Optimum Conditions for Enhancing Chitosan-Assisted Coagulation in Drinking Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Water Sample
2.2. Chemicals
2.3. Jar Test Experiments
2.3.1. Stirring Intensity
2.3.2. Optimum Stage for Adding Chitosane
2.3.3. Effect of Chitosan Concentration
2.4. Comparing the Performance of Various Coagulants
3. Results
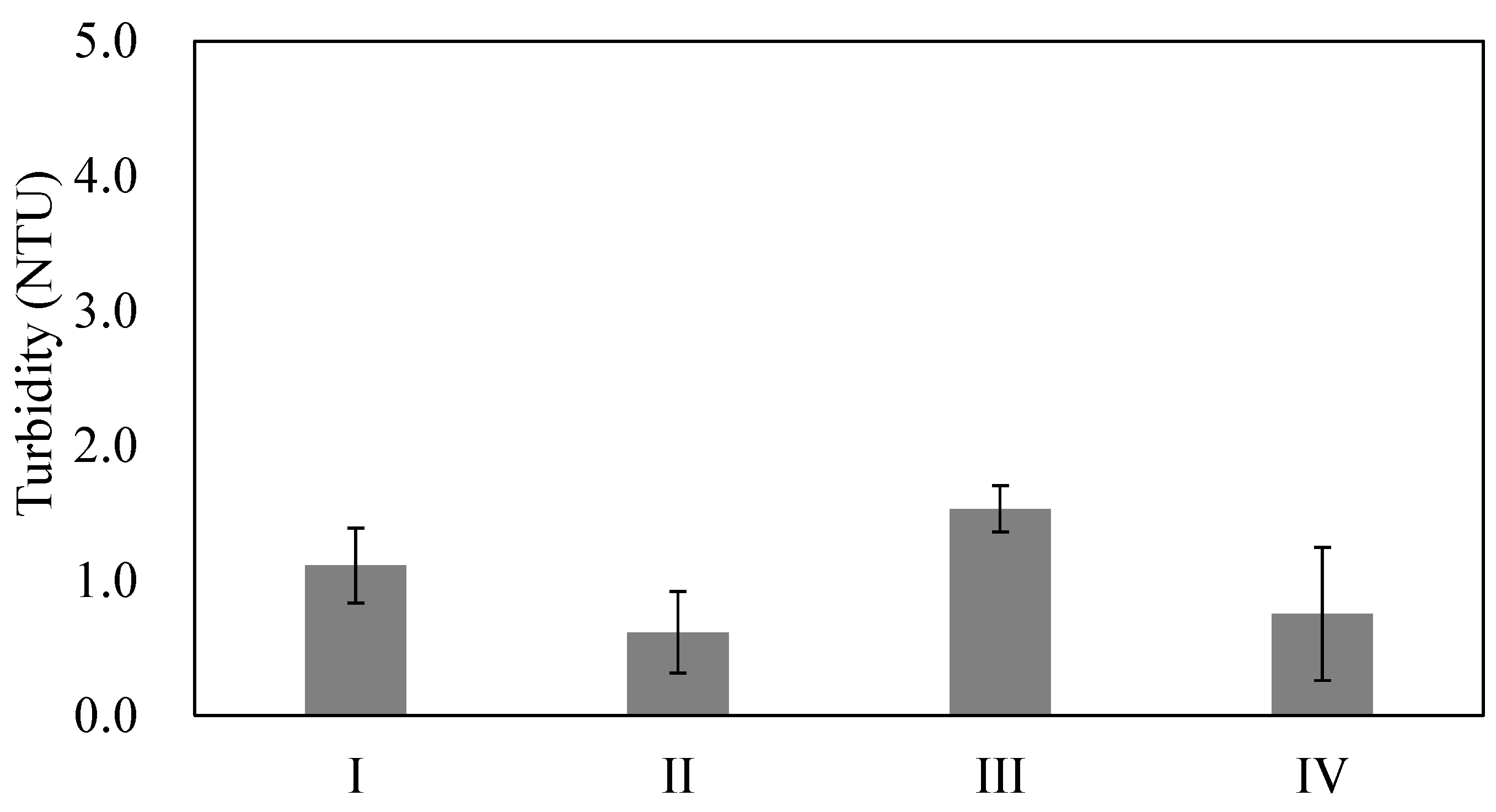
3.1. Effects of Stirring Intensity
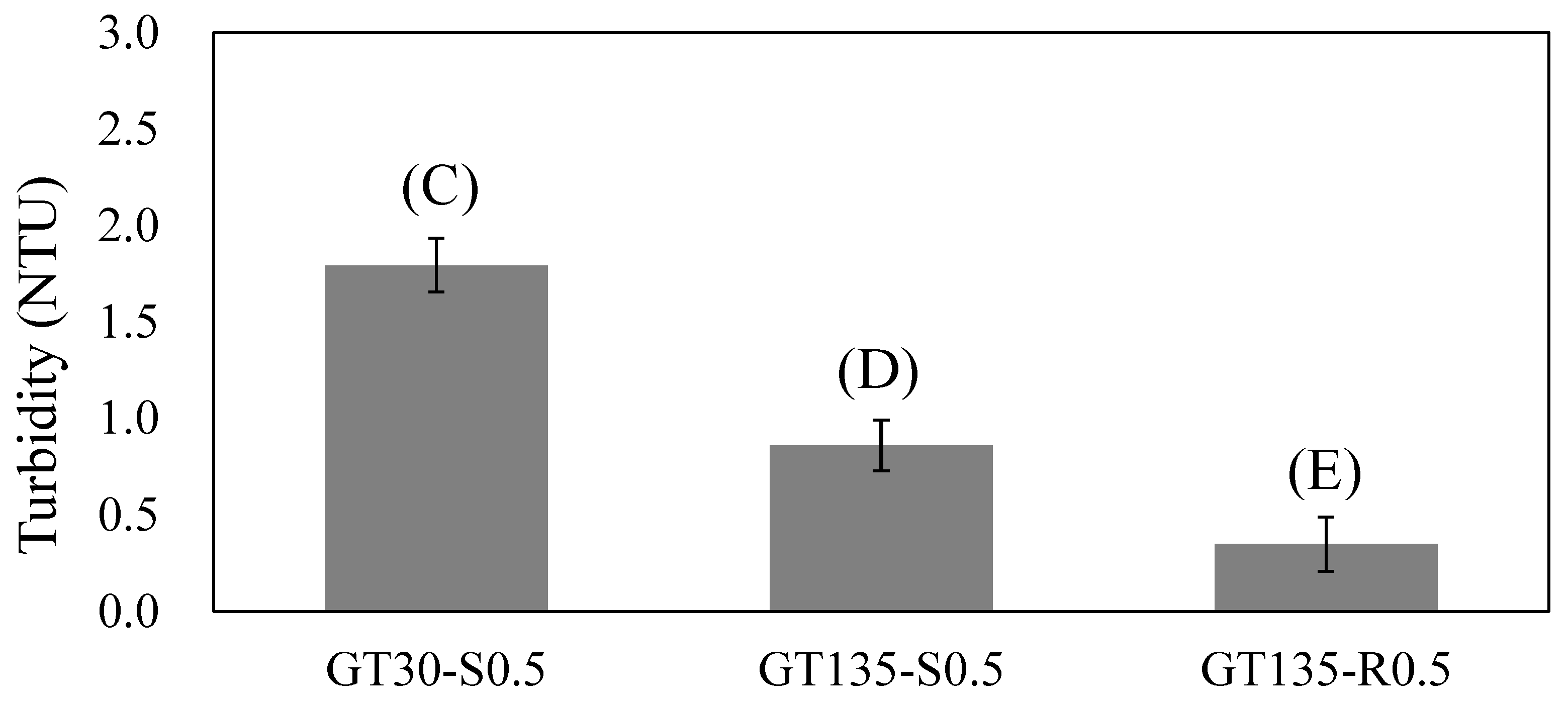
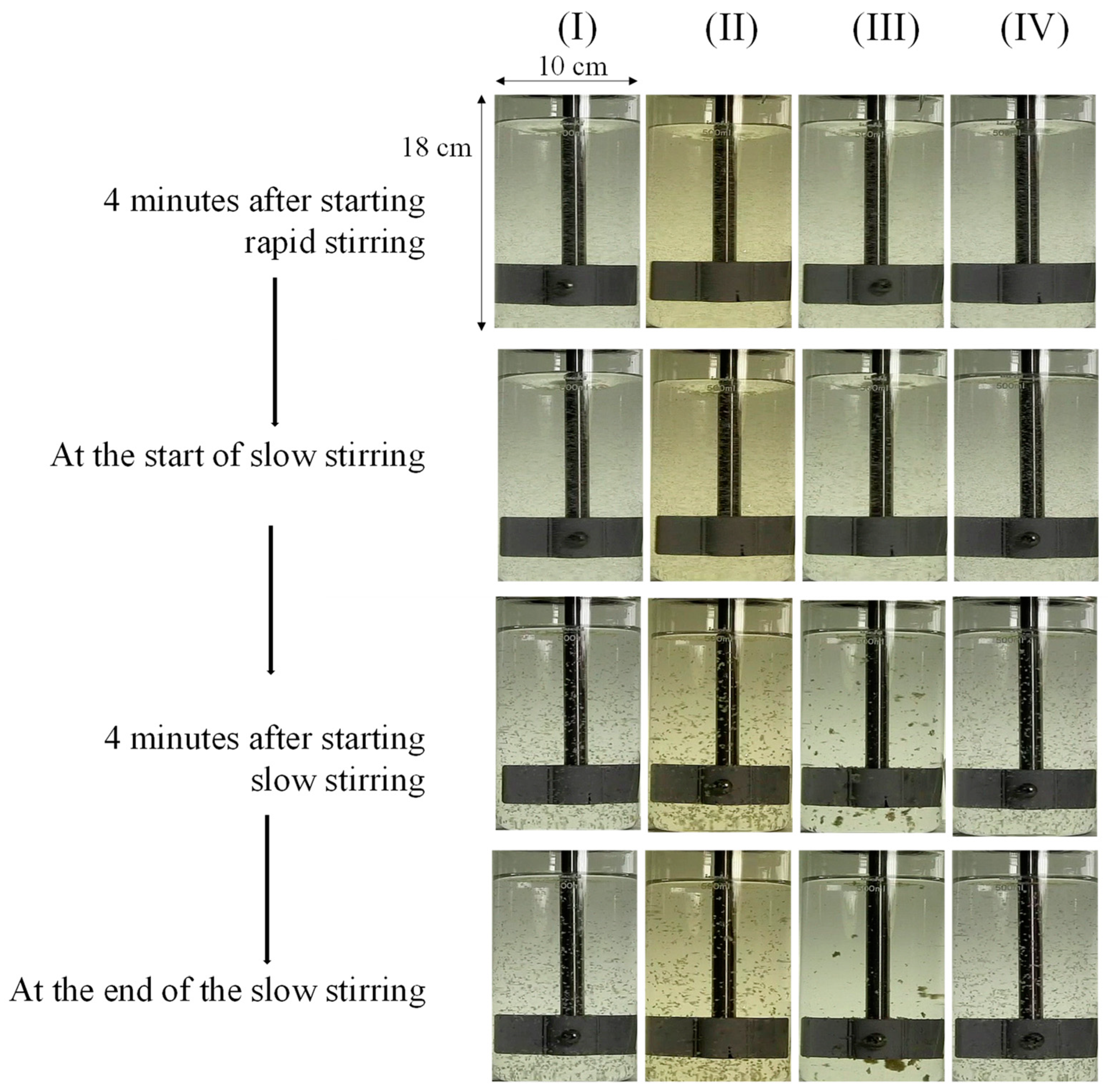
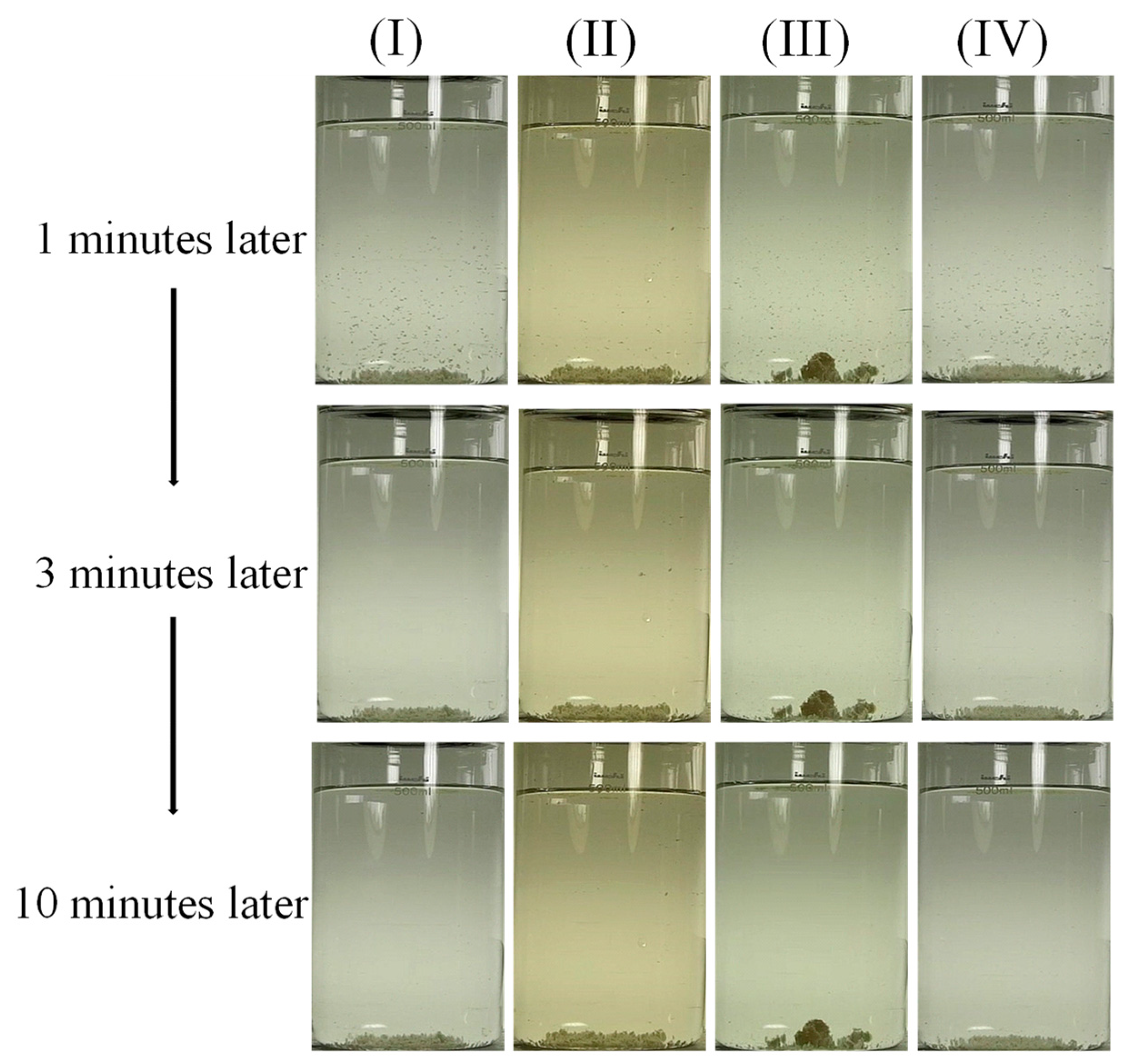
3.2. Effects of the Chitosan Addition Stage
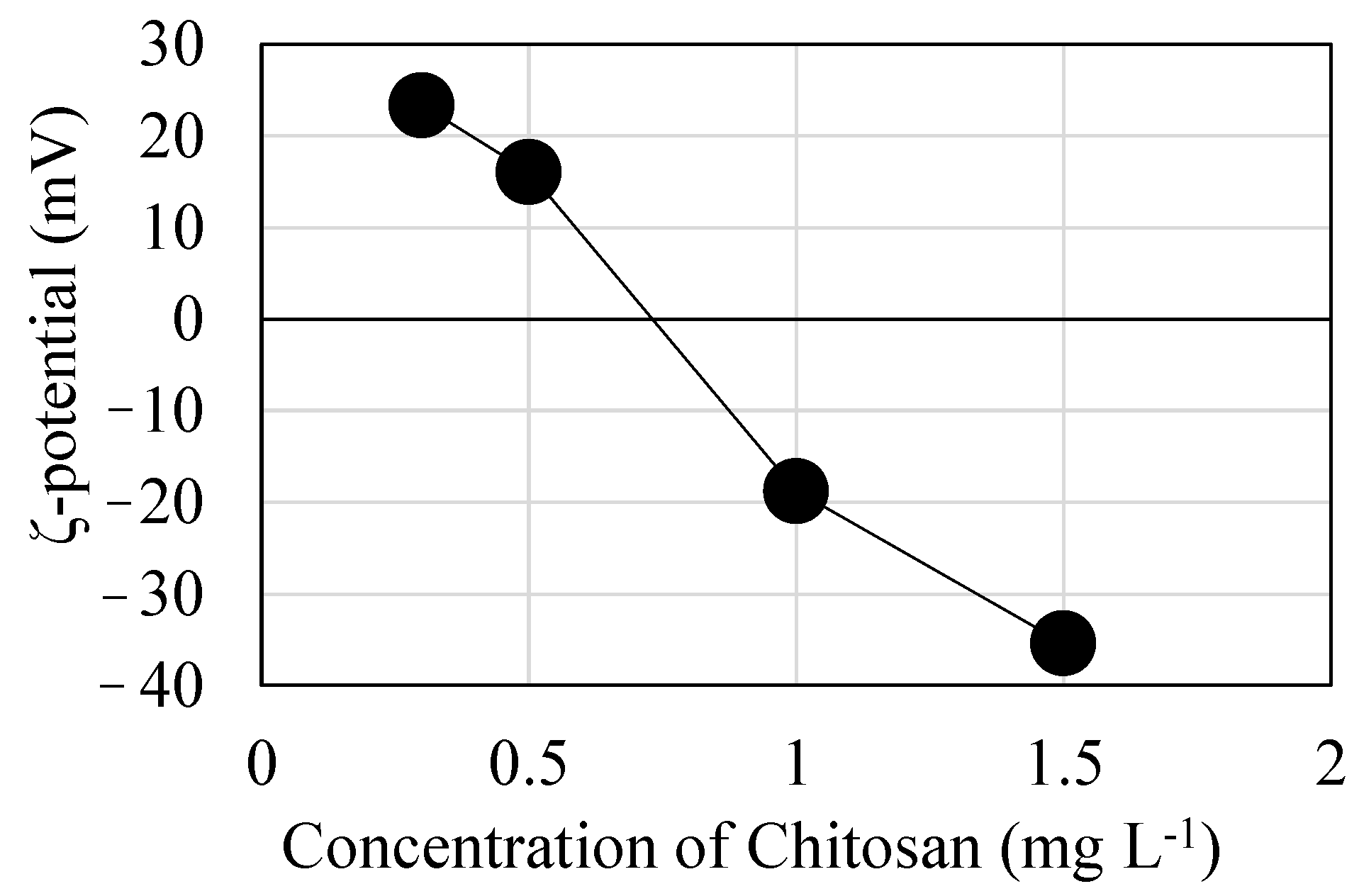
3.3. Effects of the Amount of Chitosan Added
4. Discussion
4.1. Effects of Rapid Stirring for Prompt Dispersion of Chitosan as the Coagulant Aid
4.2. Optimum Concentration of Chitosan as a Coagulant Aid
4.3. Effects of Chitosan as Coagulant Aid on the Removal of Kaolin Flocs
5. Conclusions
- (1)
- Rapid stirring is preferable for increasing the rate of floc formation irrespective of the coagulant type and the presence/absence of a coagulant aid.
- (2)
- Adding chitosan during earlier and more rapid stirring was favorable for facilitating floc formation, as indicated by the lower resultant residual turbidity.
- (3)
- The optimum chitosan concentration for use as a coagulant aid was determined as 0.8 mg L−1. This value could be justified in terms of the isoelectricity, where the electrostatic interfloc repulsive force was eliminated and further coagulation could be facilitated.
- (4)
- The formation of gigantic flocs did not necessarily result in a minimized resultant residual turbidity. Although the use of chitosan as a coagulant aid did not lead to the maximum floc size, the size uniformity caused by the presence of the dissolved chitosan seemed to have accelerated the decrease in residual turbidity.
- (5)
- Searching for the isoelectric condition by varying the concentration of added chitosan was useful for finding the optimum chitosan concentration at which coagulation sedimentation occurs most efficiently to decrease residual turbidity. This method was shown to be effective even when the incipient turbidity was much lower than in wastewater, where the method of minimizing the ζ potential is commonly used.
- (6)
- Using chitosan could mitigate the risk of polluting drinking water due to its harmlessness compared with using other artificial chemicals as the coagulant aid like poly-acrylamide. Considering the abundance and ubiquity of chitosan, it is a promising candidate natural resource that can replace artificial chemicals that have been used for treating drinking water.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keeley, J.; Jarvis, P.; Smith, A.D.; Judd, S.J. Coagulant recovery and reuse for drinking water treatment. Water Res. 2016, 88, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, D.; Mousavi, S.A. A comprehensive review on the coagulant recovery and reuse from drinking water treatment sludge. J. Environ. Manag. 2022, 319, 115649. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Ika, A.R. Minimization of halogenated DBP precursors by enhanced PACl coagulation: The impact of organic molecule fraction changes on DBP precursors destabilization with Al hydrates. Sci. Total Environ. 2020, 703, 134936. [Google Scholar] [CrossRef]
- Coral, L.; Zamyadi, A.; Barbeau, B.; Bassetti, F.; Lapolli, F.; Prévost, M. Oxidation of Microcystis aeruginosa and Anabaena flos-aquae by ozone: Impacts on cell integrity and chlorination by-product formation. Water Res. 2013, 47, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, D.; Ni, J.; Qu, J.; Chow, C.W.K.; Liu, H. Mechanism of natural organic matter removal by polyaluminum chloride: Effect of coagulant particle size and hydrolysis kinetics. Water Res. 2008, 42, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zhang, Z.; Liu, D.; Wu, Y.; Wang, J.; Wang, Q. Coagulation behavior of polyaluminum chloride: Effects of pH and coagulant dosage. Chin. J. Chem. Eng. 2015, 23, 1041–1046. [Google Scholar] [CrossRef]
- Chen, Y.; Nakazawa, Y.; Matsui, Y.; Shirasaki, N.; Matsushita, T. Sulfate ion in raw water affects performance of high-basicity PACl coagulants produced by Al(OH)3 dissolution and base-titration: Removal of SPAC particles by coagulation-flocculation, sedimentation, and sand filtration. Water Res. 2020, 183, 116093. [Google Scholar] [CrossRef]
- Aguilar, M.I.; Sáez, J.; Lloréns, M.; Soler, A.; Ortuño, J.F.; Meseguer, V.; Fuentes, A. Improvement of coagulation–flocculation process using anionic polyacrylamide as coagulant aid. Chemosphere 2005, 58, 47–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Yue, J.; Xing, X.; Yang, Z.; Wang, X.; Wang, Q.; Zhang, J. Enhanced removal of polyethylene terephthalate microplastics through polyaluminum chloride coagulation with three typical coagulant aids. Sci. Total Environ. 2021, 800, 149589. [Google Scholar] [CrossRef]
- Mehrnoosh, A.; Ali, K.; Sina, D.; Kamyar, Y.; Anoushiravan, M.B.; Shokooh, S.K.; Sahand, J.; Reza, S. Defluoridation of synthetic and natural waters by polyaluminum chloride-chitosan (PACl-Ch) composite coagulant. Water Supply 2018, 18, 259–269. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, Q.; Gu, Y.; Yang, W.; Chen, Y.; Lin, J.; Dong, M.; Cheng, H.; Hu, H.; Guo, Z. Enteromorpha prolifera polysaccharide based coagulant aid for humic acids removal and ultrafiltration membrane fouling control. Int. J. Biol. Macromol. 2020, 152, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Yu, Z.; Baoyu, G.; Shenglei, S.; Yan, W.; Qian, L.; Qiyan, Y. Polyacrylamide as coagulant aid with polytitanium sulfate in humic acid-kaolin water treatment: Effect of dosage and dose method. J. Taiwan Inst. Chem. Eng. 2016, 64, 173–179. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, J.; Yang, W.; Liu, M.; Yan, Y.; Jia, W. Application of laminarin as a novel coagulant aid to improve coagulation-ultrafiltration efficiency. Environ. Res. 2023, 228, 115909. [Google Scholar] [CrossRef]
- Ding, S.; Chu, W.; Bond, T.; Cao, Z.; Xu, B.; Gao, N. Contribution of amide-based coagulant polyacrylamide as precursors of haloacetamides and other disinfection by-products. Chem. Eng. J. 2018, 350, 356–363. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Karthikeyan, P.; Sirajudheen, P.; Meenakshi, S. Optimization of sustainable chitosan/Moringa. oleifera as coagulant aid for the treatment of synthetic turbid water—A systemic study. Environ. Chem. Ecotoxicol. 2020, 2, 132–140. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, B.; Yue, Q.; Wang, Y. Effect of Enteromorpha polysaccharides on coagulation performance and kinetics for dye removal. Colloids Surf. Physicochem. Eng. Asp. 2014, 456, 253–260. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Na, M.; Liana, A.E.; Liu, S.; Lim, M.; Chow, C.W.K.; Wang, D.; Drikas, M.; Amal, R. Preparation and characterisation of new-polyaluminum chloride-chitosan composite coagulant. Water Res. 2012, 46, 4614–4620. [Google Scholar] [CrossRef]
- Na, M.; Liu, S.; Chow, C.W.K.; Drikas, M.; Amal, R. Understanding effects of water characteristics on natural organic matter treatability by PACl and a novel PACl-chitosan coagulants. J. Hazard. Mater. 2013, 263, 718–725. [Google Scholar] [CrossRef]
- Qin, J.J.; Oo, M.H.; Kekre, K.A.; Knops, F.; Miller, P. Impact of coagulation pH on enhanced removal of natural organic matter in treatment of reservoir water. Sep. Purif. Technol. 2006, 49, 295–298. [Google Scholar] [CrossRef]
- Dayarathne, H.N.P.; Angove, M.J.; Jeong, S.; Aryal, R.; Paudel, S.R.; Mainali, B. Effect of temperature on turbidity removal by coagulation: Sludge recirculation for rapid settling. J. Water Process Eng. 2022, 46, 102559. [Google Scholar] [CrossRef]
- Li, T.; Zhu, Z.; Wang, D.; Yao, C.; Tang, H. Characterization of floc size, strength and structure under various coagulation mechanisms. Powder Technol. 2006, 168, 104–110. [Google Scholar] [CrossRef]
- Yu, W.Z.; Gregory, J.; Campos, L.; Li, G. The role of mixing conditions on floc growth, breakage and re-growth. Chem. Eng. J. 2011, 171, 425–430. [Google Scholar] [CrossRef]
- Rossini, M.; Garrido, J.G.; Galluzzo, M. Optimization of the coagulation–flocculation treatment: Influence of rapid mix parameters. Water Res. 1999, 33, 1817–1826. [Google Scholar] [CrossRef]
- Takaara, T.; Yamamoto, T.; Kurumada, K. Optimum Condition for Enhancing Chitosan-assisted Aggregation. In Proceedings of the 1st KOSEN Research International Symposium, Virtual, 20–21 December 2022; p. 203. [Google Scholar]
- Sun, Y.; Zhou, S.; Chiang, P.C.; Shah, K.J. Evaluation and optimization of enhanced coagulation process: Water and energy. nexus Water-Energy Nexus 2019, 2, 25–36. [Google Scholar] [CrossRef]
- Camp, T.R.; Stein, P.C. Flocculation and Flocculation Basin. Proc. ASCE 1955, 120, 1–18. [Google Scholar] [CrossRef]
- Lin, J.L.; Pan, J.R.; Huang, C. Enhanced particle destabilization and aggregation by flash-mixing coagulation for drinking water treatment. Sep. Purif. Technol. 2013, 115, 145–151. [Google Scholar] [CrossRef]
- Gregory, J. Polymer adsorption and flocculation in sheared suspensions. Colloids Surf. 1988, 31, 231–253. [Google Scholar] [CrossRef]
- Tseng, T.; Segal, B.; Edward, M. Maintaining effective turbidity removal during runoff events. In Proceedings of the AWWA Annual Conference, Dallas, TX, USA, 21–25 June 1998; pp. 21–25. [Google Scholar]
- Tambo, N.; Watanabe, Y. Physical aspect of flocculation process—I: Fundamental treatise. Water Res. 1979, 13, 429–439. [Google Scholar] [CrossRef]
- Ebie, K.; Higuchi, S.; Kawaguchi, T.; Asano, M.; Tamura, S.; Wajima, H. Effect of Optimization of G T Value on the Reducing of Settled Water Turbidity in Coagulation and Sedimentation; Hokkaido Branch, Japan Society of Civil Engineers: Tokyo, Japan, 2004; Volume 61, p. VII-8. (In Japanese) [Google Scholar]
- Zhao, J.; Su, R.; Guo, X.; Li, W.; Feng, N. Role of mixing conditions on coagulation performance and flocs breakage formed by magnesium hydroxide. J. Taiwan Inst. Chem. Eng. 2014, 45, 1685–1690. [Google Scholar] [CrossRef]



| Condition | Value |
|---|---|
| Temperature (°C) | 13.7 |
| pH | 7.31 |
| Turbidity (NTU) | 4.34 |
| Alkalinity (mg L−1) | 32.6 |
| Rapid Stirring | Chitosan | |||
|---|---|---|---|---|
| GT Value | Stage of Addition of Chitosan | Amount | ||
| ×103 | mg L−1 | |||
| A | GT30 | 30 | - | 0 |
| B | GT135 | 135 | - | 0 |
| C | GT30-S0.5 | 30 | Slow stirring as started | 0.5 |
| D | GT135-S0.5 | 135 | Slow stirring as started | 0.5 |
| E | GT135-R0.5 | 135 | Middle of rapid stirring | 0.5 |
| F | GT135-R0.8 | 135 | Middle of rapid stirring | 0.8 |
 | ||||
| Sample | ζ Potential (mV) | |
|---|---|---|
| A | GT-30 | −29.2 |
| B | GT135 | −29.6 |
| C | GT30-S0.5 | −29.7 |
| D | GT135-S0.5 | −13.5 |
| E | GT135-R0.5 | −9.5 |
| F | GT135-R0.8 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaara, T.; Kurumada, K. Optimum Conditions for Enhancing Chitosan-Assisted Coagulation in Drinking Water Treatment. Sustainability 2023, 15, 14197. https://doi.org/10.3390/su151914197
Takaara T, Kurumada K. Optimum Conditions for Enhancing Chitosan-Assisted Coagulation in Drinking Water Treatment. Sustainability. 2023; 15(19):14197. https://doi.org/10.3390/su151914197
Chicago/Turabian StyleTakaara, Tomoko, and Kenichi Kurumada. 2023. "Optimum Conditions for Enhancing Chitosan-Assisted Coagulation in Drinking Water Treatment" Sustainability 15, no. 19: 14197. https://doi.org/10.3390/su151914197
APA StyleTakaara, T., & Kurumada, K. (2023). Optimum Conditions for Enhancing Chitosan-Assisted Coagulation in Drinking Water Treatment. Sustainability, 15(19), 14197. https://doi.org/10.3390/su151914197






