1. Introduction
In recent years, with the deterioration of the global environment, drought has become a major non-biological limiting factor for agricultural production worldwide [
1,
2,
3], and it is also one of the most severe challenges facing the world’s agricultural production [
4,
5]. With the further deterioration of the global environment, the scope and extent of drought tend to further increase [
6]. At the same time, the world’s water shortage has also sounded the alarm for world food production. Therefore, identifying how to obtain the maximum economic, ecological, and social benefits with efficient water consumption is a very important development strategy issue.
Photosynthesis is the primary process by which plants generate energy and accumulate substances. When photosynthesis is disrupted, it affects the overall growth, development, and physiological and biochemical mechanisms of the plant [
7,
8,
9,
10,
11]. Drought stress significantly impacts photosynthesis, as it is highly sensitive to water scarcity. The decrease in light energy use efficiency is a major factor contributing to the loss of crop yield under water stress conditions [
12,
13]. The main effect of drought stress on plants is a reduction in their CO
2 assimilation capacity, which in turn restricts plant growth. [
14].
In recent years, there has been a growing number of studies focusing on the molecular mechanisms underlying plant photosynthesis, specifically exploring the genes associated with metabolic adjustment [
15,
16,
17,
18]. These studies primarily investigate the enzymes involved in plant photosynthesis [
19,
20]. Throughout the process of long-term species evolution, certain plants have developed adaptive strategies by altering their metabolic pathways to suit their environment. For instance, in C
3 barley (
Hordeum vulgare), the glume exhibits a higher content of PEPCase compared with the leaves. PEPCase is a crucial enzyme in the C
4 pathway, suggesting the potential existence of the C
4 pathway in C
3 plants [
21]. Similarly, in the leaves of
Glycine max, a C
3 crop, key enzymes in the C
4 pathway are present, forming a relatively complete C
4 pathway [
22]. Consequently, it can be inferred that the C
4 pathway exists in C
3 plants.
P. cornutum (L.) Gaertn. is endemic to China. Growing on the sandy land of the Inner Mongolia Plateau, it has strong vitality and excellent drought resistance. This plant possesses rich nutritional value and can be utilized as food [
23], feed, and medicine and also for health care, windbreak, and sand fixation [
24,
25]. Additionally, it demonstrates good stress resistance [
26,
27] and plays a crucial role in preventing desertification, protecting the ecological environment, and maintaining ecological balance [
28]. However,
P. cornutum is a wild plant that is distributed in deserts. Unfortunately, its natural resources are diminishing, and the species is currently endangered.
In this study, we investigated the transcriptome sequencing and biological carbon sequestration pathways in the leaves of P. cornutum under drought stress. The aim of this study is to understand the molecular mechanism underlying biological carbon sequestration under drought stress and to contribute to the development of high-quality germplasm for plant water-saving technologies. Additionally, the findings of this study can serve as a theoretical foundation for sustainable agricultural development.
2. Materials and Methods
2.1. Basic Condition of the Test Site
The experiment was conducted in the awning of Science and Technology Park of Inner Mongolia Agricultural University. The culture substrate was composed of sand and decomposed barnyard manure at a volume ratio of 4:1. The substrate contained total N (2.20 g·kg−1), available P (0.94 g·kg−1), and rapid available K (1.13 g·kg−1), and the maximum water holding capacity (WHC) in the field was 13.74 g·100 g−1. The seedling bowl for planting was 12.5 cm high × 12.5 cm diameter. During the test, the temperature range was 20 ± 5 °C and the humidity range was 60 ± 5%.
2.2. Materials and Experimental Design
Seeds of P. cornutum were collected from the Mu Us Desert in Ordos, Inner Mongolia Autonomous Region of China. P. cornutum seeds were disinfected using a 2% hypochlorous acid solution for 10 min, rinsed with distilled water, and then placed in an incubator for germination. In April of the following year, the germinated seeds were sown in a seedling bowl containing 600 g of substrate. From the time of sowing until the start of the experiment, the soil water content in the pots was maintained at 70–80% of the water holding capacity (WHC) using a weighing method. Seedlings with identical morphology were selected at the 6-leaf stage. The degree of drought stress was determined based on the soil water content, which was divided into the following categories: control (73.65 ± 2.33%), moderate drought stress (50.22 ± 3.65%), and severe drought stress (32.47 ± 1.37%). The evening before the experiment, the pots were filled with water to reach 70–80% WHC after being weighed. Samples were taken at 7:00 every morning, with eight pots randomly selected as one treatment. The first day of sampling served as the control.
2.3. RNA Isolation and Library Preparation
A Quick RNA Isolation Kit (Huayueyang, Beijing, China) was utilized for the extraction of total RNA, adhering to the protocol provided by the manufacturer. Following extraction, the integrity of the RNA was assessed using an Agilent 2000 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Subsequent analyses were performed exclusively on samples exhibiting an RNA integrity number (RIN) of 7 or higher. For the creation of libraries, a TruSeq Stranded mRNA LTSample Prep Kit (Illumina, San Diego, CA, USA) was used, following the manufacturer’s instructions. An Illumina sequencing platform (HiSeqTM 2000) was then utilized to sequence these libraries, generating paired-end reads of 125 bp/150 bp.
2.4. Analysis of RNA Sequencing and Differentially Expressed Genes (DEGs)
The sequencing of the libraries was conducted on an Illumina HiSeq X Ten platform, resulting in the generation of paired-end reads with a length of 150 bp. Raw reads for each sample from
P. cornutum (L.) Gaertn. were obtained. To process the raw data (raw reads), the Trimmomatic software [
29] (v0.40) was used. The reads that contained ploy-N and those with low quality were eliminated to obtain clean reads. After the removal of adaptor sequences and low-quality reads, the clean reads were assembled into clusters of expressed sequence tags (contigs) and further de novo assembled into transcripts with the application of Trinity [
30] (version: 2.4) using the paired-end method. The longest transcript in terms of similarity and length was selected as the unigene for subsequent analyses.
2.5. Functional Annotation
Functional annotation of the unigenes was achieved by aligning the unigenes with the NCBI non-redundant (NR), Swiss-Prot, evolutionary genealogy of genes: Non-supervised Orthologous Groups (eggNOG) and Clusters of Orthologous Groups for eukaryotic complete genomes (KOG) databases using the Blastx method [
31] with a threshold E-value of 10
−5. The proteins showing the highest similarities to the unigenes were utilized to assign functional annotations. Additionally, the unigenes were subjected to mapping in the Kyoto Encyclopedia of Genes and Genomes (KEGG) [
32] database to provide annotations on potential metabolic pathways. The Gene Ontology (GO) classification was performed by establishing a mapping relationship between the Swiss-Prot and GO term.
After annotation, FPKM [
33] and the read count value of each unigene were calculated using bowtie2 [
34] and eXpress [
35]. Differentially expressed genes (DEGs) between different groups were identified using the DESeq [
36] (2012) functions estimate Size Factors and nbinom Test. A threshold
p-value < 0.05 and fold change > 2 or fold change < 0.5 was established to determine significantly differential expression. The expression pattern of genes in different groups and samples was showcased with the implementation of hierarchical cluster analysis on DEGs. Subsequently, DEGs underwent GO enrichment and KEGG pathway enrichment analysis separately using the hypergeometric distribution method.
2.6. Analysis of Quantitative Real-Time PCR
While RNA sequencing (RNA-seq) is a robust technique utilized to investigate the complete transcriptome, when examining billions of short reads obtained from RNA-seq, some errors may occur in the assembly of the transcriptome. Therefore, 8 single genes were randomly selected to verify the sequencing and transcription abundance results of the expressed products, and the Power SYBR
® Premix Ex Taq
TM II Kit (TaKaRa) was used for qRT-PCR analysis. The experimental parameters included heating the reaction mixture at a temperature of 95 °C for a duration of 10 min. Subsequently, a series of 40 cycles were performed, comprising a denaturation step at 95 °C for 15 s, followed by an annealing step at 60 °C for 5 min. Finally, the reaction was subjected to dissociation at 94 °C for a period of 90 s, then cooled to 45 °C for three minutes, followed by a final denaturation step at 94 °C for 10 s. R1_Unigene_BMK.22570 was used as a reference [
25]. The 2
−ΔΔCt method was used to calculate the relative expression level of the selected single gene for standardization analysis. Primers were selected using Premier 5 (
Table 1).
4. Discussion
Photosynthesis, a crucial plant process, is significantly impacted by drought stress. Plants have different photosynthetic mechanisms, namely, the C3, C4, and CAM pathways. P. cornutum, an edible and medicinal plant, is a wild species found in deserts, and these plants are widely distributed in the arid and semi-arid regions of China. They have developed a variety of physiological and biochemical strategies to adapt to and tolerate conditions of drought stress. The molecular mechanism underlying drought stress tolerance in P. cornutum is currently not understood. In order to gain insight into this, we utilized Illumina DNA sequencing technology to identify genes that are involved in the response to drought stress. This discovery will serve as a starting point for future research aimed at understanding the molecular mechanism and eventually contributing to the development of sustainable agricultural practices for P. cornutum under drought conditions.
In this investigation, we used advanced Illumina sequencing technology to examine the transcriptome of
P. cornutum seedlings experiencing drought-related pressures. The total number of sequenced bases was 5,833,832,040 nt, with a Q20 percentage of 97.89%. After filtration, the N percentage was 0, and the GC percentage was 46.15%. These results indicate that the transcriptome sequencing data are highly reliable and can provide valuable original data for subsequent data assembly. It is worth noting that our Illumina sequencing results for reads and unigenes differed from those of Wang et al. [
25]. This discrepancy may be attributed to variations in the organs and treatments used in the same plant, which can lead to significant differences in the number of reads and unigenes.
Under moderate drought stress, 463 genes were upregulated, and 812 genes were downregulated. On the other hand, under extremely severe drought stress, 1296 genes were upregulated and 1484 genes were downregulated. These findings indicate that as the intensity of drought increases, the number of differentially expressed genes also increases. Interestingly, the number of genes affected by moderate drought stress was smaller compared with extremely severe drought stress. This suggests that
P. cornutum is less affected by genes under moderate drought stress, which could explain its ability to withstand extremely severe drought. Similar results were observed in maize seedlings by Min H et al. [
37]. Additionally, it has been demonstrated that plants are more vulnerable to drought stress and regulate the expression of a larger number of genes in response to stress [
38].
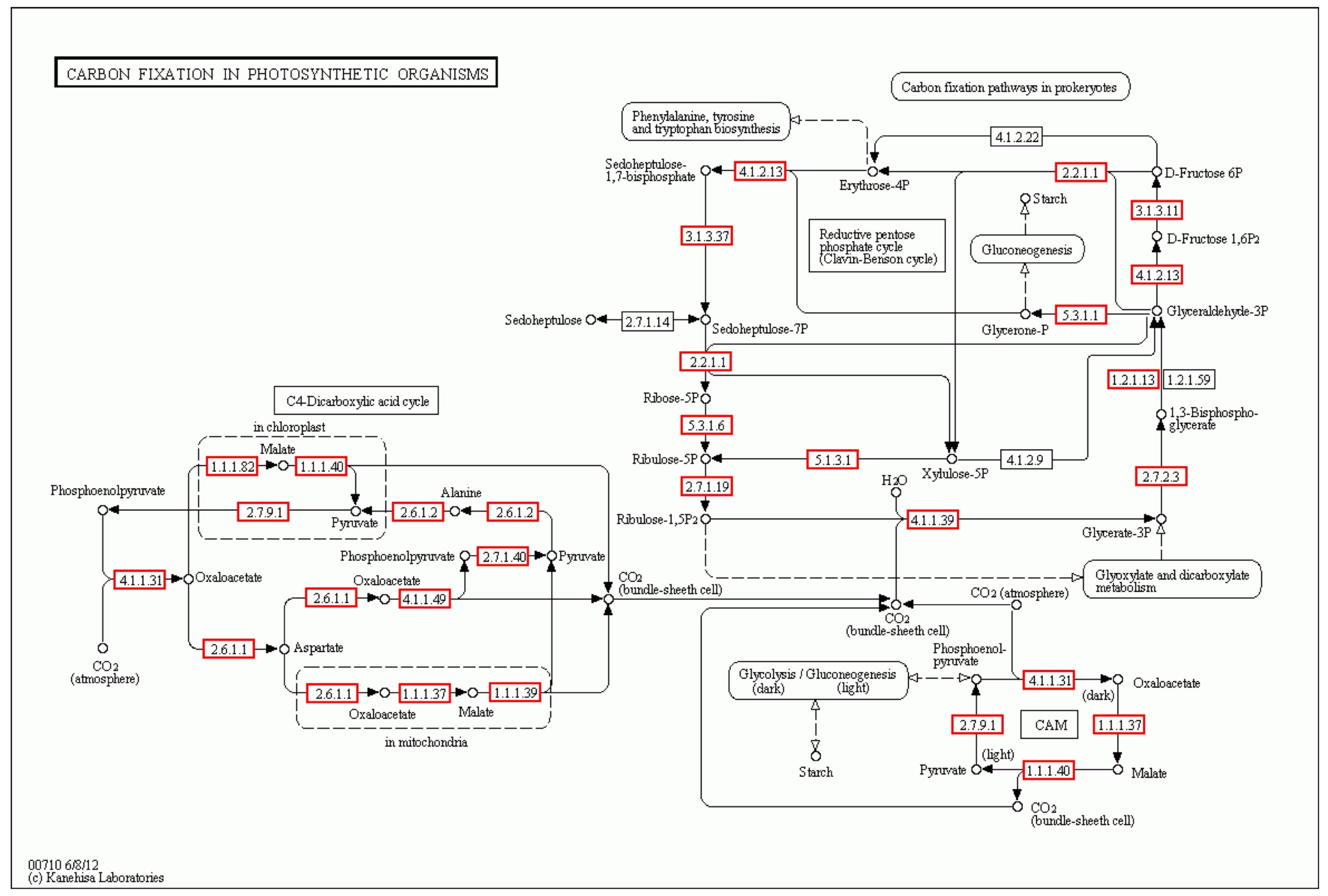
Previous studies have shown that CO
2 assimilation occurs using the high-energy substances ATP and NADPH, which provide assimilation power to convert CO
2 into stable chemical energy, such as sugar and starch [
39]. This study discovered the presence of a C
4 photosynthetic pathway in
P. cornutum, which enables this species to effectively utilize CO
2 even under water stress.
Under moderate drought stress, the uptake of CO2 by P. cornutum was inhibited, but it had little effect on the CO2 assimilation stage, light energy acceptance, and conversion of light energy into chemical energy. However, under extremely severe drought stress, the photosynthetic pathway of P. cornutum was severely inhibited. In the C4 photosynthetic pathway, some sites showed upregulation of DEGs, and the gene expression in some sites of the C4 cycle was only upregulated during the CO2 assimilation stage. This suggests that under extremely severe drought stress, although the photosynthetic acceptance and transmission ability of P. cornutum were inhibited, its ability to assimilate CO2 using high-energy substances was enhanced to some extent. This can partially alleviate the inhibition of drought on photosynthesis and ensure the material demand of seedling under severe drought stress.