Microplastic Abundance in Rainbow Trout Life Cycle: Step by Step
Abstract
:1. Introduction
2. Material and Methods
2.1. Taking Samples from Different Stages of O. mykiss and Feed
2.2. Microplastic Isolation Procedures
2.2.1. MP Isolation from Fish Feed
2.2.2. MP Isolation from Fish Life Stages and Feeds
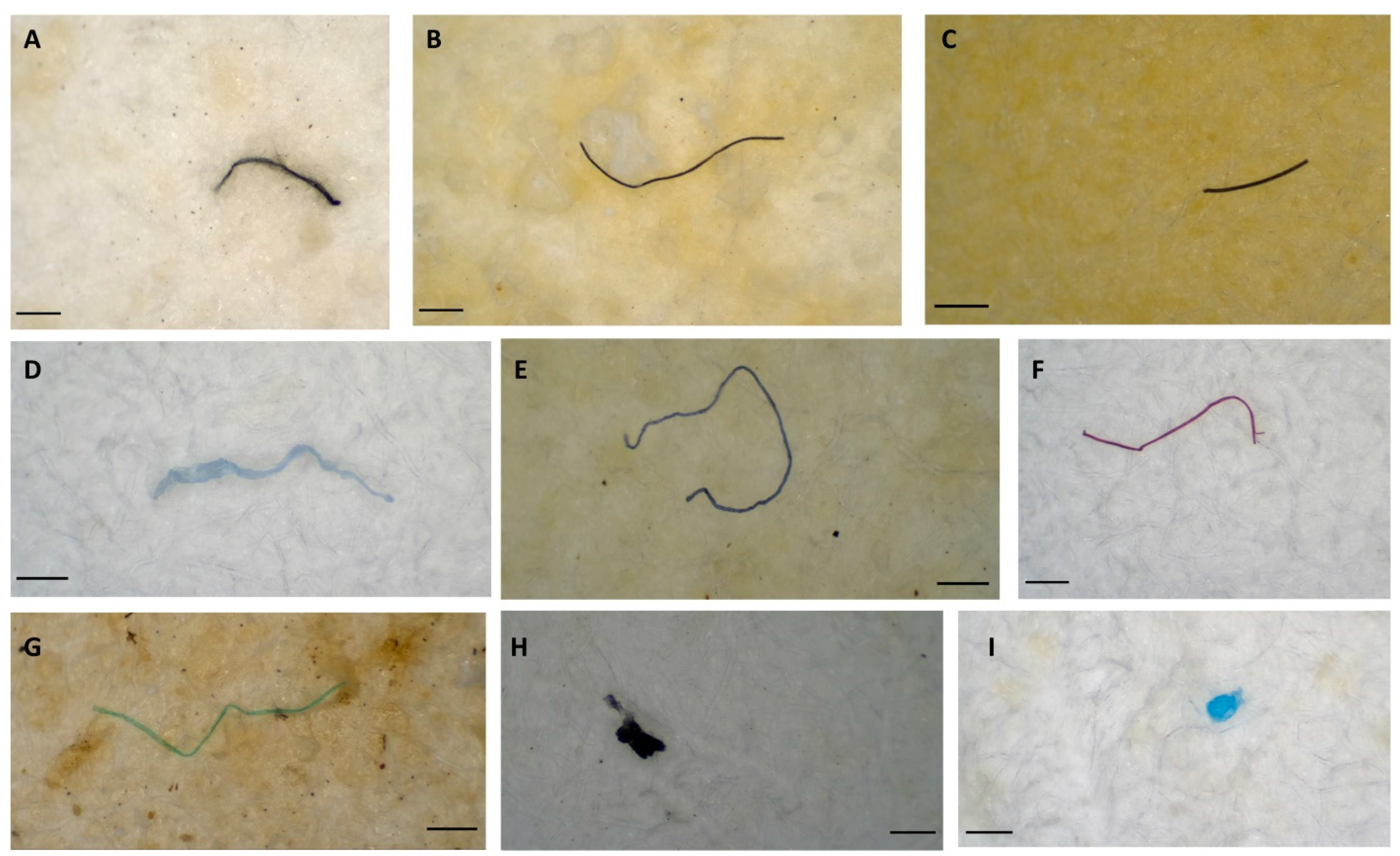
2.3. MP Characterization
2.4. Quality Control and Contamination Prevention
3. Statistical Analyzes
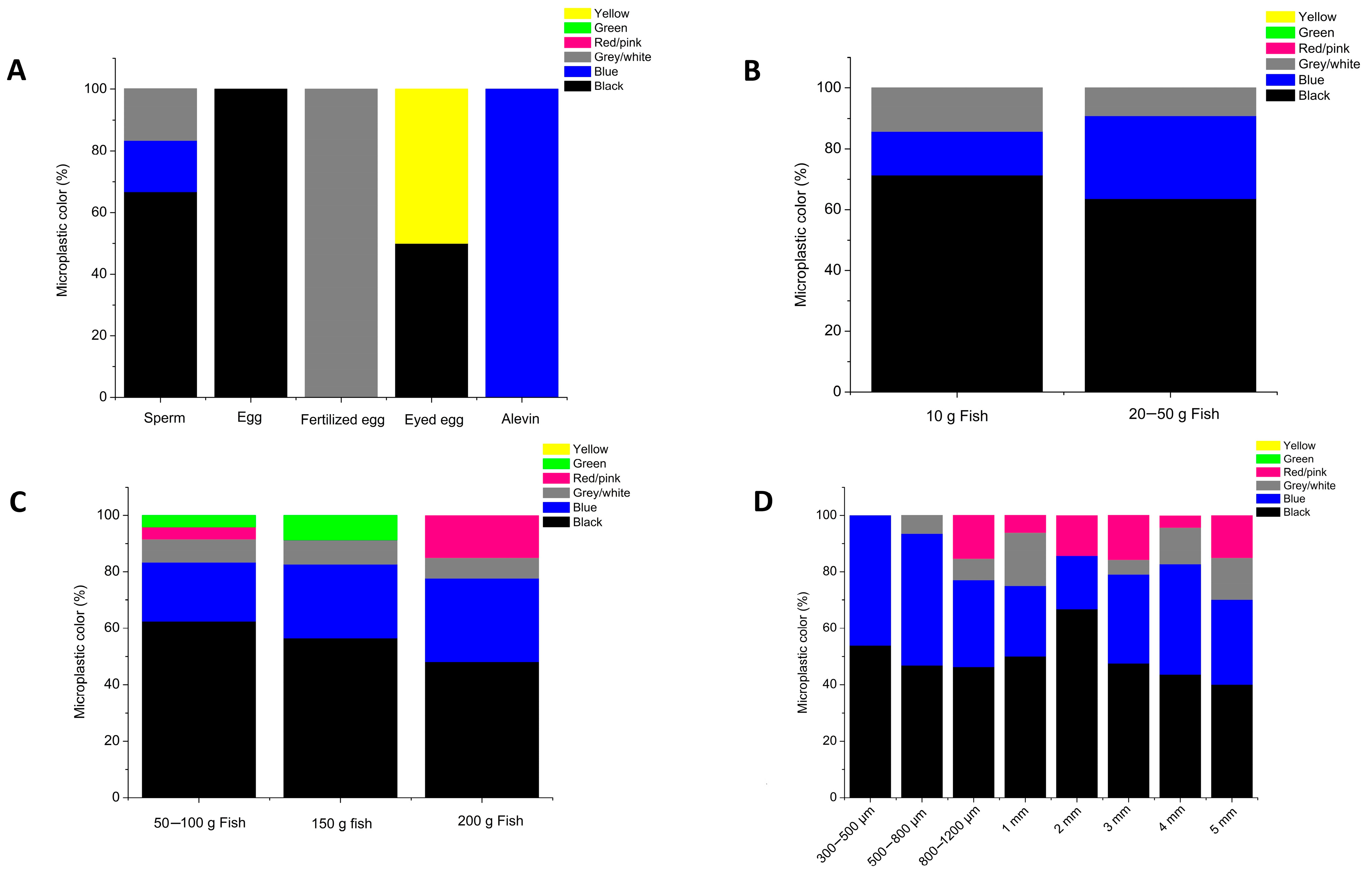
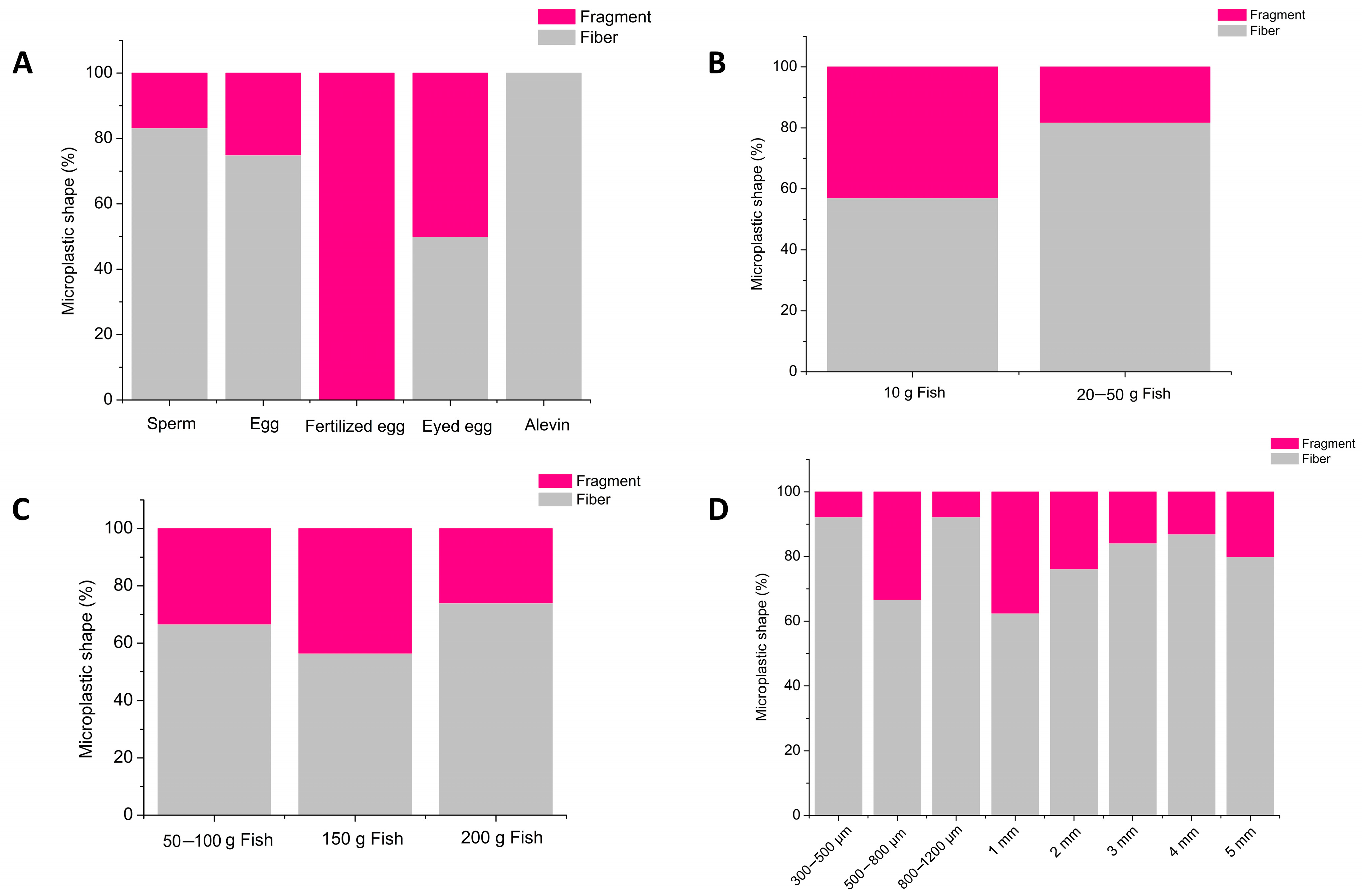
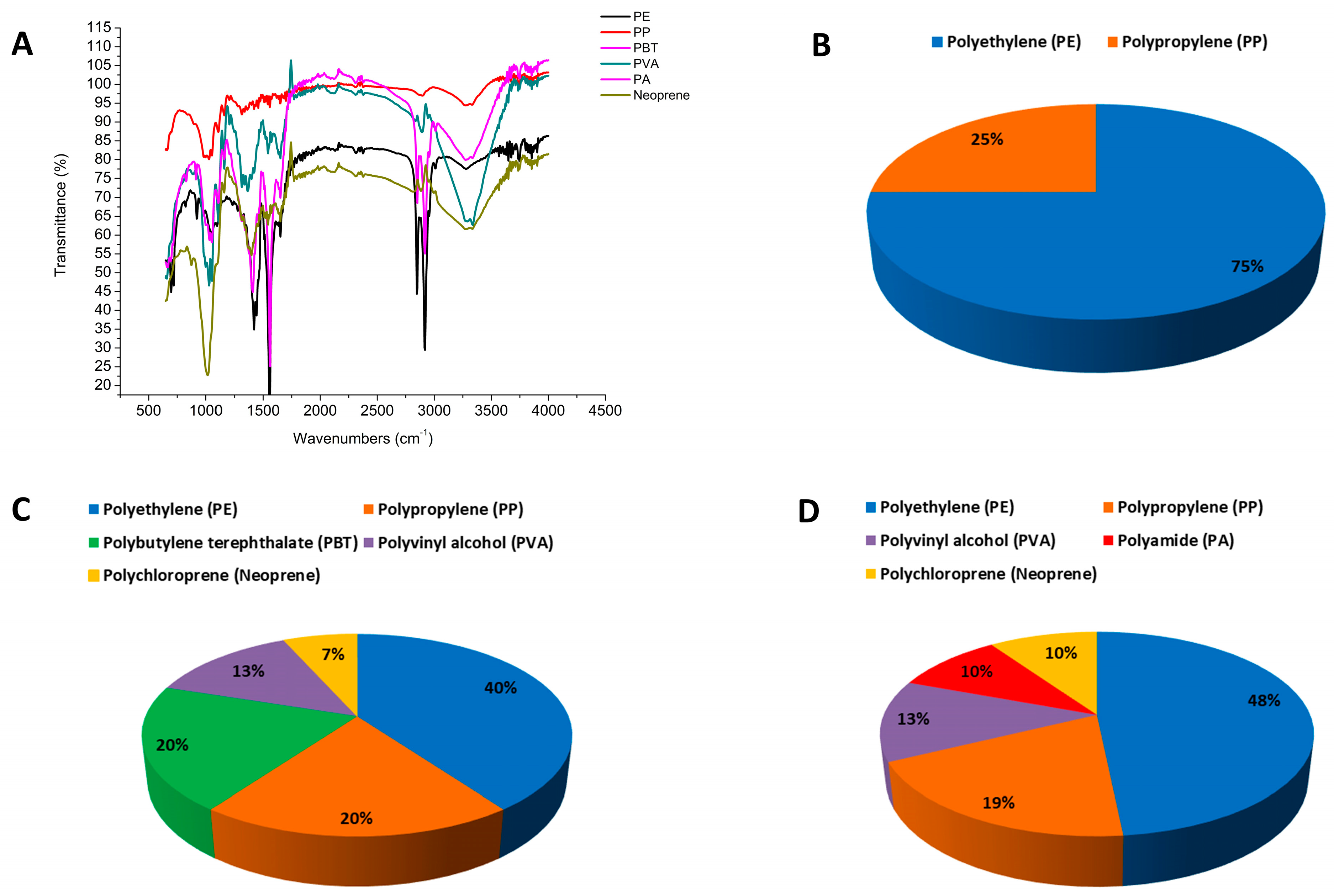
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kershaw, P.J.; Turra, A.; Galgani, F. Guidelines for the Monitoring and Assessment of Plastic Litter and Microplastics in the Ocean; GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2019. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, J.; Zhou, T.; Zhao, Y.; Yu, F.; Ma, J. Laboratory simulation of microplastics weathering and its adsorption behaviors in an aqueous environment: A systematic review. Environ. Pollut. 2020, 265, 114864. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Berlino, M.; Mangano, M.C.; Sarà, G. Microplastics and the functional traits of fishes: A global meta-analysis. Glob. Chang. Biol. 2021, 27, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Matos, J.; Regueiro, L.; González-García, S.; Vázquez-Rowe, I. Environmental performance of rainbow trout (Oncorhynchus mykiss) production in Galicia-Spain: A Life Cycle Assessment approach. Sci. Total Environ. 2023, 856, 159049. [Google Scholar] [CrossRef] [PubMed]
- TUIK. 2022. Available online: https://data.tuik.gov.tr/Bulten/Index?p=Fishery-Products-2022-49678 (accessed on 20 August 2023).
- Ghafarifarsani, H.; Yousefi, M.; Hoseinifar, S.H.; Paolucci, M.; Lumsangkul, C.; Jaturasitha, S.; Van Doan, H. Beneficial effects of Persian shallot (Allium hirtifolium) extract on growth performance, biochemical, immunological and antioxidant responses of rainbow trout Oncorhynchus mykiss fingerlings. Aquaculture 2022, 555, 738162. [Google Scholar] [CrossRef]
- Aas, T.S.; Åsgård, T.; Ytrestøyl, T. Utilization of feed resources in the production of rainbow trout (Oncorhynchus mykiss) in Norway in 2020. Aquac. Rep. 2022, 26, 101317. [Google Scholar] [CrossRef]
- Malone, E.W.; Perkin, J.S.; Keith Gibbs, W.; Padgett, M.; Kulp, M.; Moore, S.E. High and dry in days gone by: Life-history theory predicts Appalachian mountain stream fish assemblage transformation during historical drought. Ecol. Freshw. Fish. 2022, 31, 29–44. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, J. Occurrence and ecological impact of microplastics in aquaculture ecosystems. Chemosphere 2021, 274, 129989. [Google Scholar] [CrossRef]
- Atamanalp, M.; Kırıcı, M.; Köktürk, M.; Kırıcı, M.; Kocaman, E.M.; Ucar, A.; Parlak, V.; Özcan, S.; Yanık, T.; Alak, G. Polyethylene exposure in rainbow trout; suppresses growth and may act as a promoting agent in tissue-based oxidative response, DNA damage and apoptosis. Process Saf. Environ. Prot. 2023, 174, 960–970. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Prinz, N.; Korez, Š. Understanding how microplastics affect marine biota on the cellular level is important for assessing ecosystem function: A review. In YOUMARES 9-The Oceans: Our Research, Our Future: Proceedings of the 2018 Conference for Young Marine Researcher in Oldenburg, Germany; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 101–120. [Google Scholar]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Zhu, X.; Sun, C.; Teng, J.; Chen, L.; Shan, E.; Zhao, J. Microplastics in fish meals: An exposure route for aquaculture animals. Sci. Total Environ. 2022, 807, 151049. [Google Scholar] [CrossRef]
- Clere, I.K.; Ahmmed, F.; Peter III, J.G.; Fraser-Miller, S.J.; Gordon, K.C.; Komyakova, V.; Allan, B.J. Quantification and characterization of microplastics in commercial fish from southern New Zealand. Mar. Pollut. Bull. 2022, 184, 114121. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Guilhermino, L. Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. Exposure and risk to human consumers. J. Hazard. Mater. 2020, 393, 122419. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Q.; Li, R.; Zhao, Y.; Geng, J.; Wang, G. Physiological responses of lettuce (Lactuca sativa L.) to microplastic pollution. Environ. Sci. Pollut. Res. 2020, 27, 30306–30314. [Google Scholar] [CrossRef] [PubMed]
- Baalkhuyur, F.M.; Qurban, M.A.; Panickan, P.; Duarte, C.M. Microplastics in fishes of commercial and ecological importance from the Western Arabian Gulf. Mar. Pollut. Bull. 2020, 152, 110920. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, J.; Brown, R.J.; Kim, K.H. Environmental fate, ecotoxicity biomarkers, and potential health effects of micro-and nano-scale plastic contamination. J. Hazard. Mater. 2021, 403, 123910. [Google Scholar] [CrossRef]
- Alak, G.; Uçar, A.; Parlak, V.; Atamanalp, M. Identification, characterisation of microplastic and their effects on aquatic organisms. Chem. Ecol. 2022, 38, 967–987. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Eroldoğan, O.T.; Evliyaoğlu, E.; Turchini, G.M.; Wu, X.G. Fish out, plastic in: Global pattern of plastics in commercial fishmeal. Aquaculture 2021, 534, 736316. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in fish and fishmeal: An emerging environmental challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, Y.; Xie, S.; Chen, Y.; Li, X.; Wang, J.; Zou, J. Microplastics and their potential effects on the aquaculture systems: A critical review. Rev. Aquac. 2021, 13, 719–733. [Google Scholar] [CrossRef]
- Walkinshaw, C.; Tolhurst, T.J.; Lindeque, P.K.; Thompson, R.; Cole, M. Detection and characterisation of microplastics and microfibres in fishmeal and soybean meal. Mar. Pollut. Bull. 2022, 185, 114189. [Google Scholar] [CrossRef] [PubMed]
- Wootton, N.; Nursey-Bray, M.; Reis-Santos, P.; Gillanders, B.M. Perceptions of plastic pollution in a prominent fishery: Building strategies to inform management. Mar. Policy 2022, 135, 104846. [Google Scholar] [CrossRef]
- Miloloža, M.; Kučić Grgić, D.; Bolanča, T.; Ukić, Š.; Cvetnić, M.; Ocelić Bulatović, V.; Dionysiou, D.D.; Kušić, H. Ecotoxicological assessment of microplastics in freshwater sources—A review. Water 2020, 13, 56. [Google Scholar] [CrossRef]
- Hanachi, P.; Karbalaei, S.; Walker, T.R.; Cole, M.; Hosseini, S.V. Abundance and properties of microplastics found in commercial fish meal and cultured common carp (Cyprinus carpio). Environ. Sci. Pollut. Res. 2019, 26, 23777–23787. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Franzellitti, S.; Canesi, L.; Auguste, M.; Wathsala, R.H.; Fabbri, E. Microplastic exposure and effects in aquatic organisms: A physiological perspective. Environ. Toxicol. Pharmacol. 2019, 68, 37–51. [Google Scholar] [CrossRef]
- Hodkovicova, N.; Hollerova, A.; Svobodova, Z.; Faldyna, M.; Faggio, C. Effects of plastic particles on aquatic invertebrates and fish—A review. Environ. Toxicol. Pharmacol. 2022, 96, 104013. [Google Scholar] [CrossRef]



| Life Stage of Fish | Feed Size | Sampling MP |
|---|---|---|
| Sperm | - | 20 mL |
| Egg | - | 20 mL |
| Fertilized egg | - | 20 mL |
| Alevin or such fry | - | 20 mL |
| 0.5–1.2 g | 300–500 µm 500–800 µm 800–1200 µm | Whole fish |
| 1.3–10 g | 1 mm | Whole fish |
| 11–30 g | 2 mm | Whole fish |
| 31–70 g | 3 mm | Muscle, gills, and GIT |
| 71–100 g | 4 mm | Muscle, gills, and GIT |
| ≥101 g | 5 mm | Muscle, gills, and GIT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alak, G.; Köktürk, M.; Atamanalp, M.; Kocaman, E.M.; Ucar, A.; Esenbuğa, N.; Özcan, S.; Parlak, V. Microplastic Abundance in Rainbow Trout Life Cycle: Step by Step. Sustainability 2023, 15, 14255. https://doi.org/10.3390/su151914255
Alak G, Köktürk M, Atamanalp M, Kocaman EM, Ucar A, Esenbuğa N, Özcan S, Parlak V. Microplastic Abundance in Rainbow Trout Life Cycle: Step by Step. Sustainability. 2023; 15(19):14255. https://doi.org/10.3390/su151914255
Chicago/Turabian StyleAlak, Gonca, Mine Köktürk, Muhammed Atamanalp, Esat Mahmut Kocaman, Arzu Ucar, Nurinisa Esenbuğa, Sinan Özcan, and Veysel Parlak. 2023. "Microplastic Abundance in Rainbow Trout Life Cycle: Step by Step" Sustainability 15, no. 19: 14255. https://doi.org/10.3390/su151914255
APA StyleAlak, G., Köktürk, M., Atamanalp, M., Kocaman, E. M., Ucar, A., Esenbuğa, N., Özcan, S., & Parlak, V. (2023). Microplastic Abundance in Rainbow Trout Life Cycle: Step by Step. Sustainability, 15(19), 14255. https://doi.org/10.3390/su151914255








