Reactive Transport Modeling and Sensitivity Analysis of CO2–Rock–Brine Interactions at Ebeity Reservoir, West Kazakhstan
Abstract
:1. Introduction
2. Lithology and Sample Characterization
3. Geochemical Modeling
3.1. Estimation of Geochemical Equilibrium
3.2. Reactive Transport Modeling
3.3. Sensitivity Analysis
3.3.1. Reactive Surface Area
3.3.2. Impurities in the Injected CO2
4. Results and Discussion
4.1. Estimation of the Equilibrium of CO2–Rock–Brine Interaction
4.2. Reactive Transport Modeling of Injected CO2
4.3. Sensitivity Analysis
4.3.1. Effect of Mineral Surface Area
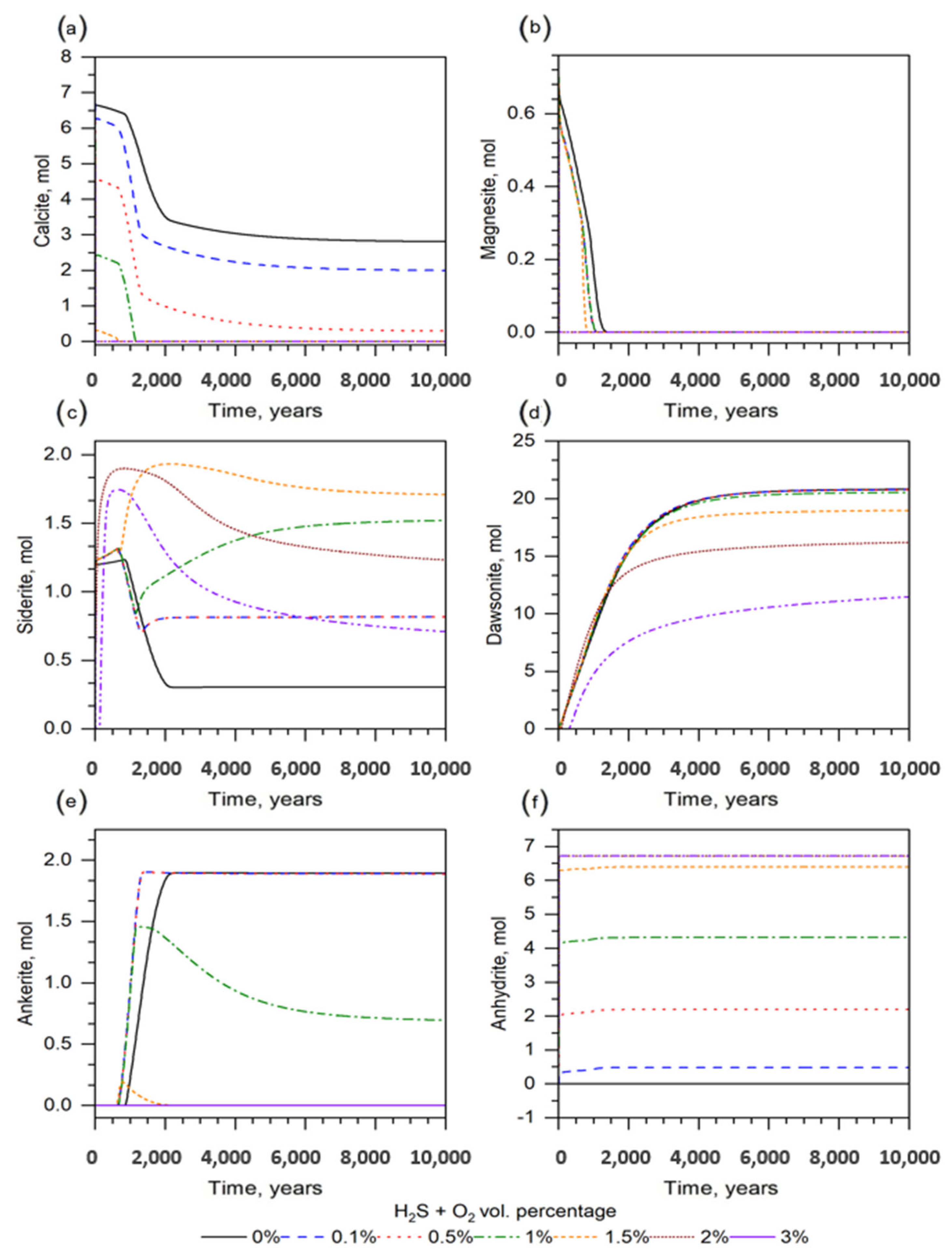
4.3.2. Effect of Impurities (H2S–O2 Gases)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nordbotten, J.M.; Celia, M.A.; Bachu, S. Injection and Storage of CO2 in Deep Saline Aquifers: Analytical Solution for CO2 Plume Evolution During Injection. Transp. Porous Media 2005, 58, 339–360. [Google Scholar] [CrossRef]
- Jenkins, C.R.; Cook, P.J.; Ennis-King, J.; Undershultz, J.; Boreham, C.; Dance, T.; de Caritat, P.; Etheridge, D.M.; Freifeld, B.M.; Hortle, A.; et al. Safe storage and effective monitoring of CO2 in depleted gas fields. Proc. Natl. Acad. Sci. USA 2012, 109, E35–E41. [Google Scholar] [CrossRef] [PubMed]
- Bachu, S.; Adams, J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 2003, 44, 3151–3175. [Google Scholar] [CrossRef]
- Ministry of Ecology and Natural Resources of the Republic of Kazakhstan. Updated Nationally Determined Contribution of the Republic of Kazakhstan to the Global Response to Climate Change. 2023. Available online: https://climatepromise.undp.org/what-we-do/where-we-work/kazakhstan (accessed on 3 August 2023).
- Climate Action Tracker. Countries: Kazakhstan. 2022. Available online: https://climateactiontracker.org/countries/kazakhstan/ (accessed on 3 August 2023).
- Kerimray, A.; Baigarin, K.; de Miglio, R.; Tosato, G. Climate change mitigation scenarios and policies and measures: The case of Kazakhstan. Clim. Policy 2016, 16, 332–352. [Google Scholar] [CrossRef]
- Abuov, Y.; Seisenbayev, N.; Lee, W. CO2 storage potential in sedimentary basins of Kazakhstan. Int. J. Greenh. Gas Control 2020, 103, 103186. [Google Scholar] [CrossRef]
- Duffy, O.B.; Fernandez, N.; Hudec, M.R.; Jackson, M.P.A.; Burg, G.; Dooley, T.P.; Jackson, C.A.L. Lateral mobility of mini basins during shortening: Insights from the SE Precaspian Basin, Kazakhstan. J. Struct. Geol. 2017, 97, 257–276. [Google Scholar] [CrossRef]
- Barde, J.-P.; Gralla, P.; Harwijanto, J.; Marsky, J. Permo-Triassic reservoirs of the Saigak Field, Precaspian Basin, Kazakhstan. Pet. Geosci. 2002, 8, 177–187. [Google Scholar] [CrossRef]
- Wigand, M.; Carey, J.W.; Schütt, H.; Spangenberg, E.; Erzinger, J. Geochemical effects of CO2 sequestration in sandstones under simulated in situ conditions of deep saline aquifers. Appl. Geochem. 2008, 23, 2735–2745. [Google Scholar] [CrossRef]
- Ajayi, T.; Salgado Gomes, J.; Bera, A. A review of CO2 storage in geological formations emphasising modeling, monitoring, and capacity estimation approaches. Pet. Sci. 2019, 16, 1028–1063. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Xu, T.; Cheng, H.; Zheng, Y.; Xiong, P. Long-term variations of CO2 trapped in different mechanisms in deep saline formations: A case study of the Songliao Basin, China. Int. J. Greenh. Gas Control 2009, 3, 161–180. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Mineral sequestration of carbon dioxide in a sandstone-shale system. Chem. Geol. 2005, 217, 295–318. [Google Scholar] [CrossRef]
- Lu, H.Y.; Lin, C.K.; Lin, W.; Liou, T.S.; Chen, W.F.; Chang, P.Y. A natural analog for CO2 mineral sequestration in Miocene basalt in the Kuanhsi-Chutung area, Northwestern Taiwan. Int. J. Greenh. Gas Control 2011, 5, 1329–1338. [Google Scholar] [CrossRef]
- Pham, V.T.H.; Aagaard, P.; Hellevang, H. On the potential for CO2mineral storage in continental flood basalts—PHREEQC batch- and 1D diffusion–reaction simulations. Geochem. Trans. 2012, 13, 5. [Google Scholar]
- Spycher, N.F.; Llanos, E.M.; Vu, H.P.; Haese, R.R. Reservoir Scale Reactive-Transport Modeling of a Buoyancy-Controlled CO2 Plume with Impurities (SO2, NO2, O2). Int. J. Greenh. Gas Control 2019, 89, 40–51. [Google Scholar] [CrossRef]
- Tambach, T.J.; Koenen, M.; Wasch, L.J.; van Bergen, F. Geochemical evaluation of CO2 injection and containment in a depleted gas field. Int. J. Greenh. Gas Control 2015, 32, 61–80. [Google Scholar] [CrossRef]
- Utomo, G.P.; Güleç, N. Preliminary geochemical investigation of a possible CO2 injection in the Ungaran geothermal field, Indonesia: Equilibrium and kinetic modeling. Greenh. Gases Sci. Technol. 2021, 11, 3–18. [Google Scholar] [CrossRef]
- Wolf, J.L.; Niemi, A.; Bensabat, J.; Rebscher, D. Benefits and restrictions of 2D reactive transport simulations of CO2 and SO2 co-injection into a saline aquifer using Toughreact v3.0-omp. Int. J. Greenh. Gas Control 2016, 54, 610–626. [Google Scholar] [CrossRef]
- Ulmishek, G.F. Petroleum Geology and Resources of the North Caspian Basin, Kazakhstan and Russia; United States Geological Survey: Reston, VA, USA, 2001. [Google Scholar]
- Barde, J.-P.; Gralla, P.; Harwijanto, J.; Marsky, J. Exploration at the eastern edge of the Precaspian Basin: Impact of data integration on Upper Permian and Triassic prospectivity. AAPG Bull. 2002, 86, 399–416. [Google Scholar] [CrossRef]
- Dudlyke, D.; Lindskog, J. Condor Petroleum Inc.: ‘Cracking the Code’—Unlocking Fresh Oil Potential within a Prolific Basin; Dundee Capital Markets: Montreal, QC, Canada, 2013. [Google Scholar]
- Bethke, C.M. Geochemical and Biogeochemical Reaction Modeling, 2nd ed.; Cambridge University Press: Cambridge, UK, 2007; pp. 1–543. ISBN 9780521875547. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; Water-Resources Investigations Report; United States Geological Survey: Reston, VA, USA, 1999. [Google Scholar] [CrossRef]
- Fatah, A.; Mahmud, H.B.; Bennour, Z.; Gholami, R.; Hossain, M. Geochemical modeling of CO2 interactions with shale: Kinetics of mineral dissolution and precipitation on geological time scales. Chem. Geol. 2022, 592, 120742. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Wissmeier, L. PhreeqcRM: A reaction module for transport simulators based on the geochemical model PHREEQC. Adv. Water Resour. 2015, 83, 176–189. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1979; Volume 7632, 604p. [Google Scholar]
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.N.; Raven, M.D.; Stanjek, H.; Krooss, B.M. Carbon dioxide storage potential of shales. Int. J. Greenh. Gas Control 2008, 2, 297–308. [Google Scholar] [CrossRef]
- Lahann, R.; Mastalerz, M.; Rupp, J.A.; Drobniak, A. Influence of CO2 on New Albany Shale composition and pore structure. Int. J. Coal Geol. 2013, 108, 2–9. [Google Scholar] [CrossRef]
- Steefel, C.I.; Lasaga, A.C. A coupled model for transport of multiple chemical species and kinetic precipitation/dissolution reactions with application to reactive flow in single phase hydrothermal systems. Am. J. Sci. 1994, 294, 529–592. [Google Scholar] [CrossRef]
- Voigt, M.; Marieni, C.; Clark, D.E.; Gíslason, S.R.; Oelkers, E.H. Evaluation and refinement of thermodynamic databases for mineral carbonation. Energy Procedia 2018, 146, 81–91. [Google Scholar] [CrossRef]
- Klajmon, M.; Havlová, V.; Červinka, R.; Mendoza, A.; Franců, J.; Berenblyum, R.; Arild, O. REPP-CO2: Equilibrium Modelling of CO2-Rock-Brine Systems. Energy Procedia 2017, 114, 3364–3373. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, G.; Apps, J.; Zhu, C. Comparison of thermodynamic data files for PHREEQC. Earth-Sci. Rev. 2022, 225, 103888. [Google Scholar] [CrossRef]
- Palandri, J.; Reed, M. Determination of in situ composition of sedimentary formation waters. Geochim. Cosmochim. Acta 2001, 65, 1741–1767. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, L.; Tan, C.; Ren, S.; Zhuang, Y.; Enechukwu, C. Injection of supercritical CO2 for geothermal exploitation from sandstone and carbonate reservoirs: CO2–water–rock interactions and their effects. J. CO2 Util. 2017, 20, 113–128. [Google Scholar] [CrossRef]
- Király, C.; Szabó, Z.; Szamosfalvi, Á.; Kónya, P.; Szabó, C.; Falus, G. How much CO2 is trapped in carbonate minerals of a natural CO2 occurrence? Energy Procedia 2017, 125, 527–534. [Google Scholar] [CrossRef]
- Yu, L.; Wu, K.; Liu, L.; Liu, N.; Ming, X.; Oelkers, E.H. Dawsonite and ankerite formation in the LDX-1 structure, Yinggehai basin, South China sea: An analogy for carbon mineralisation in subsurface sandstone aquifers. Appl. Geochem. 2020, 120, 104663. [Google Scholar] [CrossRef]
- Mohamed, I.M.; He, J.; Nasr-El-Din, H.A. Sulfate Precipitation during CO2 Sequestration in Carbonate Rock. In Proceedings of the Society of Petroleum Engineers—Middle East Turbomachinery Symposium 2011, METS—1st SPE Project and Facilities Challenges Conference at METS, Doha, Qatar, 13–16 February 2011; pp. 25–40. [Google Scholar] [CrossRef]
- Ma, B.; Cao, Y.; Zhang, Y.; Eriksson, K.A. Role of CO2-Water-Rock Interactions and Implications for CO2 Sequestration in Eocene Deeply Buried Sandstones in the Bonan Sag, Eastern Bohai Bay Basin, China. Chem. Geol. 2020, 541, 119585. [Google Scholar] [CrossRef]
- Hellevang, H.; Pham, V.T.H.; Aagaard, P. Kinetic modelling of CO2-water-rock interactions. Int. J. Greenh. Gas Control 2013, 15, 3–15. [Google Scholar] [CrossRef]
- Palandri, J.L.; Kharaka, Y.K. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geochemical Modeling; United States Geological Survey: Reston, VA, USA, 2004. [Google Scholar]
- Marty, N.C.M.; Claret, F.; Lassin, A.; Tremosa, J.; Blanc, P.; Madé, B.; Giffaut, E.; Cochepin, B.; Tournassat, C. A database of dissolution and precipitation rates for clay-rock minerals. Appl. Geochem. 2015, 55, 108–118. [Google Scholar] [CrossRef]
- Beckingham, L.E.; Mitnick, E.H.; Steefel, C.I.; Zhang, S.; Voltolini, M.; Swift, A.M.; Yang, L.; Cole, D.R.; Sheets, J.M.; Ajo-Franklin, J.B.; et al. Evaluation of mineral reactive surface area estimates for prediction of reactivity of multi-mineral sediment. Geochim. Cosmochim. Acta 2016, 188, 310–329. [Google Scholar] [CrossRef]
- Golubev, S.V.; Bénézeth, P.; Schott, J.; Dandurand, J.L.; Castillo, A. Siderite dissolution kinetics in acidic aqueous solutions from 25 to 100 °C and 0 to 50 atm pCO2. Chem. Geol. 2009, 265, 13–19. [Google Scholar] [CrossRef]
- Waldmann, S.; Busch, A.; van Ojik, K.; Gaupp, R. Importance of mineral surface areas in Rotliegend sandstones for modeling CO2-water-rock interactions. Chem. Geol. 2014, 378–379, 89–109. [Google Scholar] [CrossRef]
- Leal, A.M.M.; Blunt, M.J.; LaForce, T.C. A chemical kinetics algorithm for geochemical modeling. Appl. Geochem. 2015, 55, 46–61. [Google Scholar] [CrossRef]
- Tarokh, A.; Makhnenko, R.Y.; Kim, K.; Zhu, X.; Popovics, J.S.; Segvic, B.; Sweet, D.E. Influence of CO2 injection on the poromechanical response of Berea sandstone. Int. J. Greenh. Gas Control 2020, 95, 102959. [Google Scholar] [CrossRef]
- Verma, Y.; Vishal, V.; Ranjith, P.G. Sensitivity Analysis of Geomechanical Constraints in CO2 Storage to Screen Potential Sites in Deep Saline Aquifers. Front. Clim. 2021, 3, 115. [Google Scholar] [CrossRef]
- Balashov, V.N.; Guthrie, G.D.; Hakala, J.A.; Lopano, C.L.; Rimstidt, J.D.; Brantley, S.L. Predictive modeling of CO2 sequestration in deep saline sandstone reservoirs: Impacts of geochemical kinetics. Appl. Geochem. 2013, 30, 41–56. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Zhu, C.; Xiao, Y. Coupled reactive flow and transport modeling of CO2 sequestration in the Mt. Simon sandstone formation, Midwest USA. Int. J. Greenh. Gas Control 2011, 5, 294–307. [Google Scholar] [CrossRef]
- Qin, F.; Beckingham, L.E. The impact of mineral reactive surface area variation on simulated mineral reactions and reaction rates. Appl. Geochem. 2021, 124, 104852. [Google Scholar] [CrossRef]
- Pearce, J.K.; Kirste, D.M.; Dawson, G.K.W.; Rudolph, V.; Golding, S.D. Geochemical modeling of experimental O2–SO2–CO2 reactions of reservoir, cap-rock, and overlying cores. Appl. Geochem. 2019, 109, 104400. [Google Scholar] [CrossRef]
- Mohd Amin, S.; Weiss, D.J.; Blunt, M.J. Reactive transport modeling of geologic CO2 sequestration in saline aquifers: The influence of pure CO2 and mixtures of CO2 with CH4 on the sealing capacity of cap rock at 37 °C and 100 bar. Chem. Geol. 2014, 367, 39–50. [Google Scholar] [CrossRef]
- Mokhatab, S.; Poe, W.A.; Mak, J.Y. Basic Concepts of Natural Gas Processing. In Handbook of Natural Gas Transmission and Processing; Gulf Professional Publishing: Houston, TX, USA, 2019; pp. 177–189. [Google Scholar] [CrossRef]
- Pham, V.T.H.; Lu, P.; Aagaard, P.; Zhu, C.; Hellevang, H. On the Potential of CO2–Water–Rock Interactions for CO2 Storage Using a Modified Kinetic Model. Int. J. Greenh. Gas Control 2011, 5, 1002–1015. [Google Scholar] [CrossRef]
- Fatah, A.; Bennour, Z.; Mahmud, H.; Gholami, R.; Hossain, M. Surface wettability alteration of shales exposed to CO2: Implication for the long-term integrity of geological storage sites. Int. J. Greenh. Gas Control 2021, 110, 103426. [Google Scholar] [CrossRef]
- He, H.; Chong, T.; Gang, J.; Ke, H. Evaluating the CO2 Geological Storage Suitability of Coal-Bearing Sedimentary Basins in China. Environ. Monit. Assess. 2020, 192, 462. [Google Scholar] [CrossRef]
- Johnson, J.; Nitao, J.; Knauss, K. Reactive transport modelling of CO2 storage in saline aquifers to elucidate fundamental processes, trapping mechanisms and sequestration partitioning. Geol. Soc. Lond. Spec. Publ. 2004, 233, 107–128. [Google Scholar] [CrossRef]
- Fauziah, C.A.; Al-Khdheeawi, E.A.; Iglauer, S.; Barifcani, A. Effect of Clay Minerals Heterogeneity on Wettability Measurements: Implications for CO2 Storage. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 2–6 November 2020. [Google Scholar] [CrossRef]
- Knauss, K.; Wolery, T. Muscovite dissolution kinetics as a function of pH and time at 70 °C. Geochim. Cosmochim. Acta 1989, 53, 1493–1501. [Google Scholar] [CrossRef]
- Hellevang, H.; Declercq, J.; Aagaard, P. Why is Dawsonite Absent in CO2 Charged Reservoirs? Oil Gas Sci. Technol. Rev. d’IFP Energ. Nouv. 2011, 66, 119–135. [Google Scholar] [CrossRef]
- Koukouzas, N.; Kypritidou, Z.; Purser, G.; Rochelle, C.; Vasilatos, C.; Tsoukalas, N. Assessment of the impact of CO2 storage in sandstone formations by experimental studies and geochemical modeling: The case of the Mesohellenic Trough, NW Greece. Int. J. Greenh. Gas Control 2018, 71, 116–132. [Google Scholar] [CrossRef]
- Hellevang, H.; Aagaard, P.; Oelkers, E.; Kvamme, B. Can Dawsonite Permanently Trap CO2? Environ. Sci. Technol. 2005, 39, 8281–8287. [Google Scholar] [CrossRef] [PubMed]
- Shabani, B.; Lu, P.; Kammer, R.; Zhu, C. Effects of Hydrogeological Heterogeneity on CO2 Migration and Mineral Trapping: 3D Reactive Transport Modeling of Geological CO2 Storage in the Mt. Simon Sandstone, Indiana, USA. Energies 2022, 15, 2171. [Google Scholar] [CrossRef]
- Wang, J.; David, R.; Anthony, E.J.; Wildgust, N.; Aiken, T. Effects of Impurities on CO2 Transport, Injection and Storage. Energy Procedia 2011, 4, 3071–3078. [Google Scholar] [CrossRef]
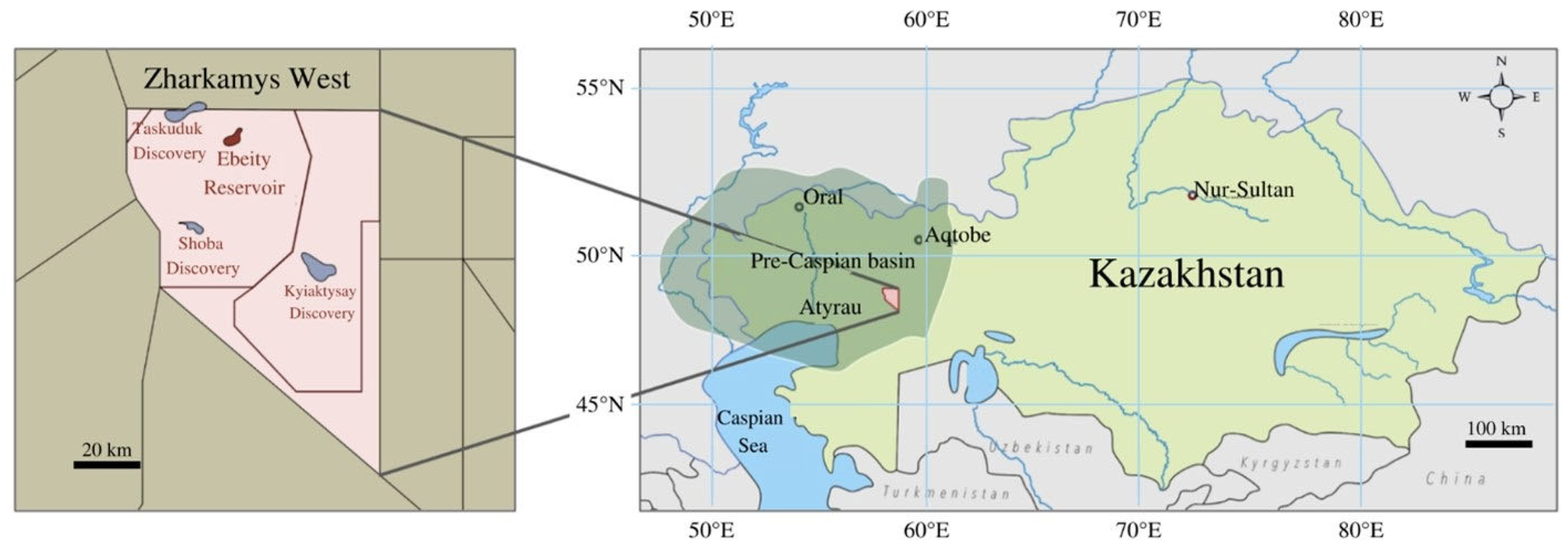


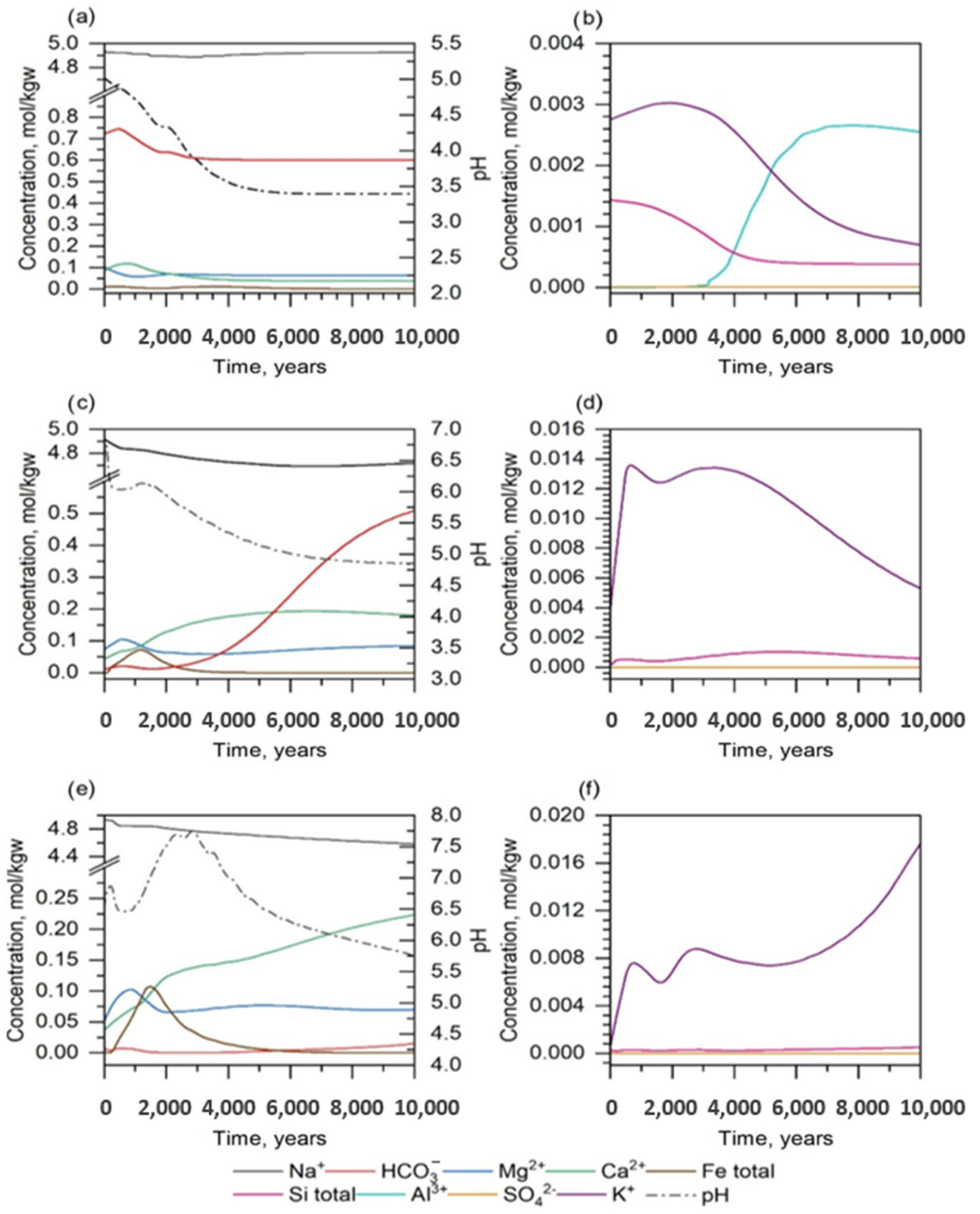
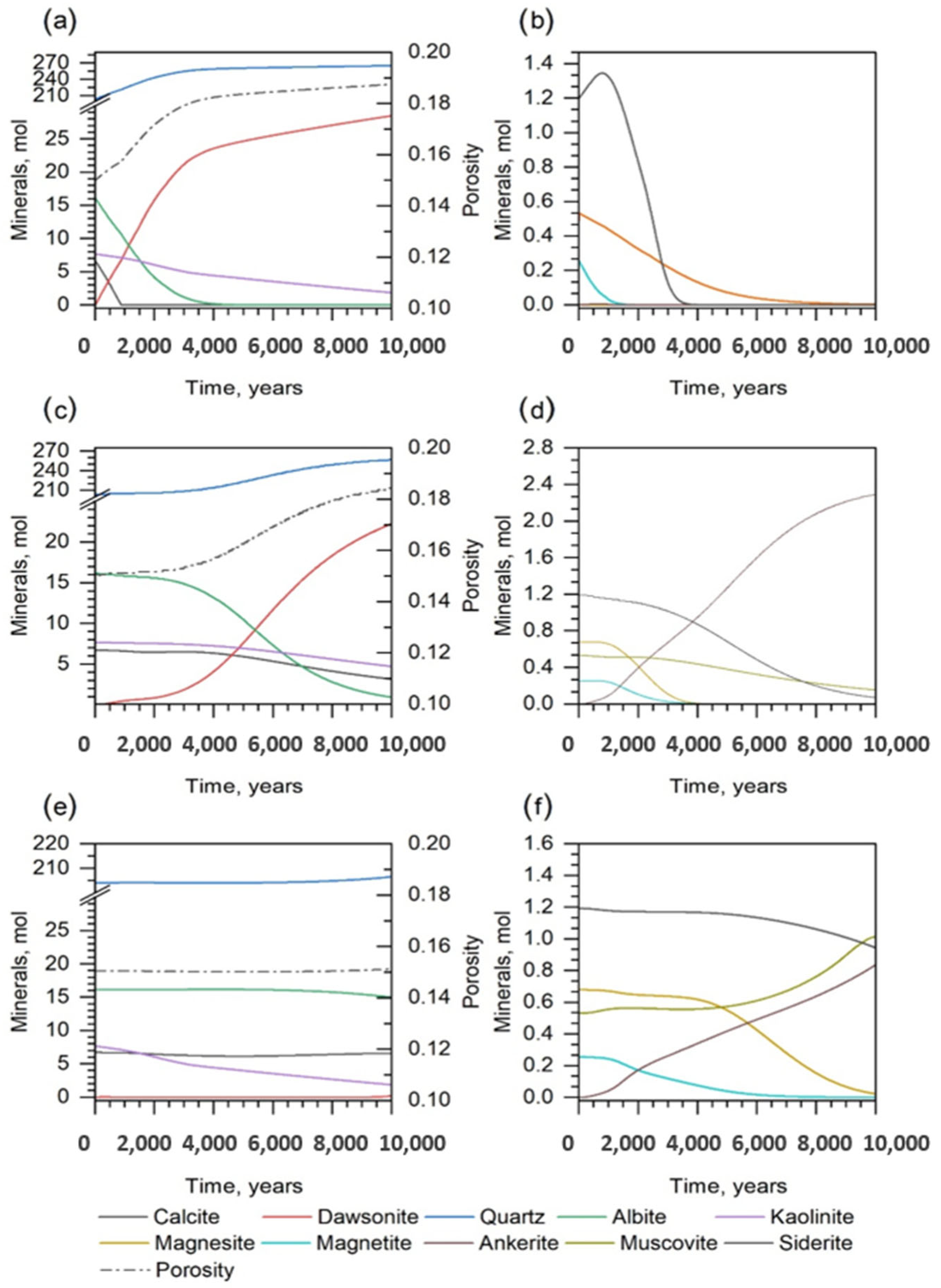

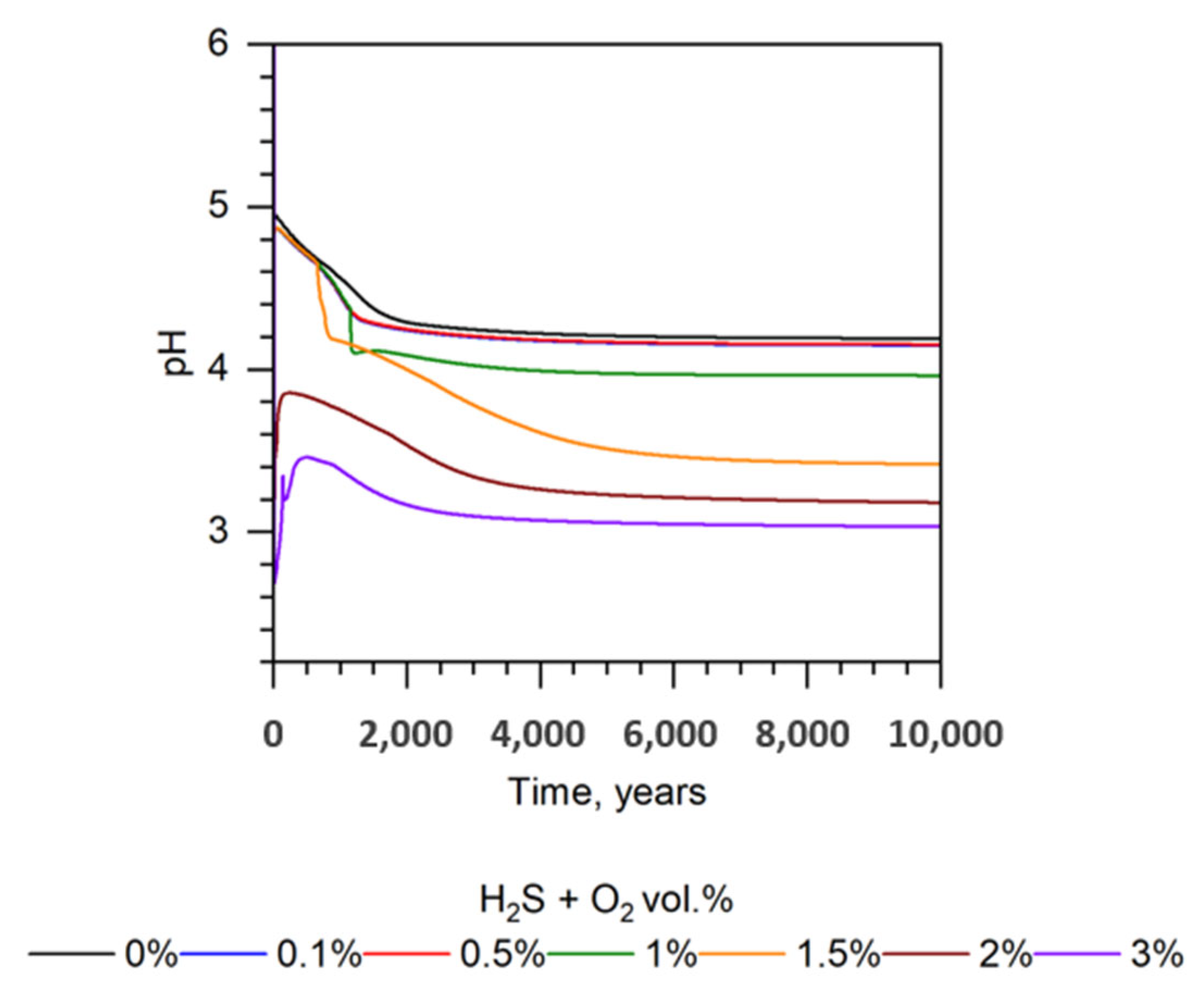

| Mineral | Chemical Formula | Weight Percent [%] | Concentration [mol/kgw] |
|---|---|---|---|
| Primary | |||
| Quartz | SiO2 | 62.2 | 204.002 |
| Albite | NaAlSi3O8 | 21.6 | 16.183 |
| Calcite | CaCO3 | 3.4 | 6.694 |
| Kaolinite | Al2Si2O5(OH)4 | 10.1 | 7.712 |
| Magnesite | MgCO3 | 0.30 | 0.701 |
| Siderite | FeCO3 | 0.70 | 1.191 |
| Muscovite | KAl3Si3O10(OH)2 | 1.0 | 0.494 |
| Magnetite | Fe3O4 | 0.30 | 0.255 |
| Secondary | |||
| Dawsonite | NaAlCO3(OH)2 | 0.0 | 0.0 |
| Ankerite | Ca (Mg, Fe) (CO3)2 | 0.0 | 0.0 |
| Parameter | Value | Elements | Concentration [mg/kgw] |
|---|---|---|---|
| Density at 20.0 °C (kg/L) | 1.1596 | Cations | |
| Resistance at 20.0 °C (Ω) | 0.0547 | Na | 100,513 |
| Temperature (°C) | 40 | Ca | 1830 |
| pH | 5.97 | K | 1400 |
| pe | −4.00 | Mg | 876 |
| Sr | 61 | ||
| Fe | 34 | ||
| Anions | |||
| Cl− | 165,211 | ||
| SO42− | 0.1 |
| Parameter | Unit | ||
|---|---|---|---|
| Brine composition | mol/kgw | Al | 1.16 × 10−8 |
| C | 8.39 × 10−3 | ||
| Ca | 4.56 × 10−2 | ||
| Cl | 5.23 | ||
| Fe | 7.46 × 10−4 | ||
| K | 4.02 × 10−2 | ||
| Mg | 7.48 × 10−2 | ||
| Na | 4.87 | ||
| S | 1.85 × 10−3 | ||
| Si | 1.84 × 10−4 | ||
| - kg/m3 | pH | 6.552 | |
| Density | 1.16 × 103 | ||
| Mineral composition | mol/kgw | Albite | 16.18 |
| Quartz | 204 | ||
| Kaolinite | 7.71 | ||
| Calcite | 6.69 | ||
| Siderite | 1.19 | ||
| Magnesite | 0.7 | ||
| Magnetite | 0.26 | ||
| Muscovite | 0.49 | ||
| Ankerite | 0 | ||
| Anhydrite | 0 | ||
| Dawsonite | 0 | ||
| Temperature | °C | 40 | |
| Pressure | bar | 88.8 | |
| Mineral | Neutral Mechanism | Acid Mechanism | Reactive Surface Area | ||||
|---|---|---|---|---|---|---|---|
| logknu25 | Enu [kJ mol−1] | logknu25 | Enu [kJ mol−1] | nH | S [m2/g mineral] | Ref. | |
| Quartz | −13.99 | 87.7 | - | - | - | 0.111 | [43] |
| Albite | −11.84 | 69.8 | −10.16 | 65.0 | 0.457 | 0.164 | [43] |
| Calcite | Equilibrium | ||||||
| Kaolinite | −13.18 | 22.2 | −11.31 | 65.9 | 0.777 | 3.17 | [43] |
| Magnetite | −10.78 | 18.6 | −8.59 | 18.6 | 0.279 | 0.1 | [40] |
| Siderite | −13.6 | 56.0 | 56.0 | 0.6 | 0.175 | [44] | |
| Muscovite | −13.55 | 22.0 | −11.85 | 22.0 | 0.370 | 0.68 | [40] |
| Ankerite | 48.0 | −7.5 | 48.0 | 0.94 | 0.18 | [45] | |
| Anhydrite | Equilibrium | ||||||
| Magnesite | −9.34 | 23.5 | −6.38 | 14.4 | 1.00 | 0.8 | [46] |
| Dawsonite | Equilibrium | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seisenbayev, N.; Absalyamova, M.; Alibekov, A.; Lee, W. Reactive Transport Modeling and Sensitivity Analysis of CO2–Rock–Brine Interactions at Ebeity Reservoir, West Kazakhstan. Sustainability 2023, 15, 14434. https://doi.org/10.3390/su151914434
Seisenbayev N, Absalyamova M, Alibekov A, Lee W. Reactive Transport Modeling and Sensitivity Analysis of CO2–Rock–Brine Interactions at Ebeity Reservoir, West Kazakhstan. Sustainability. 2023; 15(19):14434. https://doi.org/10.3390/su151914434
Chicago/Turabian StyleSeisenbayev, Nurlan, Miriam Absalyamova, Alisher Alibekov, and Woojin Lee. 2023. "Reactive Transport Modeling and Sensitivity Analysis of CO2–Rock–Brine Interactions at Ebeity Reservoir, West Kazakhstan" Sustainability 15, no. 19: 14434. https://doi.org/10.3390/su151914434
APA StyleSeisenbayev, N., Absalyamova, M., Alibekov, A., & Lee, W. (2023). Reactive Transport Modeling and Sensitivity Analysis of CO2–Rock–Brine Interactions at Ebeity Reservoir, West Kazakhstan. Sustainability, 15(19), 14434. https://doi.org/10.3390/su151914434









