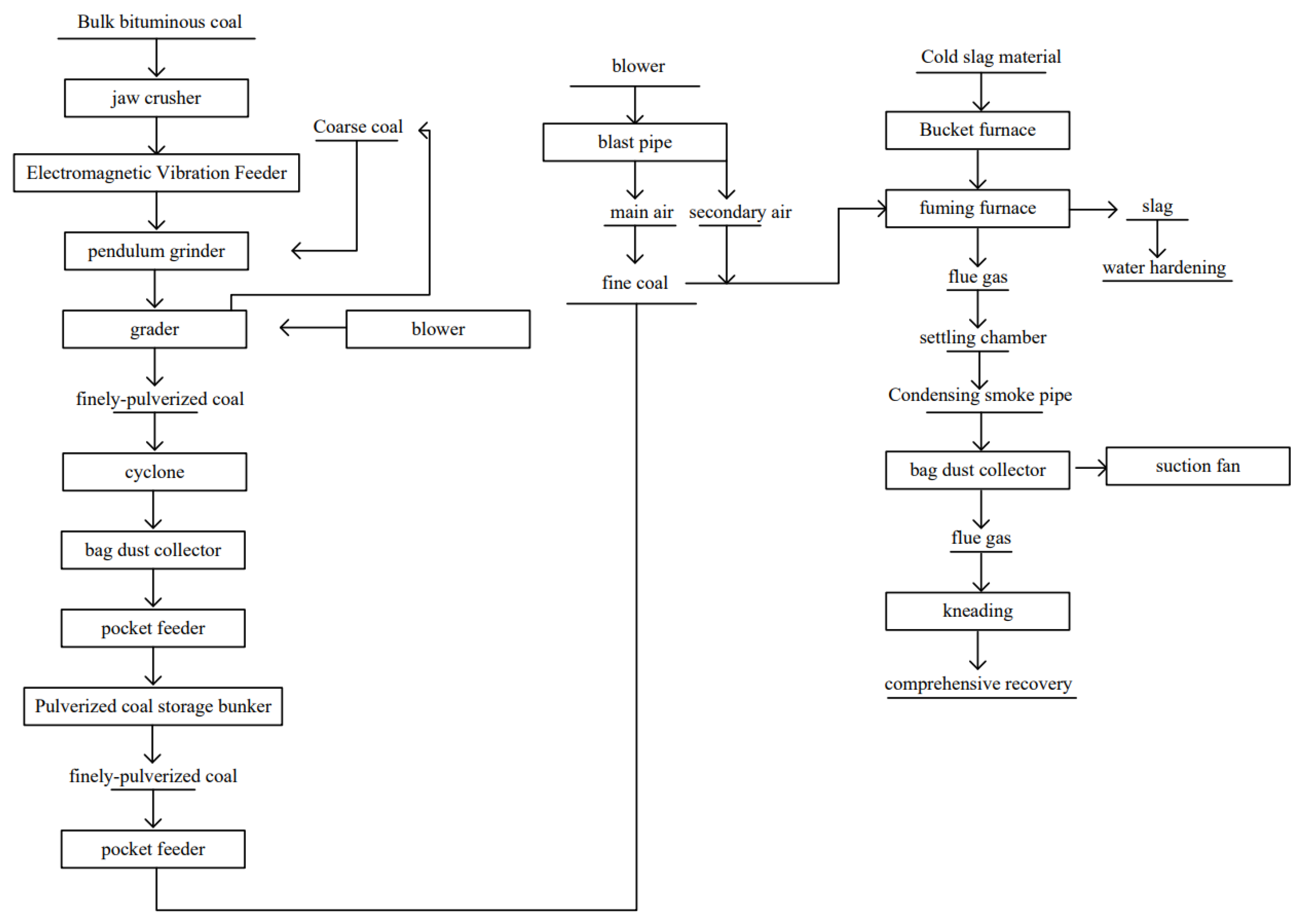
4.1. Recovery of Valuable Elements from Metallurgical Slag
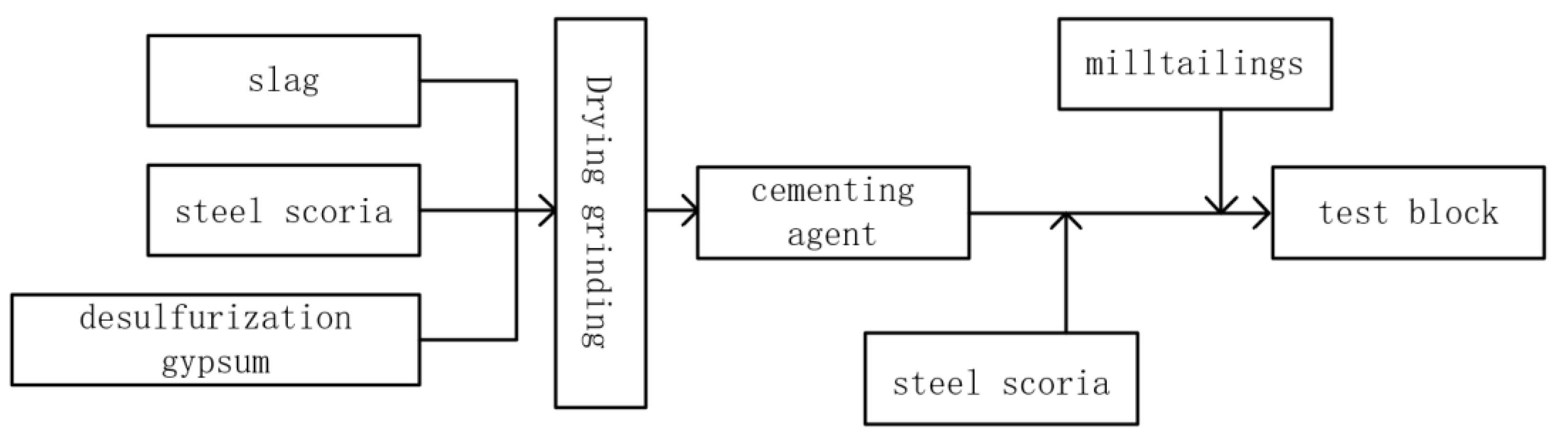
4.2. Stabilization/Solidification Method for Metallurgical Slag Treatment
5. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, C.G. Antimony Production and Commodities; Society for Mining, Metallurgy and Exploration: Englewood, CO, USA, 2019. [Google Scholar]
- Ozer, M. Flotation of Antimony Ores with High Arsenic Content. Physicochem. Probl. Miner. Process 2022, 58, 152865. [Google Scholar] [CrossRef]
- Schulz, K.J. (Ed.) Critical Mineral Resources of the United States: Economic and Environmental Geology and Prospects for Future Supply; Geological Survey: Reston, VA, USA, 2017.
- Summaries, M.C. Mineral Commodity Summaries 2022; Geological Survey: Reston, VA, USA, 2022; p. 206.
- Deng, R.-J.; Jin, C.-S.; Ren, B.-Z.; Hou, B.-L.; Hursthouse, A. The Potential for the Treatment of Antimony-Containing Wastewater by Iron-Based Adsorbents. Water 2017, 9, 794. [Google Scholar] [CrossRef]
- Hiller, E.; Lalinská, B.; Chovan, M.; Jurkovič, Ľ.; Klimko, T.; Jankulár, M.; Hovorič, R.; Šottník, P.; Fľaková, R.; Ženišová, Z.; et al. Arsenic and Antimony Contamination of Waters, Stream Sediments and Soils in the Vicinity of Abandoned Antimony Mines in the Western Carpathians, Slovakia. Appl. Geochem. 2012, 27, 598–614. [Google Scholar] [CrossRef]
- Guo, X.; Wang, K.; He, M.; Liu, Z.; Yang, H.; Li, S. Antimony Smelting Process Generating Solid Wastes and Dust: Characterization and Leaching Behaviors. J. Environ. Sci. 2014, 26, 1549–1556. [Google Scholar] [CrossRef]
- Guo, Z.; Yao, J.; Yuan, Z.; Zhu, M. Spatial Distribution and Potential Ecological Risk Assessment of Heavy Metals in Tailing, Guangxi Autonomous Region. Guangzhou Chem. Ind. 2015, 43, 156–158+178. [Google Scholar]
- Zhang, J.; Qiu, X.; Li, N. Analysis on characteristics of heavy metal pollution of antimony mine tailings pond. Non-Ferr. Met. (Min. Part) 2022, 74, 126–130. [Google Scholar]
- Brink, S.V.D.; Kleijn, R.; Sprecher, B.; Mancheri, N.; Tukker, A. Resilience in the Antimony Supply Chain. Resour. Conserv. Recycl. 2022, 186, 106586. [Google Scholar] [CrossRef]
- Li, Y. Study on the Solidification Mechanism of Antimony-Containingtailings by Using BFS-SS-FGDG Based Cementitious Materials. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2022. [Google Scholar]
- Song, S. Environmental Effects of Mining and Strategies for Integration of Mine Resources and Environment. Ph.D. Thesis, Zhongshan University, Guangzhou, China, 2004. [Google Scholar]
- Wang, K.; Xu, Z.; Cao, G.; Zang, N.; Zhou, X.; Yu, F. Construct a collaborative disposal mechanism for cross-regional sudden water pollution incidents. Environ. Econ. 2021, 18, 64–69. [Google Scholar]
- Villarroel, L.F.; Miller, J.R.; Lechler, P.J.; Germanoski, D. Lead, Zinc, and Antimony Contamination of the Rio Chilco-Rio Tupiza Drainage System, Southern Bolivia. Environ. Geol. 2006, 51, 283–299. [Google Scholar] [CrossRef]
- Punia, A. Role of Temperature, Wind, and Precipitation in Heavy Metal Contamination at Copper Mines: A Review. Environ. Sci. Pollut. Res. 2021, 28, 4056–4072. [Google Scholar] [CrossRef]
- Deng, W. State of art of antimony metallurgy in China. Min. Metall. 2017, 26, 50–54. [Google Scholar]
- Liu, F.; Le, X.C.; McKnight-Whitford, A.; Xia, Y.; Wu, F.; Elswick, E.; Johnson, C.C.; Zhu, C. Antimony Speciation and Contamination of Waters in the Xikuangshan Antimony Mining and Smelting Area, China. Environ. Geochem. Health 2010, 32, 401–413. [Google Scholar] [CrossRef]
- Tian, J.; Sun, W.; Zhang, X.; Han, H.; Yu, Z.; Yue, T.; Wang, L.; Yang, Y.; Tang, H.; Li, E. Comprehensive Utilization and Safe Disposal of Hazardous Arsenic-Alkali Slag by the Combination of Beneficiation and Metallurgy. J. Clean. Prod. 2021, 295, 126381. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, G.; Miao, Y.; Zhou, L. Harmless Disposal and Resource Utilization Technology of Arsenic Alkali Slag in Antimony Smelting Industry; Chinese Society of Environmental Sciences: Beijing, China, 2013; pp. 2319–2322. [Google Scholar]
- Baron, S.; Carignan, J.; Ploquin, A. Dispersion of Heavy Metals (Metalloids) in Soils from 800-Year-Old Pollution (Mont-Lozère, France). Environ. Sci. Technol. 2006, 40, 5319–5326. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hao, C.; Jia, Y.; Zhang, W. Space-temporal evolution and hydrochemistry analysis of Qingfeng river in Tin mine. J. North China Inst. Sci. Technol. 2021, 18, 25–31. [Google Scholar]
- Wang, J.; Chen, J.; Dong, F.; Du, M.; Fu, K. Research Progress and Prospect of Antimony Oxide Ore Beneficiation. Met. Man Mine 2021, 12, 21–27. [Google Scholar]
- Ming, T. Experimental Study on Recovering Gold from Antimony Flotation Tailings. Non-Ferr. Met. (Miner. Process Part) 2011, 406, 27–29. [Google Scholar]
- Wang, J.; Li, L.; Zhang, L.; Li, B.; Deng, R.; Shi, D. Sustainable Applications for Utilizing Antimony Tailing Coarse Aggregate (ATCA) in Concrete: Characteristic of ATCA and Toxicity Risks of Concrete. Materials 2021, 14, 5480. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, Y.; Lu, D.; Liu, Z.; Zheng, X. Pre-Concentration of Fine Antimony Oxide Tailings Using an Agitated Reflux Classifier. Powder Technol. 2020, 376, 565–572. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, D.; Wang, Y.; Chu, H.; Zheng, X.; Chen, F. Application of reflux classifier with closely spaced inclined channels in pre-concentrate process of fine antimony oxide particles. J. Cent. South Univ. 2020, 27, 3290–3301. [Google Scholar] [CrossRef]
- Kang, W. Experimental Study on Reconcentration of Fine Antimony Oxide Tailings in Xikuangshan Mining Plant. China Met. Notif. 2018, 230–231. [Google Scholar]
- Ren, L.; Wang, L.; Guo, H. Experimental Study on Mineral Processing of a Low Grade Mercury-Antimony Tailings in Northwest China. Non-Ferr. Met. (Miner. Process Part) 2020, 123–126+133. [Google Scholar] [CrossRef]
- Gao, W.; Ni, W.; Zhang, Y.; Li, Y.; Shi, T.; Li, Z. Investigation into the Semi-Dynamic Leaching Characteristics of Arsenic and Antimony from Solidified/Stabilized Tailings Using Metallurgical Slag-Based Binders. J. Hazard. Mater. 2020, 381, 120992. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Zhang, L.; Deng, R.; Zhou, S.; Wang, G. Strength and Durability Properties of Antimony Tailing Coarse Aggregate (ATCA) Concrete. Materials 2021, 14, 5606. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, X.; Qu, G.; Ning, P.; Jin, C.; Cui, Q.; Ren, Y.; He, M.; Yang, Y.; Li, J. Solidification and Stabilization of Harmful Elements in Antimony Tailings and Synergistic Utilization of Multiple Solid Wastes. Cem. Concr. Compos. 2022, 133, 104718. [Google Scholar] [CrossRef]
- Li, Y.; Ni, W.; Gao, W.; Zhang, S.; Fu, P.; Li, Y. Study on Solidification and Stabilization of Antimony-Containing Tailings with Metallurgical Slag-Based Binders. Materials 2022, 15, 1780. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Q.; Chen, Y.; Li, D.; Tian, Q.; Guo, X. Status of solid waste disposal in antimony metallurgy. Nonferr. Met. Sci. Eng. 2022, 13, 8–17. [Google Scholar]
- Luo, S.; Ma, D. Study on the Influence of Slag Composition on Antimony Content in Slag DuringVolatilization Smelting of Antimony Concentrate in Blast Furnace. Hunan Nonferr. Met. 2021, 37, 28–30+44. [Google Scholar]
- Shan, T. Study on the Recovery Process of Antimony Lead from Removal Lead Slag of Smelting Antimony. Hunan Nonferr. Met. 2014, 30, 36–38+76. [Google Scholar]
- Hao, F. Mechanism of Arsenic Fixation by Steel Slag-Based Active Materials and Its Application. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2020. [Google Scholar] [CrossRef]
- Keqiang, Q.; Rongliang, Z. Research on Preparation of Nanometer Antimony Trioxide from Slag Containing Antimony by Vacuum Evaporation Method. Vacuum 2006, 80, 1016–1020. [Google Scholar] [CrossRef]
- Mo, W. The Industrial Practices of Volatility Process for Jamesonite Smelting Slag with Fuming Furnace. Guangzhou Chem. Ind. 2012, 40, 134–136. [Google Scholar]
- He, Q.; Tan, Y. On Zinc and Indium Recycle from Blast Furnace Slag by Fuming Process. Jiangxi Nonferr. Met. 2008, 22, 29–32. [Google Scholar] [CrossRef]
- Luo, H.-L.; Liu, W.; Qin, W.-Q.; Zheng, Y.-X.; Yang, K. Cleaning of High Antimony Smelting Slag from an Oxygen-Enriched Bottom-Blown by Direct Reduction. Rare Met. 2019, 38, 800–804. [Google Scholar] [CrossRef]
- Yao, L.; Liu, D.; Ke, Y.; Li, Y.; Wang, Z.; Fei, J.; Xu, H.; Min, X. Synthesis and Hydration Characteristic of Geopolymer Based on Lead Smelting Slag. Int. J. Environ. Res. Public Health 2020, 17, 2762. [Google Scholar] [CrossRef]
- Wei, Y.; Pan, Y. Study on Lead Recovery from Lead Slag of Antimony Refining byNitric Acid Leaching Process. Non-Ferr. Met. (Smelt. Part) 2013, 8, 11–13. [Google Scholar]
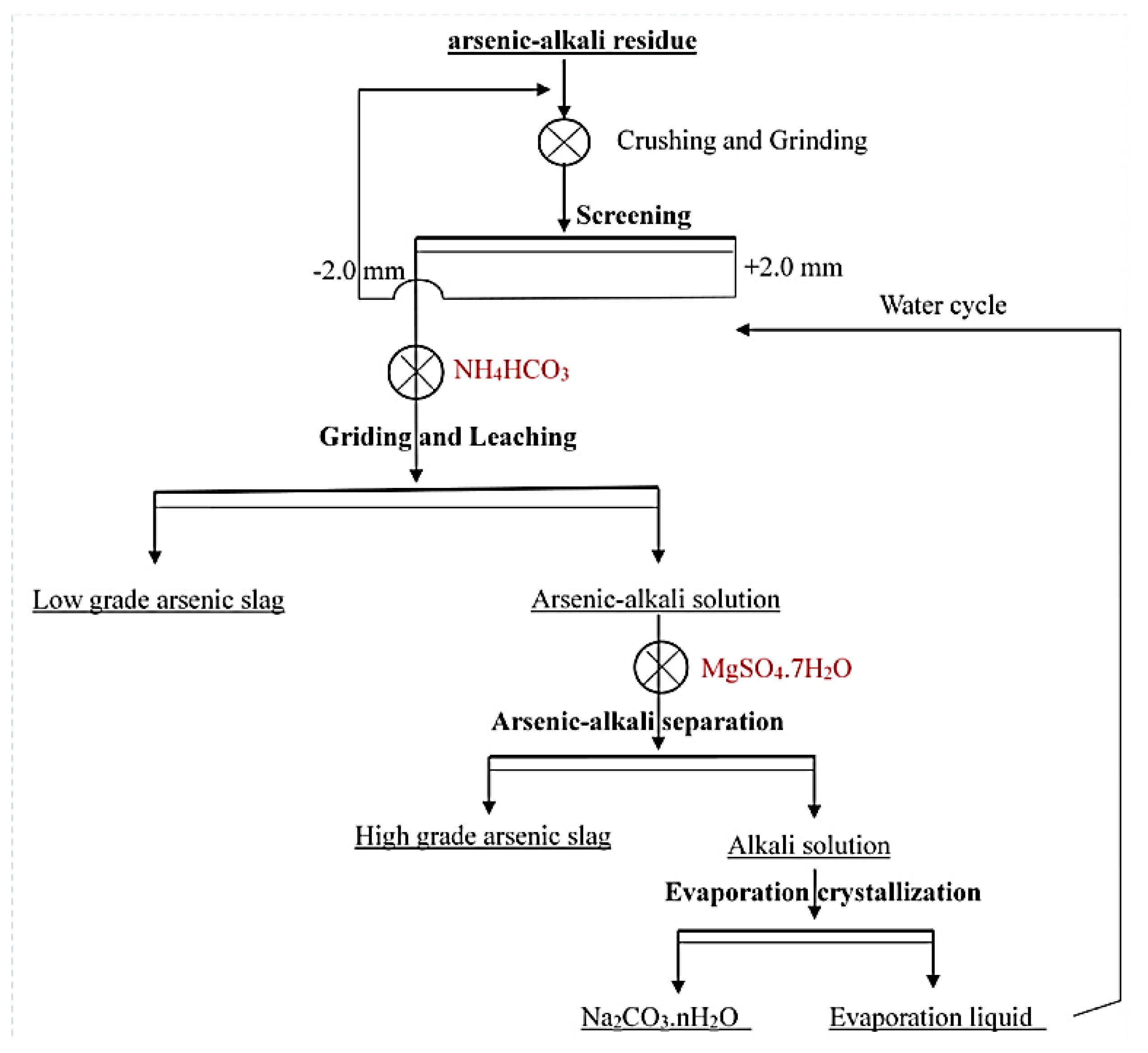
- Long, H.; Zheng, Y.; Peng, Y.; Jin, G.; Deng, W.; Zhang, S.; He, H. Separation and Recovery of Arsenic and Alkali Products during the Treatment of Antimony Smelting Residues. Miner. Eng. 2020, 153, 106379. [Google Scholar] [CrossRef]
- Deng, W.; Chai, L.; Dai, Y. ndustrial Experimental Study on Comprehensive Recoverying Valuable Resources fromAntimony Smelting Arsenic Alkali Residue. Hunan Nonferrous Met. 2014, 30, 24–27. [Google Scholar]
- Long, H.; Zheng, Y.; Peng, Y.; He, H. Recovery of Alkali, Selenium and Arsenic from Antimony Smelting Arsenic-Alkali Residue. J. Clean. Prod. 2020, 251, 119673. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Zhang, X.; Sun, W.; Han, H.; Yu, Z.; Yue, T. A Novel Scheme for Safe Disposal and Resource Utilization of Arsenic-Alkali Slag. Process Saf. Environ. Prot. 2021, 156, 429–437. [Google Scholar] [CrossRef]
- Jia, X.; Ma, L.; Liu, J.; Liu, P.; Yu, L.; Zhou, J.; Li, W.; Zhou, W.; Dong, Z. Reduction of Antimony Mobility from Sb-Rich Smelting Slag by Shewanella Oneidensis: Integrated Biosorption and Precipitation. J. Hazard. Mater. 2022, 426, 127385. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Wang, L.; Zhang, S.; Hou, H.; Zhang, J.; Chen, J.; Ma, M.; Tsang, D.C.W.; Wu, X. Stabilization Treatment of Arsenic-Alkali Residue (AAR): Effect of the Coexisting Soluble Carbonate on Arsenic Stabilization. Environ. Int. 2020, 135, 105406. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, P.; Li, Z.; Chen, J.; Ke, Y.; Hu, J.; Liu, B.; Yang, J.; Liang, S.; Su, X.; et al. Iron-Calcium Reinforced Solidification of Arsenic Alkali Residue in Geopolymer Composite: Wide PH Stabilization and Its Mechanism. Chemosphere 2023, 312, 137063. [Google Scholar] [CrossRef] [PubMed]



| Groups | Au | Ag | Sb | S | Zn | Pb | As | TiO2 | SiO2 | Al2O3 | CaO | MgO | C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| content | 2.92 | 0.50 | 1.21 | 0.76 | 0.01 | 0.10 | 0.38 | 0.59 | 64.42 | 14.50 | 1.54 | 1.81 | 1.62 |
| Groups | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | TiO2 | MnO | Pb2O5 | Na2O | FeO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| content | 88.78 | 3.86 | 0.98 | 0.61 | 0.11 | 0.42 | 0.17 | 0.03 | 0.04 | 0.02 | 0.27 | 2.98 |
| Pb | Zn | Sb | In * | FeO | SiO2 | CaO | |
|---|---|---|---|---|---|---|---|
| 1 | 3.01 | 5.04 | 0.76 | 103 | 13.54 | 25.52 | 16.73 |
| 2 | 0.91 | 6.56 | 3.21 | 110 | 16.97 | 20.44 | 14.82 |
| 3 | 0.10 | -- | 1.22 | -- | 26.64 | 42.03 | 16.11 |
| Component | As | Sb | Se | Na2CO3 | NaHCO3 |
|---|---|---|---|---|---|
| Content | 3.8 | 1.0 | 0.18 | 34.0 | 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Wang, L.; Zheng, Q.; Che, X.; Cui, X.; Wei, S.; Li, H.; Shi, X. Present Situation and Research Progress of Comprehensive Utilization of Antimony Tailings and Smelting Slag. Sustainability 2023, 15, 13947. https://doi.org/10.3390/su151813947
Yu Z, Wang L, Zheng Q, Che X, Cui X, Wei S, Li H, Shi X. Present Situation and Research Progress of Comprehensive Utilization of Antimony Tailings and Smelting Slag. Sustainability. 2023; 15(18):13947. https://doi.org/10.3390/su151813947
Chicago/Turabian StyleYu, Zeen, Lei Wang, Qi Zheng, Xiaokui Che, Xinglan Cui, Shenyu Wei, Hongxia Li, and Xinyue Shi. 2023. "Present Situation and Research Progress of Comprehensive Utilization of Antimony Tailings and Smelting Slag" Sustainability 15, no. 18: 13947. https://doi.org/10.3390/su151813947
APA StyleYu, Z., Wang, L., Zheng, Q., Che, X., Cui, X., Wei, S., Li, H., & Shi, X. (2023). Present Situation and Research Progress of Comprehensive Utilization of Antimony Tailings and Smelting Slag. Sustainability, 15(18), 13947. https://doi.org/10.3390/su151813947






