Natural Nitrogen-Bearing and Phosphorus-Bearing Nanoparticles in Surface Sediments of the Pearl River Estuary, China: Implications for Nitrogen and Phosphorus Cycling in Estuarine and Coastal Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Methods
2.3. Analysis Methods
3. Results
3.1. Total Nitrogen (TN) and Total Phosphorus (TP) in the Surface Sediments
3.2. Main Mineral Components of the Surface Sediments
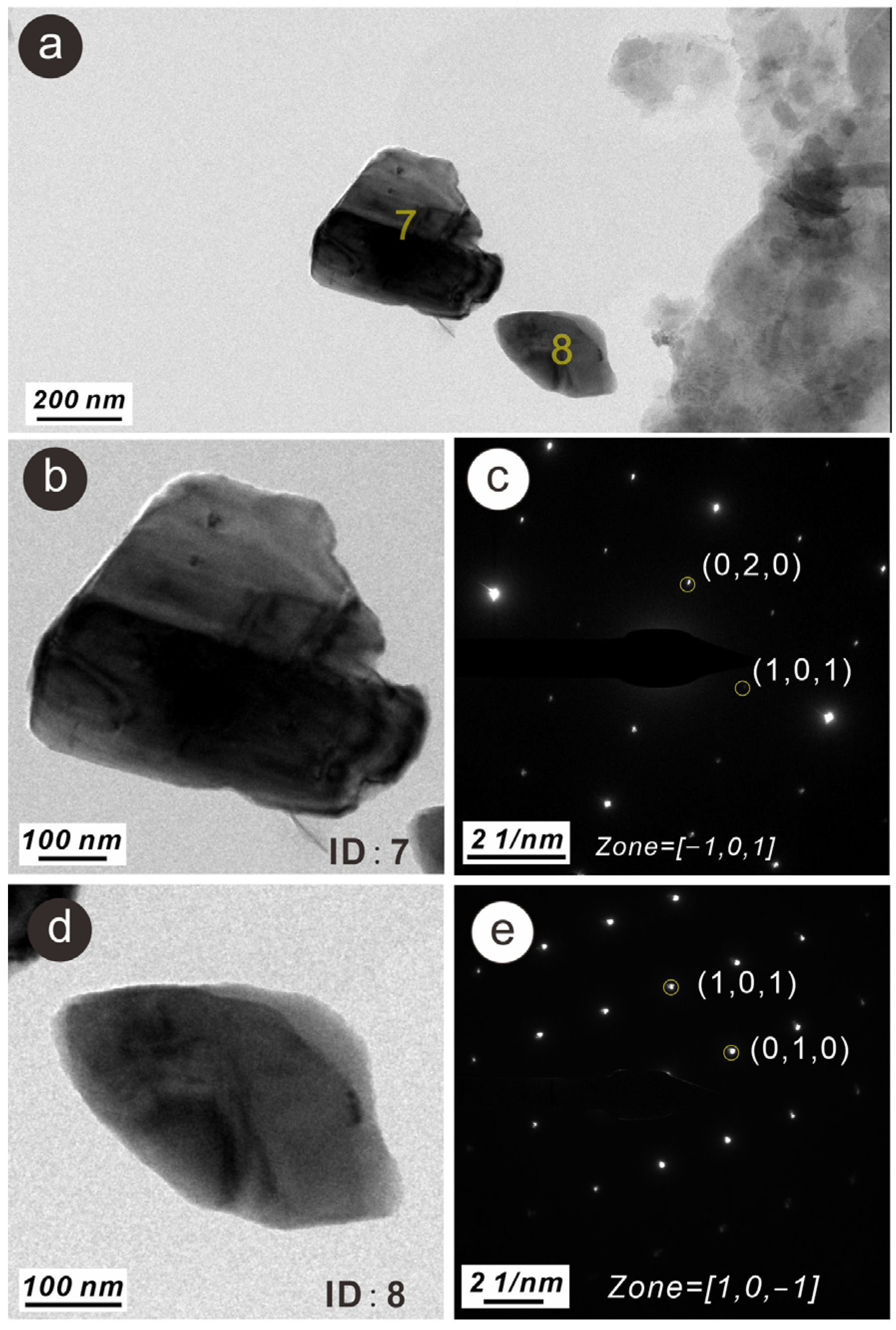
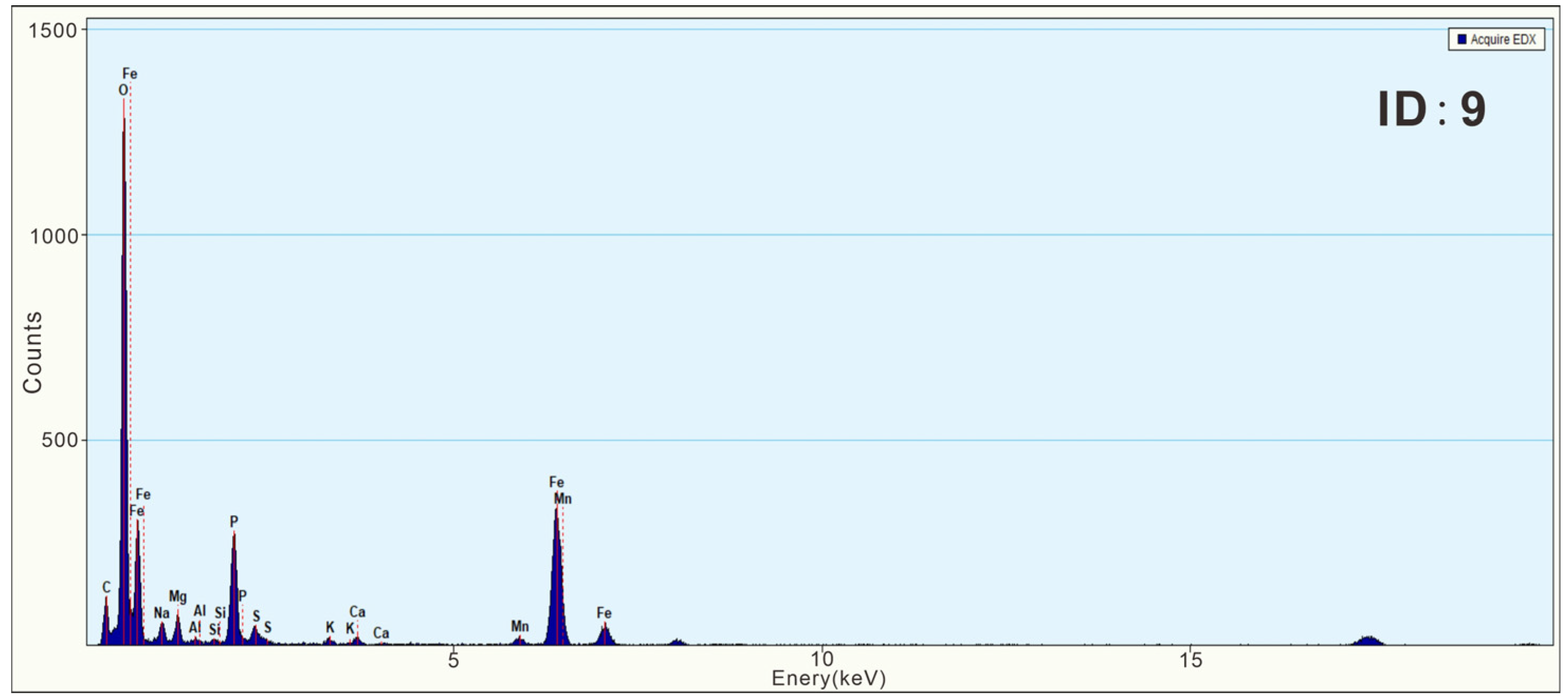
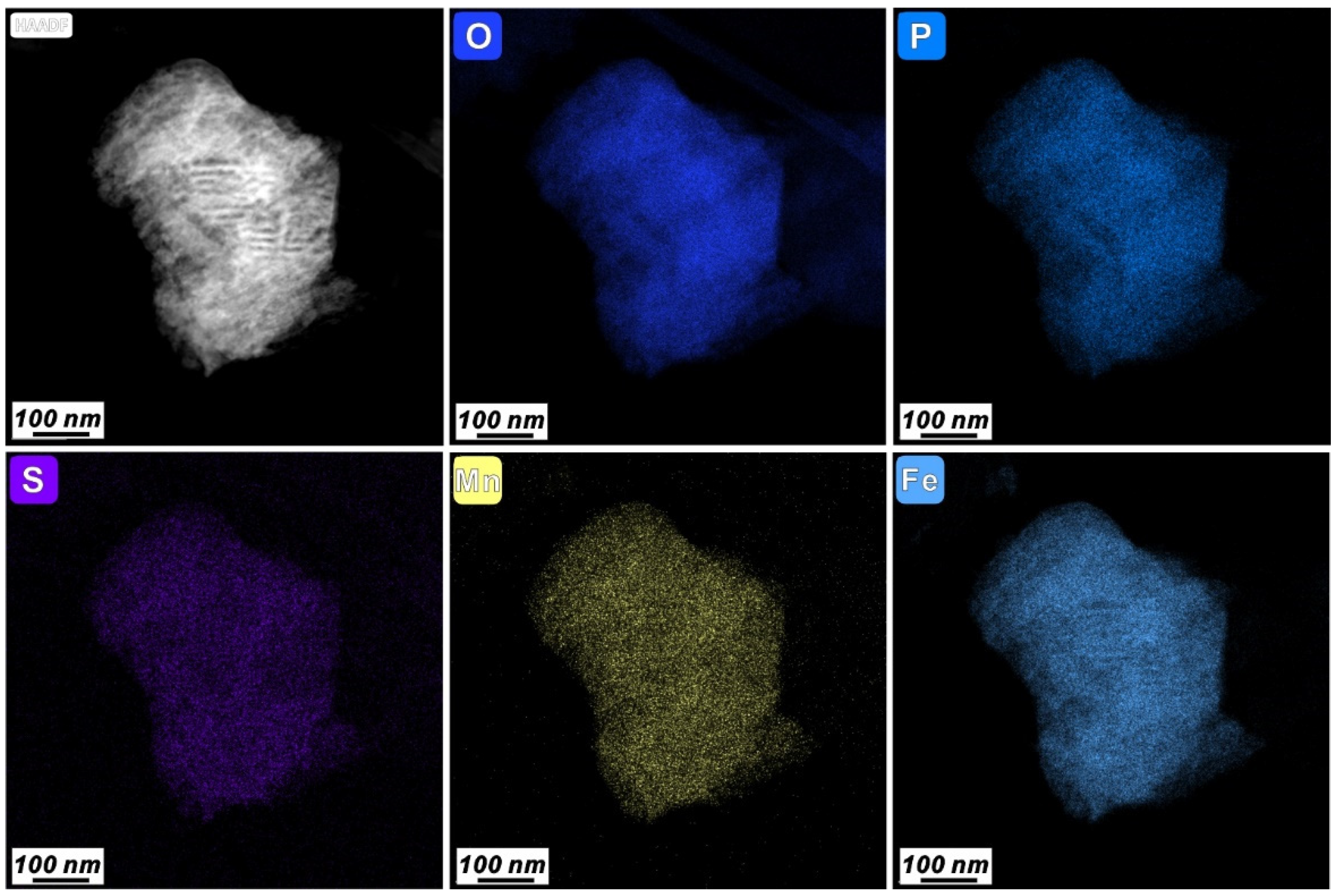
3.3. Characteristics of N-Bearing and P-Bearing Nanoparticles in the Surface Sediments
4. Discussion
4.1. Comparison of the Forms of Natural N-Bearing and P-Bearing Nanoparticles in the Surface Sediments of the Pearl River Estuary
4.2. Implications for N-Cycling and P-Cycling in Estuarine and Coastal Ecosystems
5. Conclusions
- Based on the HR-TEM observation, various forms of N and P nanoparticles were identified in surface sediments in the Pearl River Estuary of South China.
- Both N and P can be adsorbed by nanoparticles of minerals such as hematite, goethite, muscovite, anorthite and quartz in an estuarine and coastal environment, and further form N-bearing and P-bearing nanoparticles. These nanoparticles contained small amounts of N (1.52–3.73 wt%) and P (0.22–1.12 wt%), mainly single crystal or polycrystalline in form, with sizes ranging from 10 nm × 50 nm to 250 nm × 400 nm.
- P could exist in the form of Ca and Fe phosphate nanoparticles in the estuarine sediments. These phosphate nanoparticles have higher phosphorus content (5.02–9.97 wt%) and are mainly amorphous, with sizes ranging from 50 nm × 120 nm to 250 nm × 400 nm.
- N-bearing and P-bearing nanoparticles could influence the migration, precipitation and release processes of N and P, and play a certain role in the N-cycling and P-cycling of estuarine and coastal ecosystems. The water environmental management should also pay attention to sediment, especially the N-bearing and P-bearing nanoparticles.
- The further investigation of P-bearing nanoparticles in sediments may provide nanoscale evidence for phosphorus enrichment and mineralization in marine environment.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crooks, S.; Turner, R.K. Integrated Coastal Management: Sustaining Estuarine Natural Resources. Adv. Ecol. Res. 1999, 29, 241–289. [Google Scholar] [CrossRef]
- Statham, P.J. Nutrients in estuaries—An overview and the potential impacts of climate change. Sci. Total. Environ. 2012, 434, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Booi, S.; Mishi, S.; Andersen, O. Ecosystem Services: A Systematic Review of Provisioning and Cultural Ecosystem Services in Estuaries. Sustainability 2022, 14, 7252. [Google Scholar] [CrossRef]
- Nixon, S.W. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 1995, 41, 199–219. [Google Scholar] [CrossRef]
- Paerl, H.W. Controlling Eutrophication along the Freshwater–Marine Continuum: Dual Nutrient (N and P) Reductions are Essential. Estuaries Coasts 2009, 32, 593–601. [Google Scholar] [CrossRef]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Am. Assoc. Adv. Sci. 2009, 323, 5917. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Eutrophication: Time to Adjust Expectations—Response. Science 2009, 324, 724–725. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahid, M. Improving Water Quality Using Metal−Organic Frameworks. ACS Symp. Ser. 2021, 1395, 171–191. [Google Scholar] [CrossRef]
- Khan, M.S.; Khalid, M.; Shahid, M. What triggers dye adsorption by metal organic frameworks? The current perspectives. Mater. Adv. 2020, 1, 1575–1601. [Google Scholar] [CrossRef]
- Seitzinger, S.; Harrison, J.A.; Böhlke, J.K.; Bouwman, A.F.; Lowrance, R.; Peterson, B.; Tobias, C.; Van Drecht, G. Denitrification across Landscapes and Waterscapes: A Synthesis. Ecol. Appl. 2006, 16, 2064–2090. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.-S.; Ohandja, D.-G.; Voulvoulis, N. The role of sediments as a source of metals in river catchments. Chemosphere 2012, 88, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Jin, X.; Liu, D.; Tang, W.Z.; Shan, B.Q. Assessment of the sediment quality of freshwater ecosystems in eastern China based on spatial and temporal variation of nutrients. Environ. Sci. Pollut. Res. 2017, 24, 19412–19421. [Google Scholar] [CrossRef]
- Carpenter, S.R. Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proc. Natl. Acad. Sci. USA 2005, 102, 10002–10005. [Google Scholar] [CrossRef]
- Kraal, P.; Burton, E.D.; Rose, A.L.; Cheetham, M.D.; Bush, R.T.; Sullivan, L.A. Decoupling between Water Column Oxygenation and Benthic Phosphate Dynamics in a Shallow Eutrophic Estuary. Environ. Sci. Technol. 2013, 47, 3114–3121. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, G.; Li, W.; Zhang, Y.; Zhao, L.; Gu, Z. Estimation of the algal-available phosphorus pool in sediments of a large, shallow eutrophic lake (Taihu, China) using profiled SMT fractional analysis. Environ. Pollut. 2013, 173, 216–223. [Google Scholar] [CrossRef]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Peierls, B.L.; Rossignol, K.L. Evolving Paradigms and Challenges in Estuarine and Coastal Eutrophication Dynamics in a Culturally and Climatically Stressed World. Estuaries Coasts 2014, 37, 243–258. [Google Scholar] [CrossRef]
- Huang, F.; Lin, X.; Hu, W.; Zeng, F.; He, L.; Yin, K. Nitrogen cycling processes in sediments of the Pearl River Estuary: Spatial variations, controlling factors, and environmental implications. Catena 2021, 206, 105545. [Google Scholar] [CrossRef]
- Zhao, K.; Fu, H.; Zhu, Y.; Wang, Y.; Wang, S.; Li, F. Environmental Impacts of Nitrogen and Phosphorus Nutrient Diffusion Fluxes at a Sediment-Water Interface: The Case of the Yitong River, China. Sustainability 2023, 15, 1210. [Google Scholar] [CrossRef]
- Cao, J.J.; Hu, X.Y.; Jiang, Z.T.; Li, H.W.; Zou, X.Z. Simulation of adsorption of gold nanoparticles carried by gas ascending from the Earth’s interior in alluvial cover of the middle-lower reaches of the Yangtze River. Geofluids 2010, 10, 438–446. [Google Scholar] [CrossRef]
- Yon, J.N.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414. [Google Scholar]
- Zhang, P.; Lu, J.; Zuo, L.; Wang, Y.; Liu, R.; Tao, D.; Chen, Z.; Tao, G.; Wang, K. Identification of Natural Nearly or Nanoscale Particles in Bituminous Coal: An Important Form of Elements in Coal. Sustainability 2023, 15, 6276. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, Mineral Nanoparticles, and Earth Systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef]
- Ruttenberg, K.C.; Sulak, D.J. Sorption and desorption of dissolved organic phosphorus onto iron (oxyhydr)oxides in seawater. Geochim. Cosmochim. Acta 2011, 75, 4095–4112. [Google Scholar] [CrossRef]
- Johnson, B.B.; Quill, E.; Angove, M.J. An investigation of the mode of sorption of inositol hexaphosphate to goethite. J. Colloid Interface Sci. 2012, 367, 436–442. [Google Scholar] [CrossRef]
- Wan, B.; Yan, Y.; Liu, F.; Tan, W.F.; He, J.J.; Feng, X.H. Surface speciation of myo-inositol hexakisphosphate adsorbed on TiO2 nanoparticles and its impact on their colloidal stability in aqueous suspension: A comparative study with orthophosphate. Sci. Total. Environ. 2016, 544, 134–142. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Li, J.-Y.; Xu, R.-K.; Hong, Z.-N. Sorption of organic phosphates and its effects on aggregation of hematite nanoparticles in monovalent and bivalent solutions. Environ. Sci. Pollut. Res. 2017, 24, 7197–7207. [Google Scholar] [CrossRef] [PubMed]
- Madden, A.S.; Hochella, M.F.; Luxton, T.P. Insights for size-dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption. Geochim. Cosmochim. Acta 2006, 70, 4095–4104. [Google Scholar] [CrossRef]
- Fortin, D.; Beveridge, T.J. Mechanistic routes towards biomineral surface development. In Biomineralisation: From Biology to Biotechnology and Medical Application; Baeuerlein, E., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2000. [Google Scholar]
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the Mobilization of Native Phosphorus in the Mung Bean Rhizosphere Using ZnO Nanoparticles Synthesized by Soil Fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Rathore, I.; Tarafdar, J.C. Bio-Inspired Synthesis of Nitrogen Nanoparticles and Its Application on Pearl Millet (Pennisetum americanum L.) cv. HHB 6. J. Bionanosci. 2016, 10, 300–306. [Google Scholar] [CrossRef]
- Liu, R.; Lin, X.; Wang, G.; Liu, X. Natural N-bearing nanoparticles in sediments of a shallow bay of the south china: A new N form in N-cycling. Ecol. Indic. 2021, 122, 107281. [Google Scholar] [CrossRef]
- Luo, X.; Jiao, J.J.; Moore, W.S.; Cherry, J.A.; Wang, Y.; Liu, K. Significant chemical fluxes from natural terrestrial groundwater rival anthropogenic and fluvial input in alarge-rive r deltaic estuary. Water Res. 2018, 144, 603–615. [Google Scholar] [CrossRef]
- Liu, J.; Du, J.; Wu, Y.; Liu, S. Nutrient input through submarine groundwater discharge in two major Chinese estuaries: The Pearl River Estuary and the Changjiang River Estuary. Estuar. Coast. Shelf Sci. 2018, 203, 17–28. [Google Scholar] [CrossRef]
- Yuan, X.Q.; Yang, Q.S.; Luo, X.X.; Yu, F.L.; Liu, F.; Li, J.Y.; Wang, Z.H. Distribution of grain size and organic elemental composition of the surficial sediments in Lingding Bay in the Pearl River Delta, China: A record of recent human activity. Ocean Coast. Manag. 2019, 178, 104849. [Google Scholar] [CrossRef]
- He, J.; Garzanti, E.; Cao, L.C.; Wang, H. The zircon story of the Pearl River (China) from Cretaceous to present. Earth Sci. Rev. 2020, 201, 103078. [Google Scholar] [CrossRef]
- Fu, T.; Niu, L.X.; Dang, H.M.; Yang, Q.S. Effects of seasonal variation of the river discharge input on nitrogen and phosphorus nutrients in Pearl River Estuary. Chin. J. Mar. Environ. Sci. 2023, 42, 122–130, (In Chinese with English Abstract). [Google Scholar]
- Wu, Z.; Milliman, J.D.; Zhao, D.; Cao, Z.; Zhou, J.; Zhou, C. Geomorphologic changes in the lower Pearl River Delta, 1850–2015, largely due to human activity. Geomorphology 2018, 314, 42–54. [Google Scholar] [CrossRef]
- Gao, L.-H.; Ning, J.; Bao, W.-L.T.Y.; Yan, A.; Yin, Q.-R. A study on the marine ecological security assessment of Guangdong-Hong Kong-Macao Great Bay Area. Mar. Pollut. Bull. 2022, 176, 113416. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Z.; Pei, C.M. Geology and geochemistry of source rocks of the eastern Pearl River mouth basin, South China Sea. J. Asian Earth Sci. 1993, 8, 393–406. [Google Scholar]
- Hu, D.; Clift, P.D.; Böning, P.; Hannigan, R.; Hillier, S.; Blusztajn, J.; Wan, S.M.; Fuller, D.Q. Holocene evolution in weathering and erosion patterns in the Pearl River delta. Geochem. Geophys. Geosyst. 2013, 14, 2349–2368. [Google Scholar] [CrossRef]
- GB/T 17378.5-2007; The Specificatoin for Marine Monitoring. Part 5: Sediment Analysis. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2007.
- Wang, G.Q.; Yang, T.J.; Fan, Y.T.; Bai, S.S.; Yin, P.Y. Calcium Sulfate Nanoparticles in Surface Sediments of Lingding Bay of the Pearl River Estuary, China: Implications for the Nonclassical Crystallization Pathway of Gypsum in the Natural Estuary Environment. Minerals 2023, 13, 903. [Google Scholar] [CrossRef]
- Yang, X.Z.; Wang, Q.; Qu, X.L.; Jiang, W. Bound and unbound humic acids perform different roles in the aggregation and deposition of multi-walled carbon nanotubes. Sci. Total. Environ. 2017, 586, 738–745. [Google Scholar] [CrossRef]
- Li, Y.K.; Cao, J.J.; Hopke, P.K.; Holub, R.F.; Jiang, T. The discovery of the metallic particles of groundwater from the Dongshengmiao polymetallic deposit, Inner Mongolia, and their prospecting significance. J. Geochem. Explor. 2016, 161, 49–61. [Google Scholar] [CrossRef]
- Chester, J.S.; Chester, F.M.; Kronenberg, A.K. Fracture surface energy of the Punchbowl fault, San Andreas system. Nature 2005, 437, 133–136. [Google Scholar] [CrossRef]
- Dor, O.; Ben-Zion, Y.; Rockwell, T.K.; Brune, J. Pulverized rocks in the Mojave section of the San Andreas Fault Zone. Earth Planet. Sci. Lett. 2006, 245, 642–654. [Google Scholar] [CrossRef]
- Sun, Y.; Shu, L.; Lu, X.; Liu, H.; Zhang, X.H.; Lin, A.; Kosaka, K. Recent progress in studies on the nano-sized particle layer in rock shear planes. Prog. Nat. Sci. Mater. 2008, 18, 367–373. [Google Scholar] [CrossRef]
- Wang, G.Q.; Cao, J.J.; Dai, D.L. TEM analysis of nano- or near-nanoparticles in fault gouge from the Kaxiutata iron deposit (CHN) and the implications for ore body exploration. J. Geochem. Explor. 2019, 207, 106390. [Google Scholar] [CrossRef]
- Southam, G.; Lengke, M.F.; Fairbrother, L.; Reith, F. The Biogeochemistry of Gold. Elements 2009, 5, 303–307. [Google Scholar] [CrossRef]
- Wei, X.J.; Cao, J.J.; Holub, R.F.; Hopke, P.K.; Zhao, S.J. TEM study of geogastransported nanoparticles from the Fankou lead–zinc deposit, Guangdong Province, South China. J. Geochem. Explor. 2013, 128, 124–135. [Google Scholar] [CrossRef]
- Luo, S.Y.; Cao, J.J.; Yan, H.B.; Yi, J. TEM observations of particles based on sampling in gas and soil at the Dongshengmiao polymetallic pyrite deposit, Inner Mongolia, Northern China. J. Geochem. Explor. 2015, 158, 95–111. [Google Scholar] [CrossRef]
- Yücel, M.; Gartman, A.; Chan, C.S.; Luther, G.W., III. Hydrothermal vents as a kinetically stable source of iron-sulphide-bearing nanoparticles to the ocean. Nat. Geosci. 2011, 4, 367–371. [Google Scholar] [CrossRef]
- Borm, P.J.; Kreyling, W. Toxicological Hazards of Inhaled Nanoparticles—Potential Implications for Drug Delivery. J. Nanosci. Nanotechnol. 2004, 4, 521–531. [Google Scholar] [CrossRef]
- Craft, E.S.; Abu-Qare, A.W.; Flaherty, M.M.; Garofolo, M.C.; Rincavage, H.L.; Abou-Donia, M.B. Depleted and Natural Uranium: Chemistry and Toxicological Effects. J. Toxicol. Environ. Health Part B 2004, 7, 297–317. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Tsandev, I.; Reed, D.C.; Slomp, C.P. Phosphorus diagenesis in deep-sea sediments: Sensitivity to water column conditions and global scale implications. Chem. Geol. 2012, 330–331, 127–139. [Google Scholar] [CrossRef]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V.; Loery, G.V.; Bernhardt, E.S.; Dionysiou, D.D.; Wiesner, M.R. Nanoparticle aggregation: Hallenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909. [Google Scholar] [CrossRef] [PubMed]
- Afshinnia, K.; Baalousha, M. Effect of phosphate buffer on aggregation kinetics of citrate-coated silver nanoparticles induced by monovalent and divalent electrolytes. Sci. Total Environ. 2017, 581–582, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Ingall, E.D.; Jahnke, R.A. Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochim. Cosmochim. Acta 1994, 58, 2571–2575. [Google Scholar] [CrossRef]
- Mort, H.P.; Slomp, C.P.; Gustafsson, B.G.; Andersen, T.J. Phosphorus recycling and burial in Baltic Sea sediments with contrasting redox conditions. Geochim. Cosmochim. Acta 2010, 74, 1350–1362. [Google Scholar] [CrossRef]
- Pufahl, P.K.; Groat, L.A. Sedimentary and Igneous Phosphate Deposits: Formation and Exploration: An Invited Paper. Econ. Geol. 2017, 112, 483–516. [Google Scholar] [CrossRef]
- Nelson, G.J.; Pufahl, P.K.; Hiatt, E.E. Paleoceanographic constraints on Precambrian phosphorite accumulation, Baraga Group, Michigan, USA. Sediment. Geol. 2010, 226, 9–21. [Google Scholar] [CrossRef]
- Yu, L.M.; Liu, M.X.; Dan, Y.; Said, N.; Wu, J.H.; Hou, M.C.; Zou, H. The Origin of Ediacaran Phosphogenesis Event: New Insights from Doushantou Formation in the Danzhai phosphorite deposit, South China. Ore Geol. Rev. 2022, 152, 105230. [Google Scholar] [CrossRef]
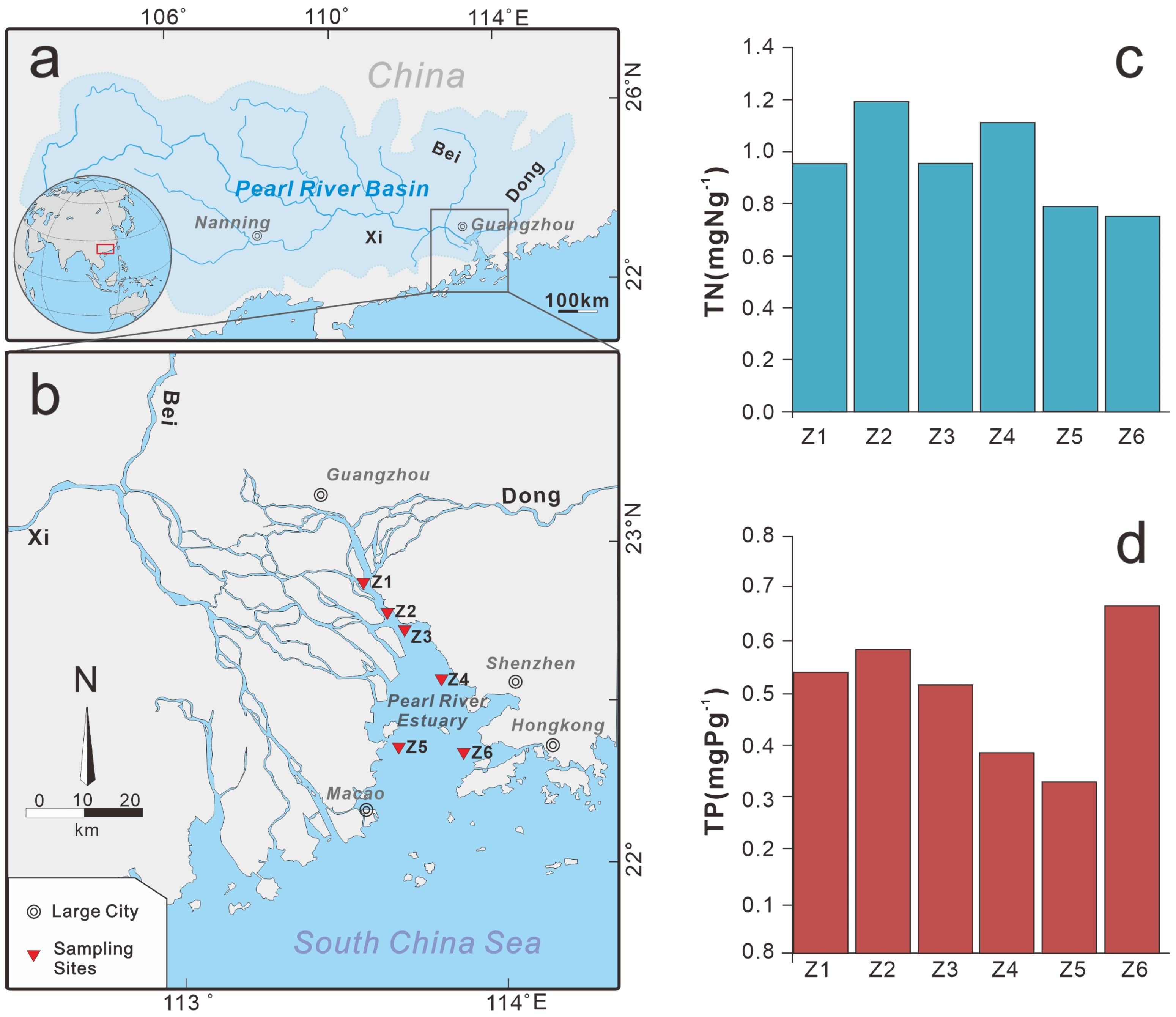
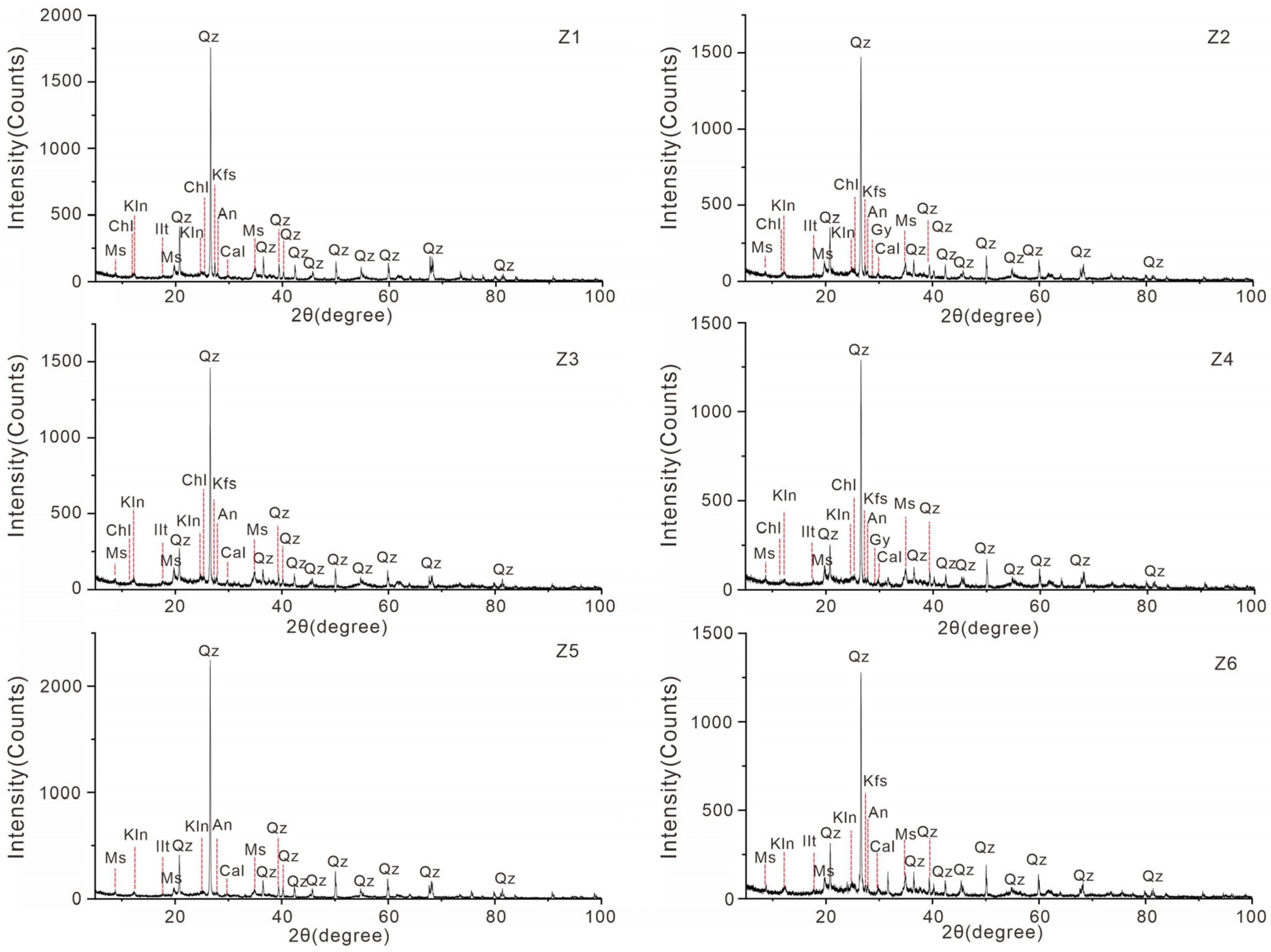
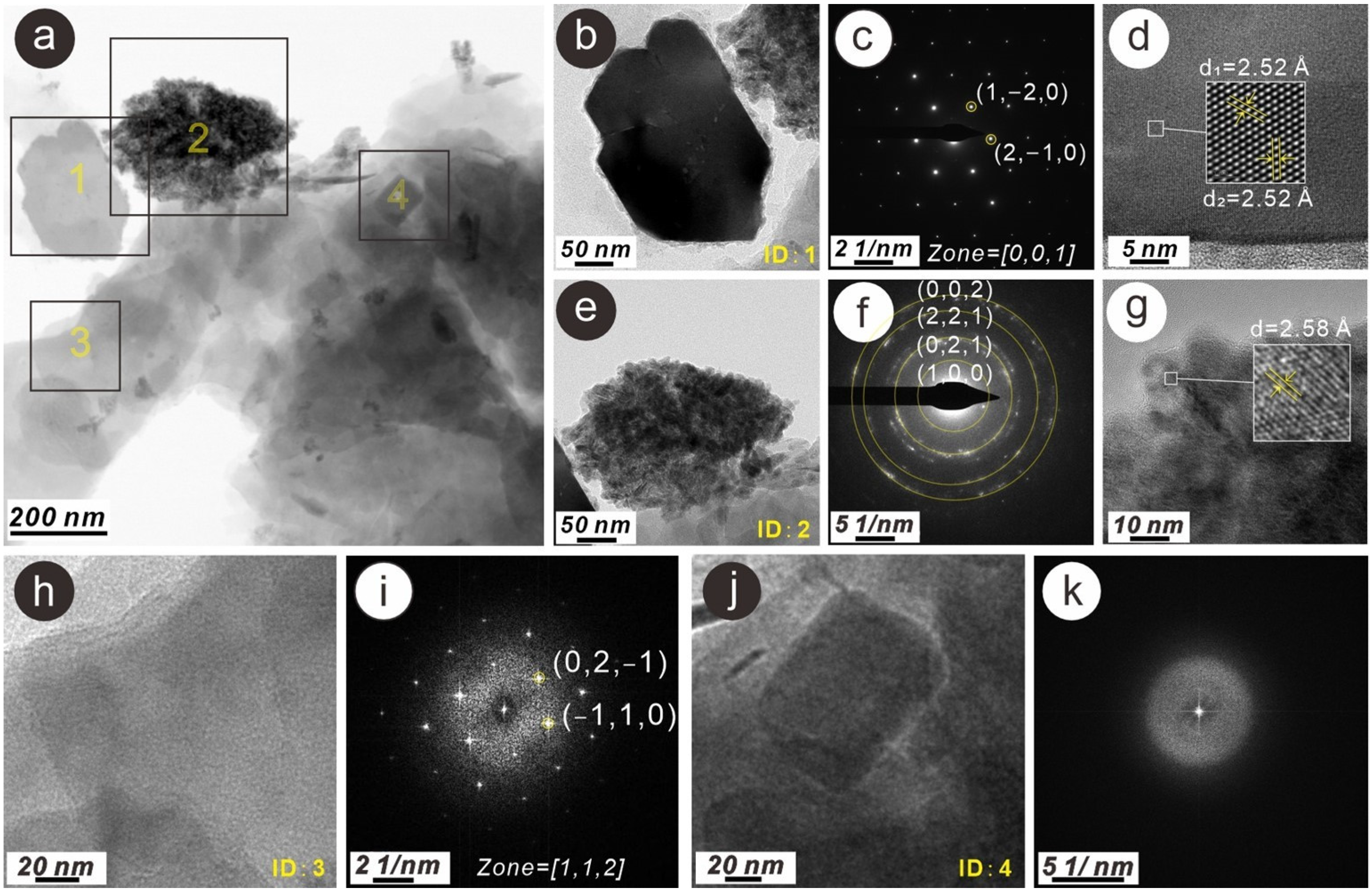


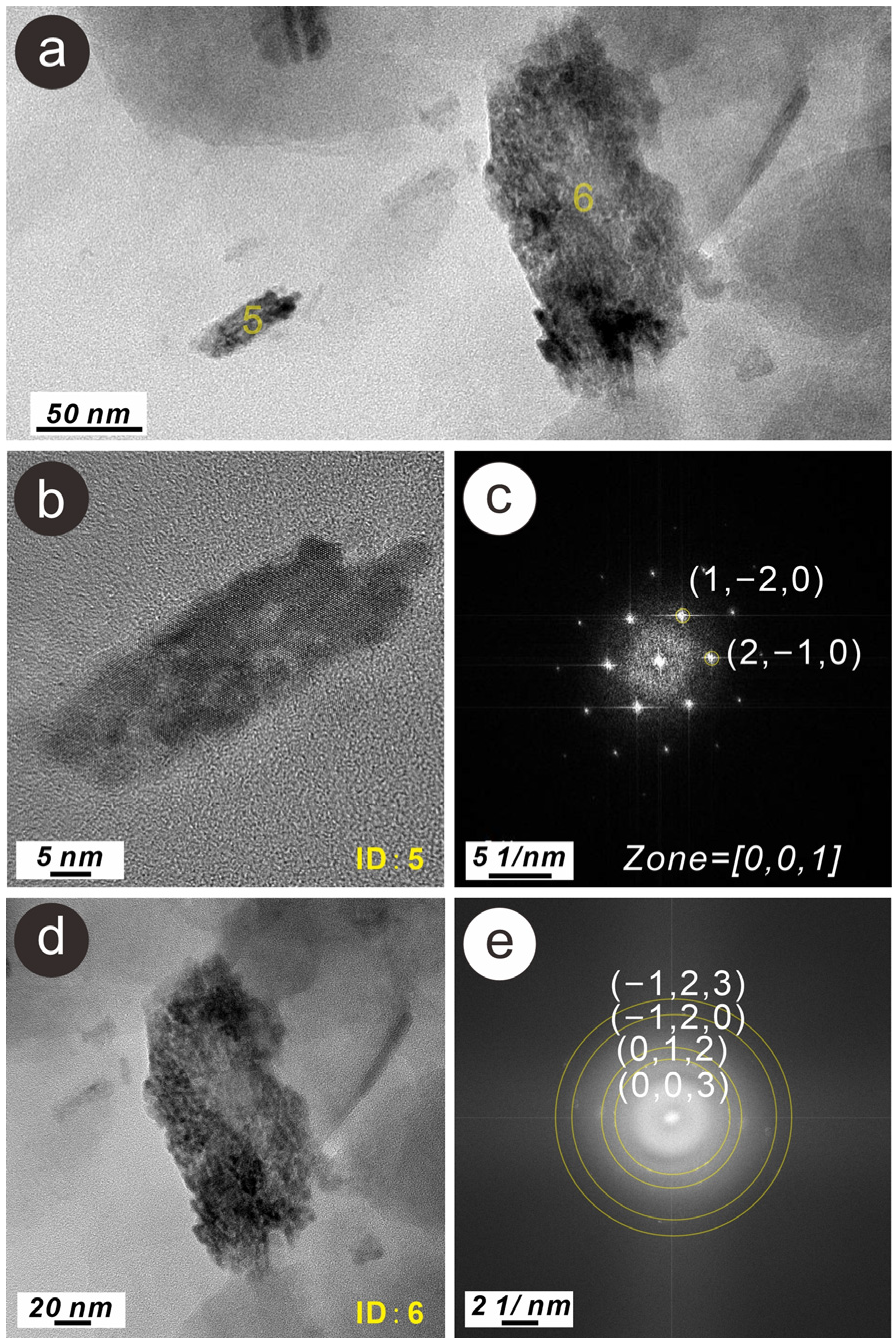



| Sample Number | Latitude (°N) | Longitude (°E) | Depth (m) | TN (mg/g) | TP (mg/g) |
|---|---|---|---|---|---|
| Z1 | 22°52′33″ | 113°33′48″ | 16.3 | 0.953 | 0.540 |
| Z2 | 22°48′49″ | 113°36′41″ | 6.0 | 1.190 | 0.584 |
| Z3 | 22°45′57″ | 113°39′10″ | 4.5 | 0.955 | 0.514 |
| Z4 | 22°34′45″ | 113°45′09″ | 13.9 | 1.113 | 0.383 |
| Z5 | 22°22′25″ | 113°40′10″ | 4.5 | 0.792 | 0.328 |
| Z6 | 22°20′25″ | 113°51′53″ | 6.5 | 0.758 | 0.668 |
| Sample Number | EDS ID | Type | Size (nm) | Shape | Crystallinity | Element | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | O | F | Na | Mg | Al | Si | P | S | Cl | K | Ca | Ti | Mn | Fe | |||||||
| Z1 | 1 | N- and P-bearing hematite nanoparticle | 150 × 200 | Irregular | Single crystal | wt% | 3.73 | 47.90 | 0.43 | 0.44 | 2.14 | 3.54 | 0.23 | 0.18 | 0.31 | 0.13 | 0.26 | 1.12 | 39.59 | ||
| at% | 6.25 | 70.21 | 0.44 | 0.42 | 1.85 | 2.96 | 0.15 | 0.12 | 0.21 | 0.08 | 0.15 | 0.54 | 16.62 | ||||||||
| Z1 | 2 | N- and P-bearing goethite nanoparticle aggregation | 150 × 200 | Irregular | Poly crystal | wt% | 3.56 | 56.76 | 0.39 | 0.80 | 4.08 | 2.39 | 1.12 | 0.66 | 0.20 | 0.13 | 0.17 | 0.84 | 28.91 | ||
| at% | 5.42 | 75.62 | 0.36 | 0.70 | 3.22 | 1.81 | 0.77 | 0.43 | 0.12 | 0.07 | 0.08 | 0.36 | 11.04 | ||||||||
| Z1 | 3 | N-bearing muscovite nanoparticle | 50 × 100 | Layer | Single crystal | wt% | 1.88 | 50.21 | 0.75 | 1.06 | 17.72 | 23.28 | 3.92 | 1.17 | |||||||
| at% | 2.70 | 63.23 | 0.79 | 0.88 | 13.24 | 16.71 | 2.03 | 0.42 | |||||||||||||
| Z1 | 4 | Ca phosphate nanoparticle | 50 × 120 | Nearly rectangle | Amorphous | wt% | 1.69 | 47.02 | 2.17 | 1.27 | 8.07 | 16.34 | 5.02 | 2.45 | 14.24 | 1.72 | |||||
| at% | 2.55 | 62.30 | 2.42 | 1.12 | 6.34 | 12.33 | 3.44 | 1.34 | 7.52 | 0.65 | |||||||||||
| Z2 | 5 | N- and P-bearing hematite nanoparticle | 10 × 50 | Strip | Single crystal | wt% | 2.15 | 43.14 | 0.74 | 0.42 | 2.33 | 2.99 | 0.69 | 0.13 | 0.10 | 0.18 | 0.10 | 0.05 | 46.99 | ||
| at% | 3.87 | 67.94 | 0.82 | 0.43 | 2.18 | 2.68 | 0.55 | 0.09 | 0.07 | 0.11 | 0.05 | 0.02 | 21.18 | ||||||||
| Z2 | 6 | N- and P-bearing hematite nanoparticle aggregation | 50 × 100 | Irregular | Poly crystal | wt% | 3.59 | 52.08 | 0.65 | 0.84 | 5.25 | 3.87 | 0.75 | 0.33 | 0.24 | 0.27 | 0.28 | 0.64 | 31.21 | ||
| at% | 5.66 | 71.82 | 0.62 | 0.76 | 4.29 | 3.04 | 0.54 | 0.23 | 0.14 | 0.14 | 0.16 | 0.28 | 12.33 | ||||||||
| Z4 | 7 | N- and P-bearing anorthite nanoparticle | 250 × 400 | Irregular | Single crystal | wt% | 2.36 | 54.61 | 0.42 | 0.29 | 13.62 | 14.95 | 0.27 | 0.10 | 0.18 | 0.14 | 12.72 | 0.33 | |||
| at% | 3.39 | 68.38 | 0.36 | 0.24 | 10.10 | 10.67 | 0.17 | 0.06 | 0.10 | 0.07 | 6.35 | 0.11 | |||||||||
| Z4 | 8 | N- and P-bearing quartz nanoparticle | 150 × 250 | Irregular | Single crystal | wt% | 1.52 | 66.52 | 0.21 | 0.15 | 0.40 | 30.61 | 0.22 | 0.09 | 0.06 | 0.05 | 0.12 | 0.06 | |||
| at% | 2.01 | 76.96 | 0.16 | 0.12 | 0.28 | 20.17 | 0.12 | 0.05 | 0.03 | 0.02 | 0.05 | 0.02 | |||||||||
| Z5 | 9 | Fe phosphate nanoparticle | 250 × 400 | Irregular | Amorphous | wt% | 51.66 | 1.13 | 1.92 | 0.37 | 0.35 | 9.97 | 1.97 | 0.18 | 1.04 | 1.58 | 29.82 | ||||
| at% | 74.09 | 1.13 | 1.82 | 0.31 | 0.28 | 7.38 | 1.40 | 0.09 | 0.59 | 0.65 | 12.25 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Yang, T.; Zhao, M.; Li, T.; Zhang, C.; Chen, Q.; Wen, X.; Dang, L. Natural Nitrogen-Bearing and Phosphorus-Bearing Nanoparticles in Surface Sediments of the Pearl River Estuary, China: Implications for Nitrogen and Phosphorus Cycling in Estuarine and Coastal Ecosystems. Sustainability 2023, 15, 14301. https://doi.org/10.3390/su151914301
Wang G, Yang T, Zhao M, Li T, Zhang C, Chen Q, Wen X, Dang L. Natural Nitrogen-Bearing and Phosphorus-Bearing Nanoparticles in Surface Sediments of the Pearl River Estuary, China: Implications for Nitrogen and Phosphorus Cycling in Estuarine and Coastal Ecosystems. Sustainability. 2023; 15(19):14301. https://doi.org/10.3390/su151914301
Chicago/Turabian StyleWang, Guoqiang, Tianjian Yang, Mengmeng Zhao, Ting Li, Cai Zhang, Qinghua Chen, Xinyue Wen, and Lirong Dang. 2023. "Natural Nitrogen-Bearing and Phosphorus-Bearing Nanoparticles in Surface Sediments of the Pearl River Estuary, China: Implications for Nitrogen and Phosphorus Cycling in Estuarine and Coastal Ecosystems" Sustainability 15, no. 19: 14301. https://doi.org/10.3390/su151914301
APA StyleWang, G., Yang, T., Zhao, M., Li, T., Zhang, C., Chen, Q., Wen, X., & Dang, L. (2023). Natural Nitrogen-Bearing and Phosphorus-Bearing Nanoparticles in Surface Sediments of the Pearl River Estuary, China: Implications for Nitrogen and Phosphorus Cycling in Estuarine and Coastal Ecosystems. Sustainability, 15(19), 14301. https://doi.org/10.3390/su151914301





