Direct Observation of Transient Flow Kinematics of Environment-Friendly Silica-Based Alcogel at Instantaneous Gelation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gelling Solution and Induction of Its Gelation
2.3. Direct Observation of Gelation in a Shaken Transparent Cylinder
2.4. Direct Observation of Gelation in a Stirred Container
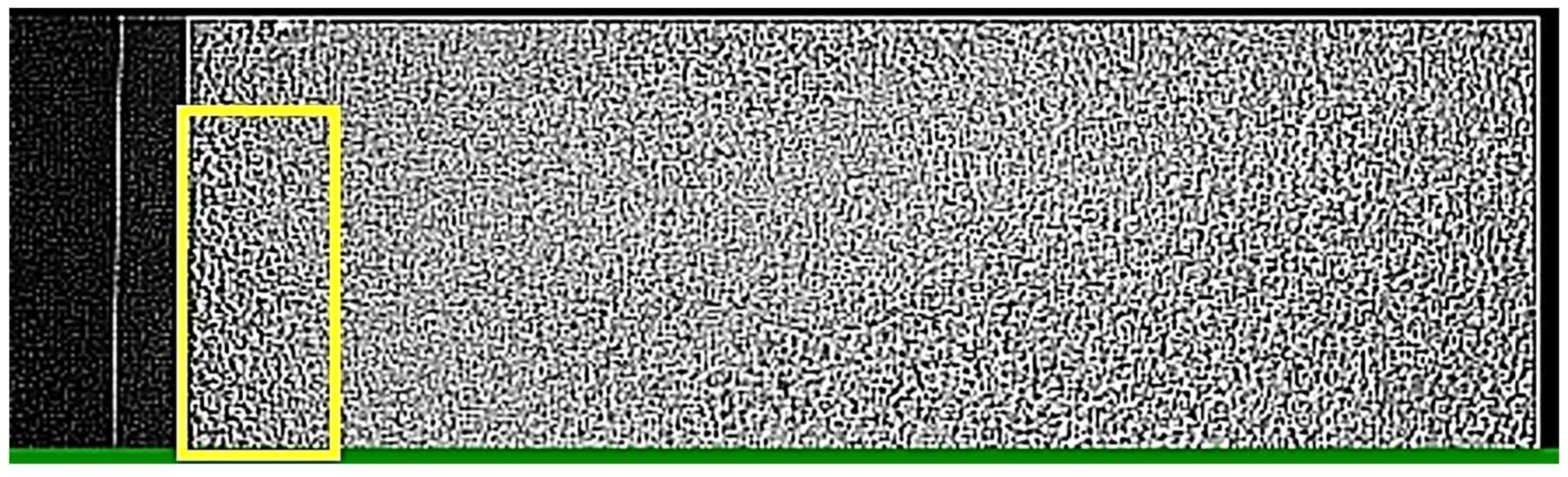
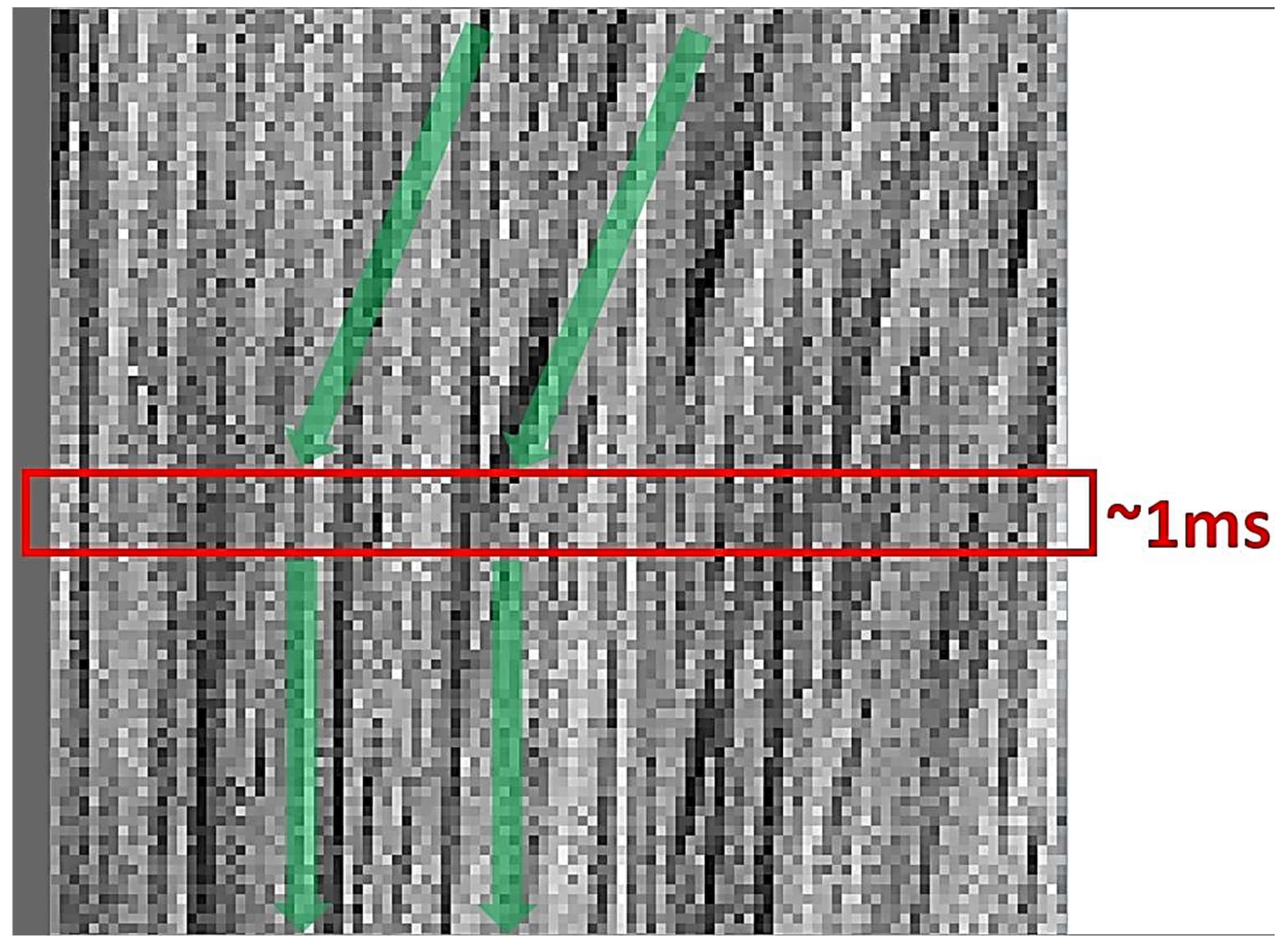
2.5. Image Analysis for Determination of Gel Time
3. Results and Discussion
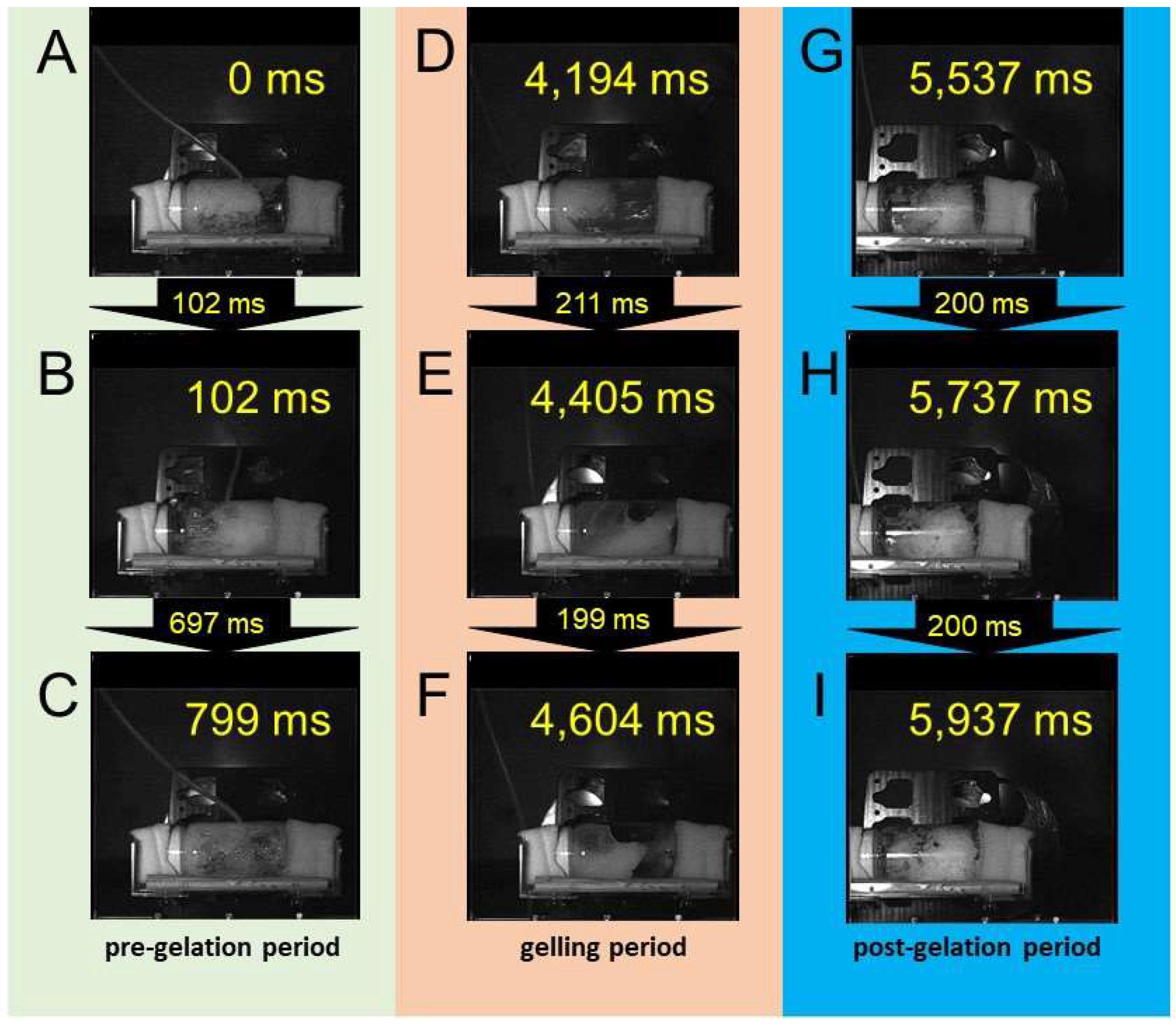
3.1. Direct Observation of Gelation in a Shaken Cylinder
3.2. Direct Observation of Gelation in a Stirred Container
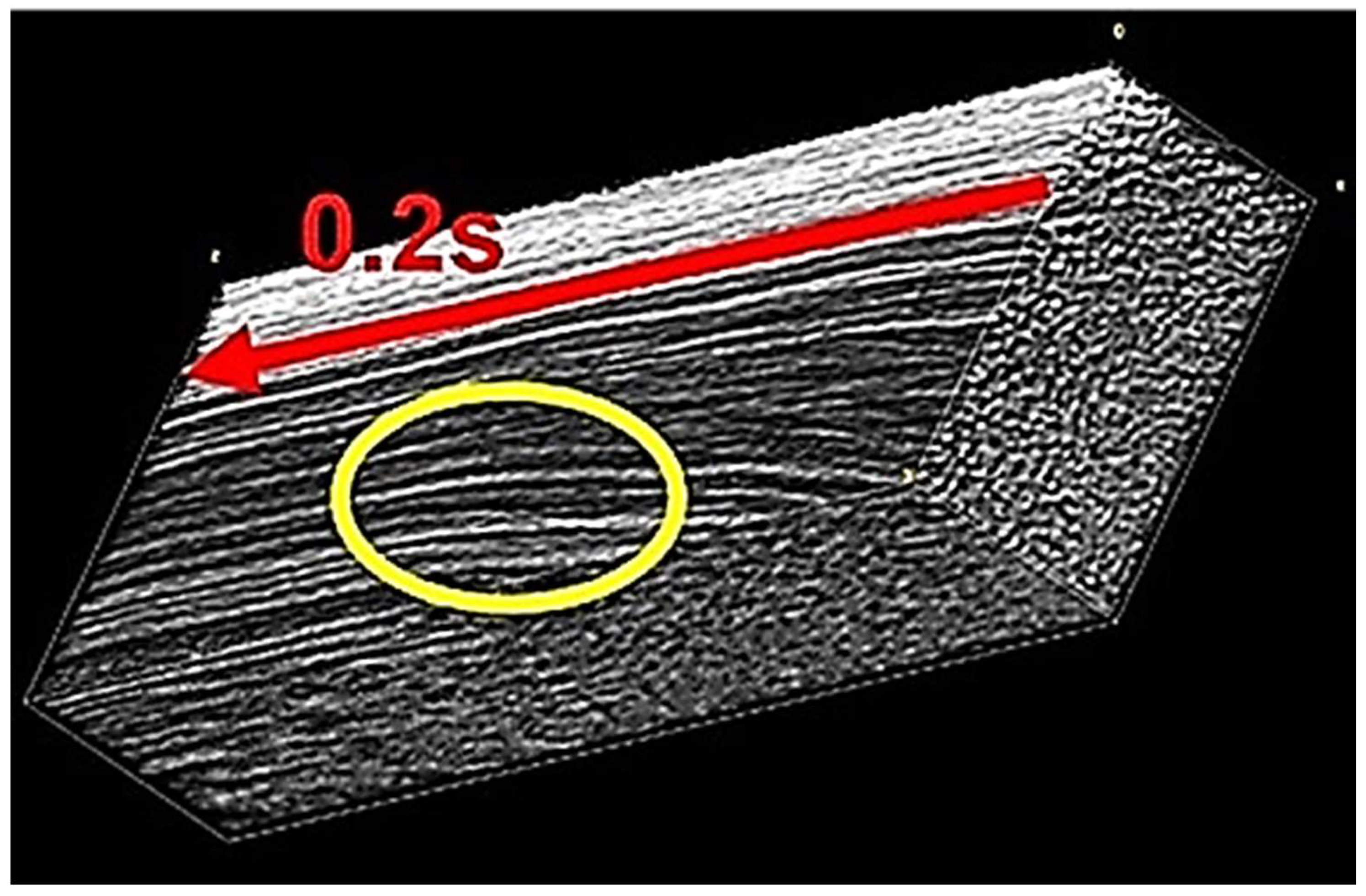
3.3. Direct Observation of Gelation near the Inner Wall of the Stirred Container
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Remzova, M.; Sasek, P.; Frankeova, D.; Slizkova, Z.; Rathousky, J. Effect of modified ethylsilicate consolidants on the mechanical properties of sandstone. Constr. Build. Mater. 2016, 112, 674–681. [Google Scholar] [CrossRef]
- Zarzuela, R.; Luna, M.; Carrascosa, L.M.; Yeste, M.P.; Garcia-Lodeiro, I.; Blanco-Varela, M.T.; Cauqui, M.; Rodríguez-Izquierdo, J.; Mosquera, M. Producing C-S-H gel by reaction between silica oligomers and portlandite: A promising approach to repair cementitious materials. Cem. Concr. Res. 2020, 130, 106008. [Google Scholar] [CrossRef]
- Khorasani, M.; Lampani, L.; Tounsi, A. A refined vibrational analysis of the FGM porous type beams resting on the silica aerogel substrate. Steel Compos. Struct. 2023, 47, 633–644. [Google Scholar] [CrossRef]
- Garg, A.; Aggarw, P.; Aggarwal, Y.; Belarbi, M.O.; Chalak, H.D.; Tounsi, A.; Gulia, R. Machine learning models for predicting the compressive strength of concrete containing nano silica. Comput. Concr. 2022, 30, 33–42. [Google Scholar] [CrossRef]
- Kurumada, K.; Otaki, K.; Ono, R.; Ue, H.; Sato, J. How long does “gelation” take?—Quest for observational methods of transient kinematics in gelation. In Proceedings of the 18th Asian Pacific Confederation of Chemical Engineering Congress (APCChE 2019), Sapporo, Japan, 23–27 September 2019. [Google Scholar]
- Chaibundit, C.; Ricardo, N.; Ricardo, N.; Muryn, C.; Madec, M.; Yeates, S.; Booth, C. Effect of ethanol on the gelation of aqueous solutions of Pluronic F127. J. Colloid Interface Sci. 2010, 351, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, N.; Ricardo, N.; Costa, F.; Chaibundit, C.; Portale, G.; Hermida-Merino, D.; Burattini, S.; Hamley, I.; Muryn, C.; Keith-Nixon, S.; et al. The effect of n-, s- and t-butanol on the micellization and gelation of Pluronic P123 in aqueous solution. J. Colloid Interface Sci. 2011, 353, 482–489. [Google Scholar] [CrossRef]
- Owen, D.; Peters, J.; Kieweg, S.; Geonnotti, A.; Schnaare, R.; Katz, D. Biophysical analysis of prototype microbicidal gels. J. Pharm. Sci. 2007, 96, 661–669. [Google Scholar] [CrossRef]
- Yuan, K.; Yang, X.; Li, D.; Wang, G.; Wang, S.; Guo, Y.; Yang, X. Incorporation of Nicandra physalodes (Linn.) Gaertn. pectin as a way to improve the textural properties of fish gelatin gels. Food Hydrocoll. 2022, 131, 107790. [Google Scholar] [CrossRef]
- Li, C.; Cai, J.; Yang, F.; Zhang, Y.; Bai, F.; Ma, Y.; Yao, B. Effect of asphaltenes on the stratification phenomenon of wax-oil gel deposits formed in a new cylindrical Couette device. J. Pet. Sci. Eng. 2016, 140, 73–84. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Hu, J.; Yu, Q.; Shi, C. Effects of anionic species of activators on the rheological properties and early gel characteristics of alkali-activated slag paste. Cem. Concr. Res. 2022, 162, 106968. [Google Scholar] [CrossRef]
- Burk, S.; Mata, A.; Snyder, M.; Schneider, S.; Osher, R.; Cionni, R. Visualizing vitreous using kenalog suspension. J. Cataract. Refract. Surg. 2003, 29, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Frank, J.; Farmer, M.; Schmidt, K.; Shalabi, S. Formation of Yogurt Microstructure and Three-Dimensional Visualization as Determined by Confocal Scanning Laser Microscopy. J. Dairy Sci. 1995, 78, 2629–2636. [Google Scholar] [CrossRef]
- Krause, J.; Lisinski, S.; Ratke, L.; Schaniel, D.; Willert, C.; Altmeyer, S.; Woike, T.; Voges, M.; Klinner, J. Observation of gelation process and particle distribution during sol–gel synthesis by particle image velocimetry. J. Sol-Gel Sci. Technol. 2008, 45, 73–77. [Google Scholar] [CrossRef]
- Winter, H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Gareche, M.; Allal, A.; Zeraibi, N.; Roby, F.; Azril, N.; Saoudi, L. Relationship between the fractal structure with the shear complex modulus of montmorillonite suspensions. Appl. Clay Sci. 2016, 123, 11–17. [Google Scholar] [CrossRef]
- Bruno, L.; Kasapis, S.; Heng, P. Effect of polymer molecular weight on the structural properties of non aqueous ethyl cellulose gels intended for topical drug delivery. Carbohydr. Polym. 2012, 88, 382–388. [Google Scholar] [CrossRef]
- Nolte, H.; John, S.; Smidsrød, O.; Stokke, B. Gelation of xanthan with trivalent metal ions. Carbohydr. Polym. 1992, 18, 243–251. [Google Scholar] [CrossRef]
- Huo, L.; Cao, W. The dual effects of non-solvent on the sol-gel transition of polyacrylonitrile solution: The promotion in thermodynamics and hindrance in kinetics. Polym. Test. 2022, 115, 107685. [Google Scholar] [CrossRef]
- Seighalani, F.; McMahon, D.; Sharma, P. Determination of critical gel-sol transition point of Highly Concentrated Micellar Casein Concentrate using multiple waveform rheological technique. Food Hydrocoll. 2021, 120, 106886. [Google Scholar] [CrossRef]
- Zammali, M.; Liu, S.; Yu, W. Symmetry breakdown in the sol-gel transition of a Guar gum transient physical network. Carbohydr. Polym. 2021, 258, 117689. [Google Scholar] [CrossRef]
- Netter, A.; Goudoulas, T.; Germann, N. Effects of Bloom number on phase transition of gelatin determined by means of rheological characterization. LWT 2020, 132, 109813. [Google Scholar] [CrossRef]
- Yang, B.; Pan, Y.; Yu, Y.; Wu, J.; Xia, R.; Wang, S.; Wang, Y.; Su, L.; Miao, J.; Qian, J.; et al. Filler network structure in graphene nanoplatelet (GNP)-filled polymethyl methacrylate (PMMA) composites: From thermorheology to electrically and thermally conductive properties. Polym. Test. 2020, 89, 106575. [Google Scholar] [CrossRef]
- Yang, D.; Yang, H. Effects of ethanol on gelation of iota-carrageenan. LWT 2020, 126, 109281. [Google Scholar] [CrossRef]
- Chen, H.; Liu, W.; Hong, M.; Zhang, E.; Dai, X.; Qiu, X.; Ji, X. Viscoelastic behavior of high molecular weight polyimide/cyclohexanone solution during sol-gel transition. Polymer 2020, 190, 122250. [Google Scholar] [CrossRef]
- Zhou, P.; Eid, M.; Xiong, W.; Ren, C.; Ai, T.; Deng, Z.; Li, J.; Li, B. Comparative study between cold and hot water extracted polysaccharides from Plantago ovata seed husk by using rheological methods. Food Hydrocoll. 2020, 101, 105465. [Google Scholar] [CrossRef]
- Yang, D.; Gao, S.; Yan, H. Effects of sucrose addition on the rheology and structure of iota-carrageenan. Food Hydrocoll. 2020, 99, 105317. [Google Scholar] [CrossRef]
- Mahjoub, H.; Zammali, M.; Abbes, C.; Othman, T. Microrheological study of PVA/borax physical gels: Effect of chain length and elastic reinforcement by sodium hydroxide addition. J. Mol. Liq. 2019, 291, 111272. [Google Scholar] [CrossRef]
- Sow, L.; Tan, S.; Yang, H. Rheological properties and structure modification in liquid and gel of tilapia skin gelatin by the addition of low acyl gellan. Food Hydrocoll. 2019, 90, 9–18. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Yang, H. Characterisation of rheology and microstructures of κ-carrageenan in ethanol-water mixtures. Food Res. Int. 2018, 107, 738–746. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.; Baum, T.; Kao, N.; Bhattacharya, S. Phase transition and anomalous rheological behaviour of polylactide/graphene nanocomposites. Compos. Part B 2018, 135, 25–34. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Yang, H. Effects of sucrose addition on the rheology and microstructure of κ-carrageenan gel. Food Hydrocoll. 2018, 75, 164–173. [Google Scholar] [CrossRef]
- Zammali, M.; Mahjoub, H.; Hanafi, M.; Ducouret, G.; Othman, T.; Narita, T. A microrheological study of physical gelation of hydrophobically modified associating polymers: Effects of temperature. Polymer 2017, 121, 204–210. [Google Scholar] [CrossRef]
- Ozaki, H.; Indei, T.; Koga, T.; Narita, T. Physical gelation of supramolecular hydrogels cross-linked by metal-ligand interactions: Dynamic light scattering and microrheological studies. Polymer 2017, 128, 363–372. [Google Scholar] [CrossRef]
- Liu, S.; Bao, H.; Li, L. Thermoreversible gelation and scaling laws for graphene oxide-filled κ-carrageenan hydrogels. Eur. Polym. J. 2016, 79, 150–162. [Google Scholar] [CrossRef]
- Lin-Gibson, S.; Walls, H.; Kennedy, S.; Welsh, E. Reaction kinetics and gel properties of blocked diisocyinate crosslinked chitosan hydrogels. Carbohydr. Polym. 2003, 54, 193–199. [Google Scholar] [CrossRef]
- Pan, Y.; Li, L. Percolation and gel-like behavior of multiwalled carbon nanotube/polypropylene composites influenced by nanotube aspect ratio. Polymer 2013, 54, 1218–1226. [Google Scholar] [CrossRef]
- Liang, G.; Cook, W.; Sautereau, H.; Tcharkhtchi, A. Diallyl orthophthalate as a reactive plasticizer for improving PVC processibility, Part II: Rheology during cure. Eur. Polym. J. 2008, 44, 366–375. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; He, J.; Hu, G. Gelation in carbon nanotube/polymer composites. Polymer 2003, 44, 7529–7532. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Zhang, L. Dynamic viscoelastic behavior of triple helical Lentinan in water: Effect of temperature. Carbohydr. Polym. 2008, 73, 26–34. [Google Scholar] [CrossRef]
- Rizvi, A.; Park, C.; Favis, B. Tuning viscoelastic and crystallization properties of polypropylene containing in-situ generated high aspect ratio polyethylene terephthalate fibrils. Polymer 2015, 68, 83–91. [Google Scholar] [CrossRef]
- Meng, J.; Hu, X.; Boey, F.; Li, L. Effect of layered nano-organosilicate on the gel point rheology of bismaleimide/diallylbisphenol A resin. Polymer 2005, 46, 2766–2776. [Google Scholar] [CrossRef]
- Ksapabutr, B.; Gulari, E.; Wongkasemjit, S. Sol-gel transition study and pyrolysis of alumina-based gels prepared from alumatrane precursor. Colloids Surf. 2004, 233, 145–153. [Google Scholar] [CrossRef]
- Lue, A.; Zhang, L. Effects of carbon nanotubes on rheological behavior in cellulose solution dissolved at low temperature. Polymer 2010, 51, 2748–2754. [Google Scholar] [CrossRef]
- Liu, S.; Li, L. Thermoreversible gelation and scaling behavior of Ca2+-induced κ-carrageenan hydrogels. Food Hydrocoll. 2016, 61, 793–800. [Google Scholar] [CrossRef]
- Sun, F.; Huang, Q.; Wu, J. Rheological behaviors of an exopolysaccharide from fermentation medium of a Cordyceps sinensis fungus (Cs-HK1). Carbohydr. Polym. 2014, 114, 506–513. [Google Scholar] [CrossRef]
- Kelarakis, A.; Yoon, K.; Somani, R.; Chen, X.; Hsiao, B.; Chu, B. Rheological study of carbon nanofiber induced physical gelation in polyolefin nanocomposite melt. Polymer 2005, 46, 11591–11599. [Google Scholar] [CrossRef]
- Lu, A.; Song, Y.; Boluk, Y. Electrolyte effect on gelation behavior of oppositely charged nanocrystalline cellulose and polyelectrolyte. Carbohydr. Polym. 2014, 114, 57–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Xu, J.; Zhang, L. Dynamic viscoelastic behavior of triple helical Lentinan in water: Effects of concentration and molecular weight. Polymer 2007, 48, 6681–6690. [Google Scholar] [CrossRef]
- Lu, A.; Wang, Y.; Boluk, Y. Investigation of the scaling law on gelation of oppositely charged nanocrystalline cellulose and polyelectrolyte. Carbohydr. Polym. 2014, 105, 214–221. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Z.; Yin, C.; Gao, Y.; Wang, Y.; Yang, M. Gelation of attractive particles in polymer melt. Polymer 2012, 53, 4293–4299. [Google Scholar] [CrossRef]
- Mo, G.; Zhang, R.; Wang, Y.; Yan, Q. Rheological and optical investigation of the gelation with and without phase separation in PAN/DMSO/H2O ternary blends. Polymer 2016, 84, 243–253. [Google Scholar] [CrossRef]
- Wang, Y.; Lue, A.; Zhang, L. Rheological behavior of waterborne polyurethane/starch aqueous dispersions during cure. Polymer 2009, 50, 5474–5481. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Tang, B.; Bi, S.; Li, L. Scaling law and microstructure of alginate hydrogel. Carbohydr. Polym. 2016, 135, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Dashtimoghadam, E.; Bahlakeh, G.; Salimi-Kenari, H.; Hasani-Sadrabadi, M.; Mirzadeh, H.; Nyström, B. Rheological Study and Molecular Dynamics Simulation of Biopolymer Blend Thermogels of Tunable Strength. Biomacromolecules 2016, 17, 3474–3484. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, S.; Xiong, S.; Huang, Q. Steady, dynamic, and creep-recovery rheological properties of myofibrillar protein from grass carp muscle. Food Hydrocoll. 2016, 61, 48–56. [Google Scholar] [CrossRef]
- Tan, L.; Pan, D.; Pan, N. Gelation behavior of polyacrylonitrile solution in relation to aging process and gel concentration. Polymer 2008, 49, 5676–5682. [Google Scholar] [CrossRef]
- Payró, E.; Llacuna, J. Rheological characterization of the gel point in sol–gel transition. J. Non-Cryst. Solids 2006, 352, 2220–2225. [Google Scholar] [CrossRef]
- Liu, X.; Qian, L.; Shu, T.; Tong, Z. Rheology characterization of sol–gel transition in aqueous alginate solutions induced by calcium cations through in situ release. Polymer 2003, 44, 407–412. [Google Scholar] [CrossRef]
- Evans, P.; Hawkins, K.; Williams, P.; Williams, R. Rheometrical detection of incipient blood clot formation by Fourier transform mechanical spectroscopy. J. Non-Newton. Fluid Mech. 2008, 148, 122–126. [Google Scholar] [CrossRef]
- Rodd, A.; Cooper-White, J.; Dunstan, D.; Boger, D. Gel point studies for chemically modified biopolymer networks using small amplitude oscillatory rheometry. Polymer 2001, 42, 185–198. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Mo, G.; Zhang, R. Rheological manifestation of the second self-similar structure in gelation process of PAN/DMSO/H2O system. Polymer 2015, 73, 149–155. [Google Scholar] [CrossRef]
- Kurumada, K.; Ue, H.; Sato, J. Direct Observation of Transient Flow Kinematics of Environment-friendly Silica-based Alcogel at Instantaneous Gelation. In Proceedings of the Kosen Research International Symposium 2023 (KRIS 2023), Tokyo, Japan, 1 September 2023. [Google Scholar]
- Rasband, W.S. ImageJ Version 1.54f, U.S.; National Institutes of Health: Bethesda, MD, USA, 2023. Available online: http://imagej.nih.gov/ij/ (accessed on 22 August 2023).
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurumada, K.; Ue, H.; Sato, J. Direct Observation of Transient Flow Kinematics of Environment-Friendly Silica-Based Alcogel at Instantaneous Gelation. Sustainability 2023, 15, 14460. https://doi.org/10.3390/su151914460
Kurumada K, Ue H, Sato J. Direct Observation of Transient Flow Kinematics of Environment-Friendly Silica-Based Alcogel at Instantaneous Gelation. Sustainability. 2023; 15(19):14460. https://doi.org/10.3390/su151914460
Chicago/Turabian StyleKurumada, Kenichi, Hidenori Ue, and Jun Sato. 2023. "Direct Observation of Transient Flow Kinematics of Environment-Friendly Silica-Based Alcogel at Instantaneous Gelation" Sustainability 15, no. 19: 14460. https://doi.org/10.3390/su151914460




