Study on Rates of NH3 Adsorption and Desorption in SCR on Various Engine Operation Conditions
Abstract
:1. Introduction
2. Experimental Setup
Experimental Apparatus
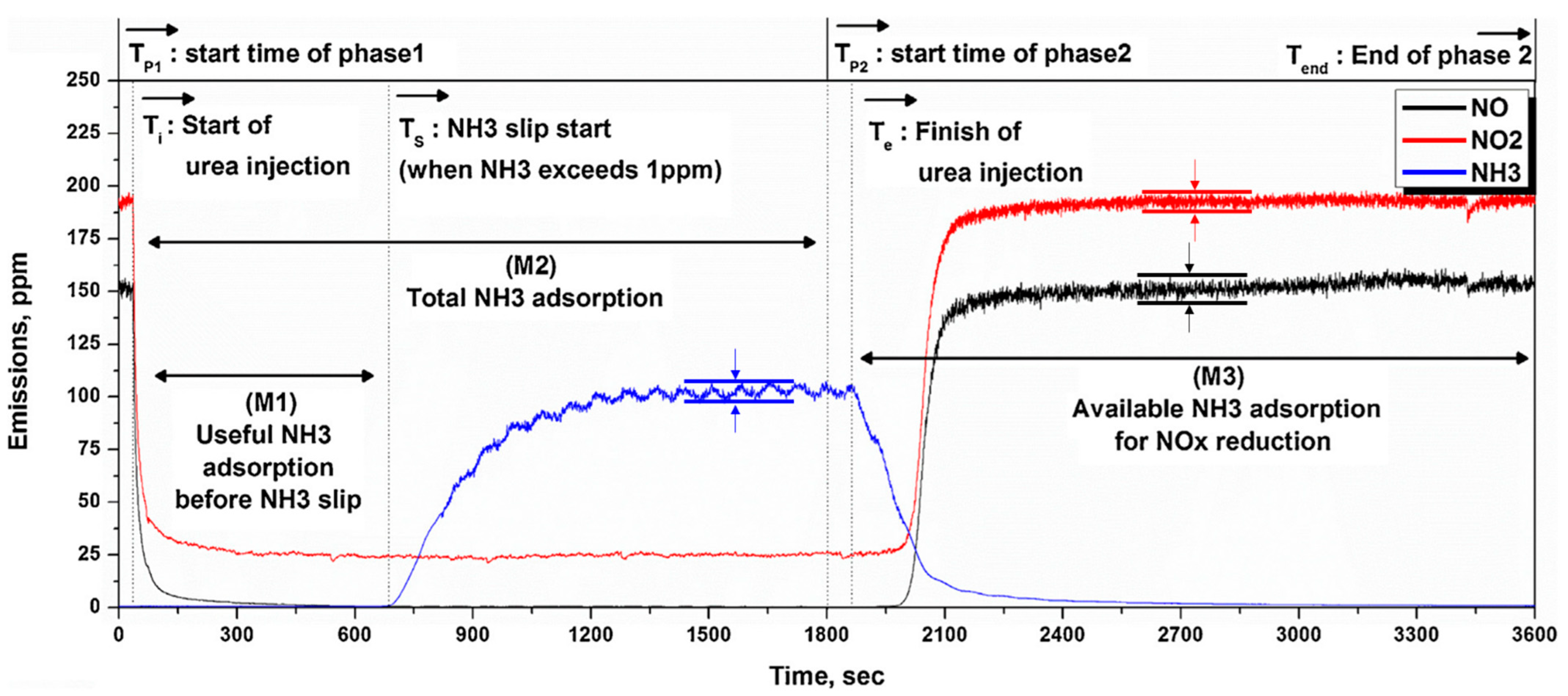
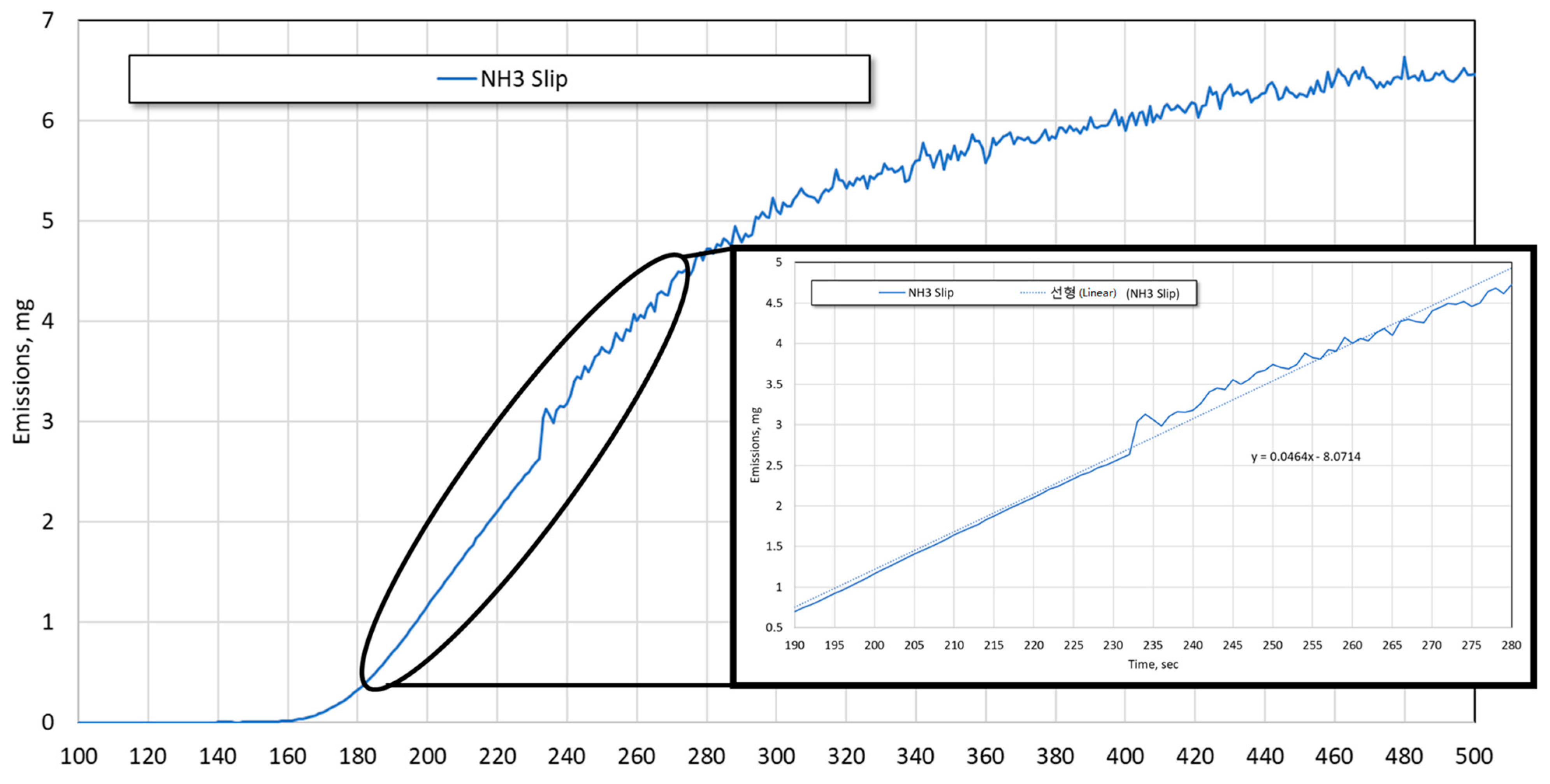
3. Methods and Analysis
4. Results and Discussion
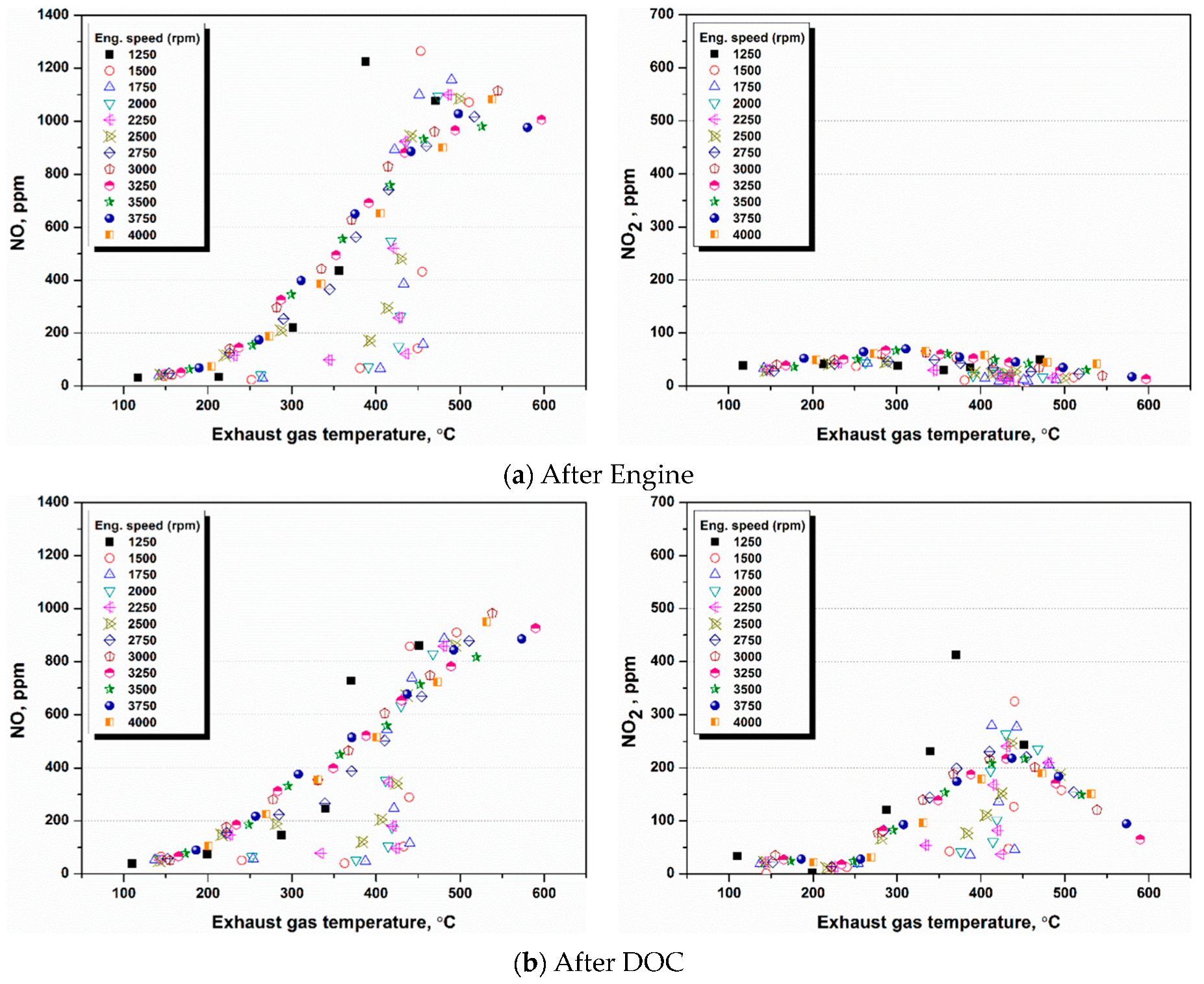
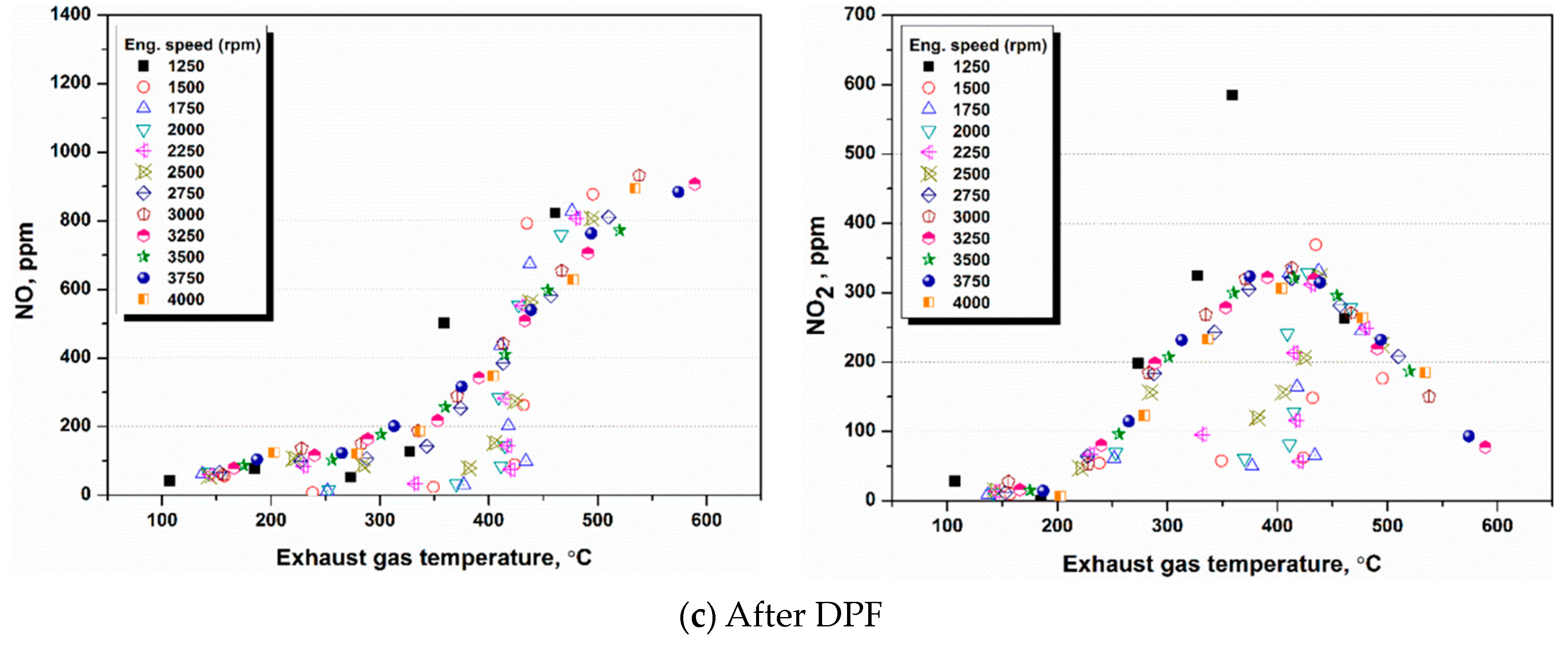
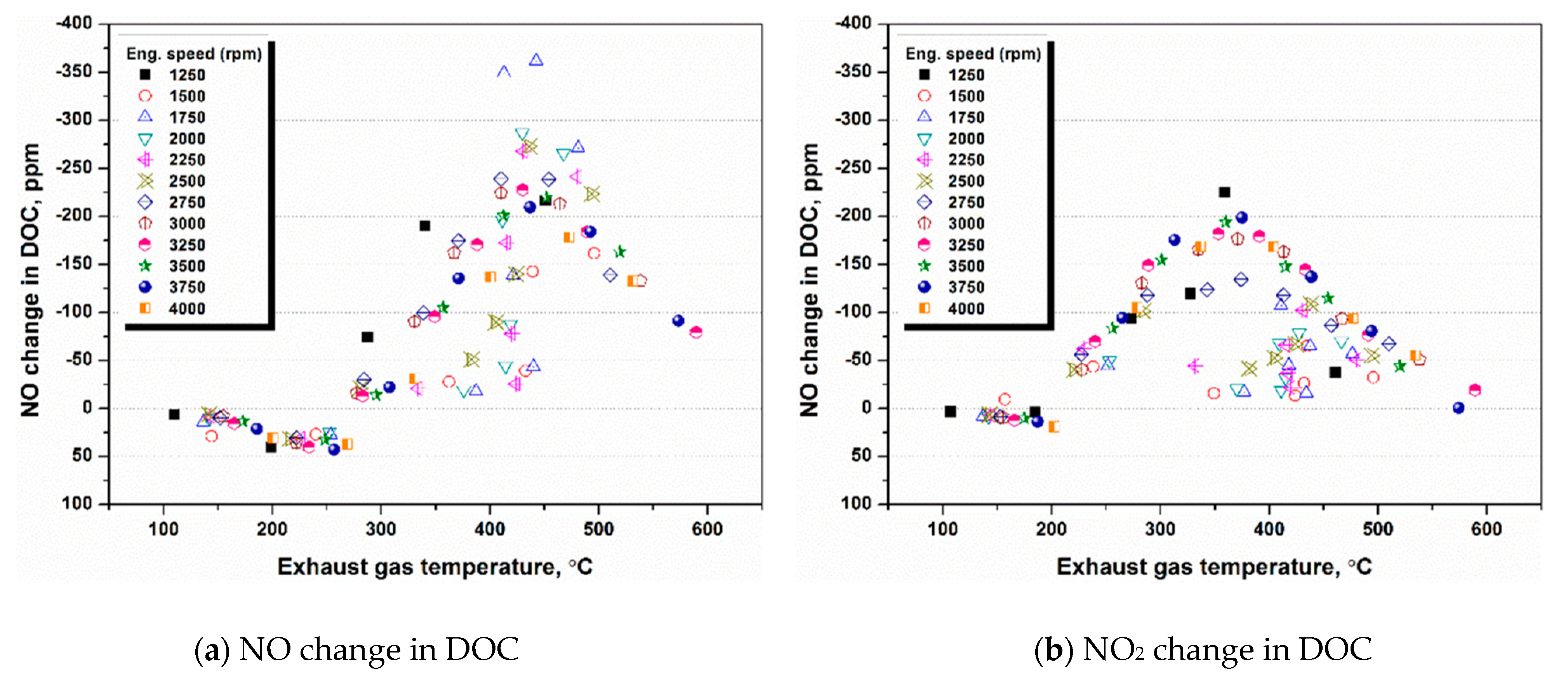
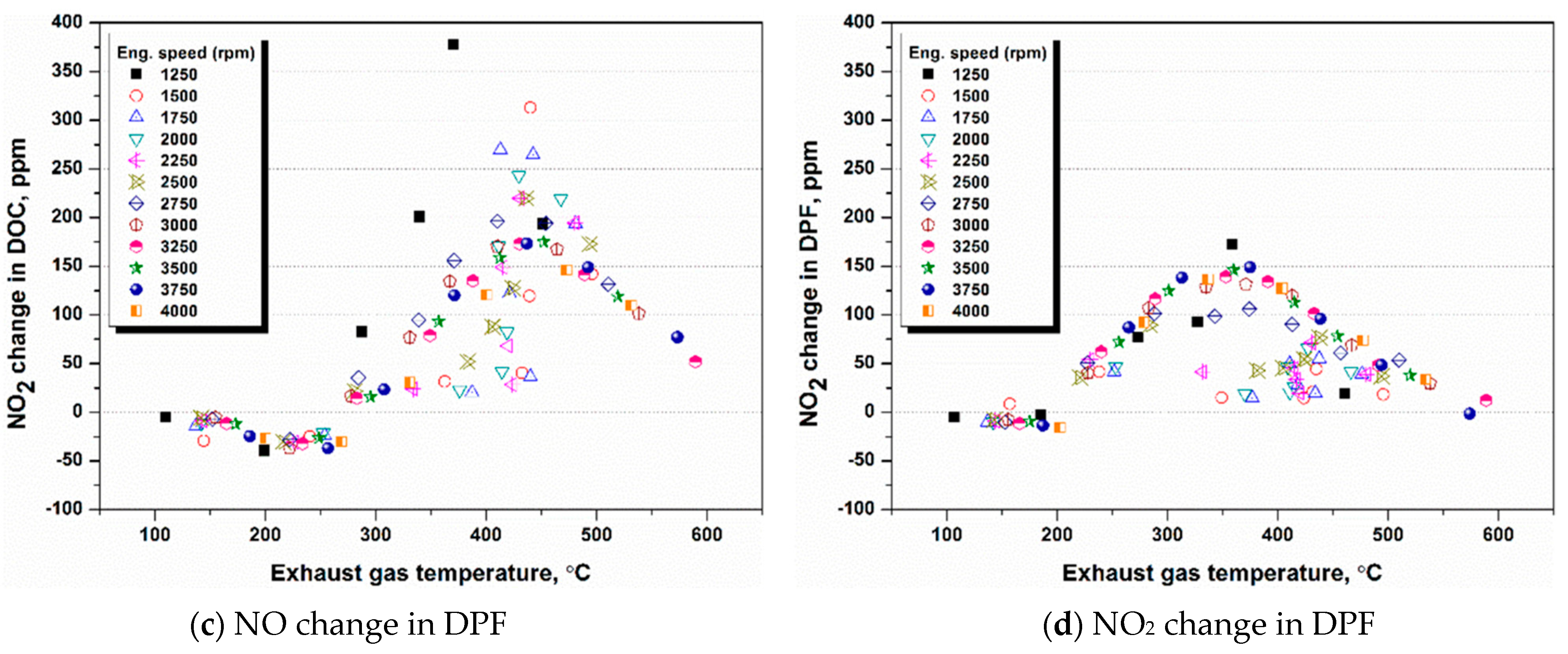
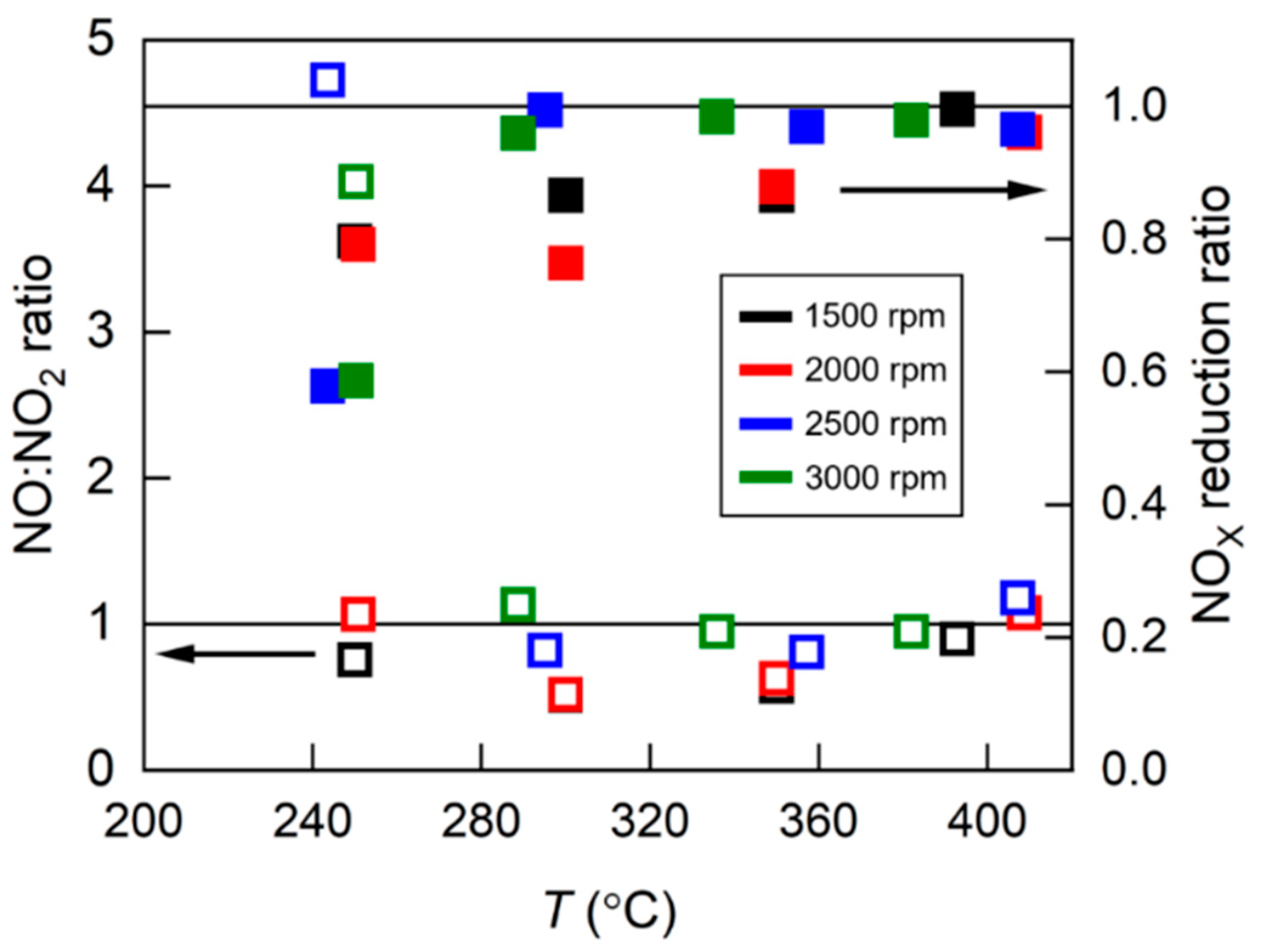
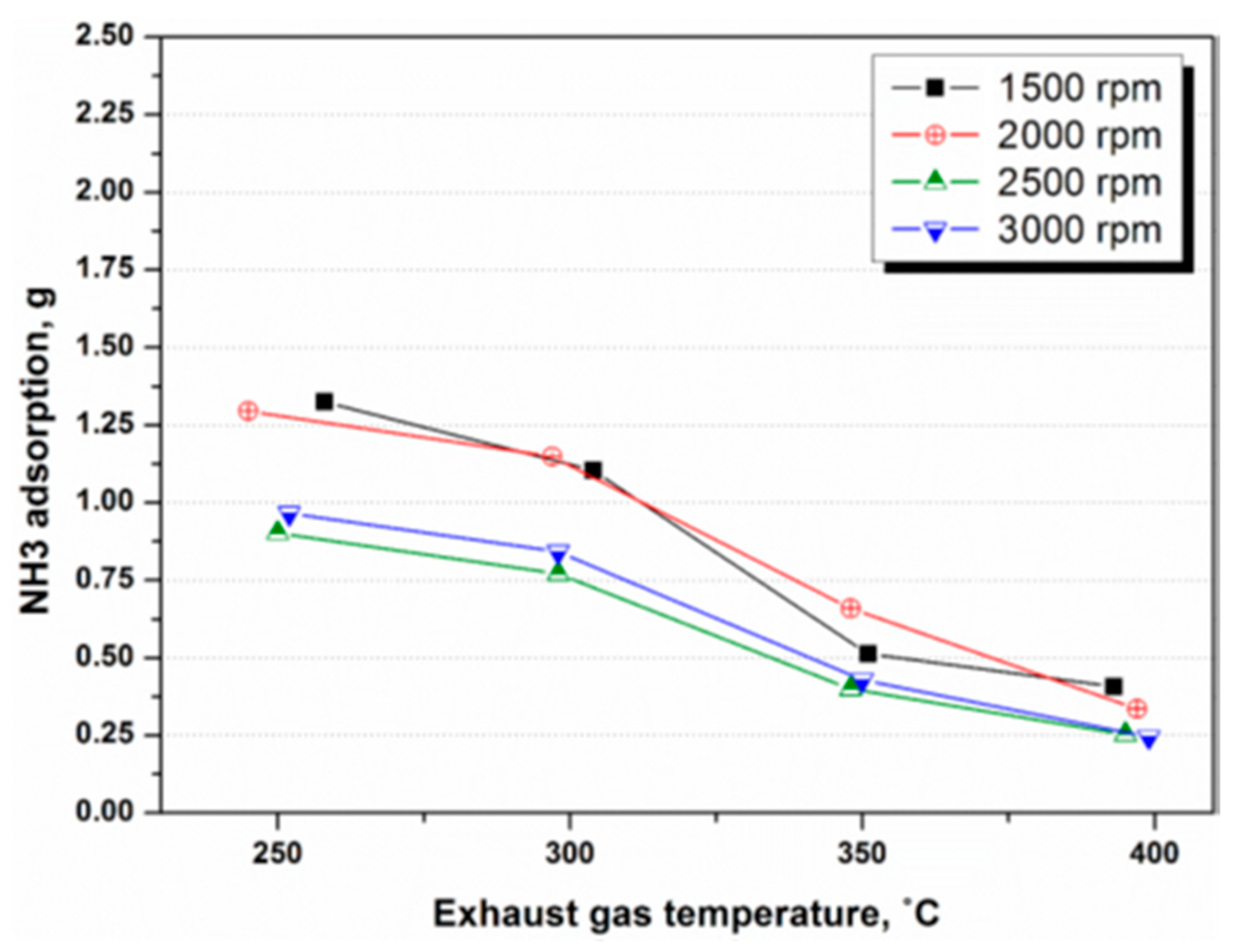
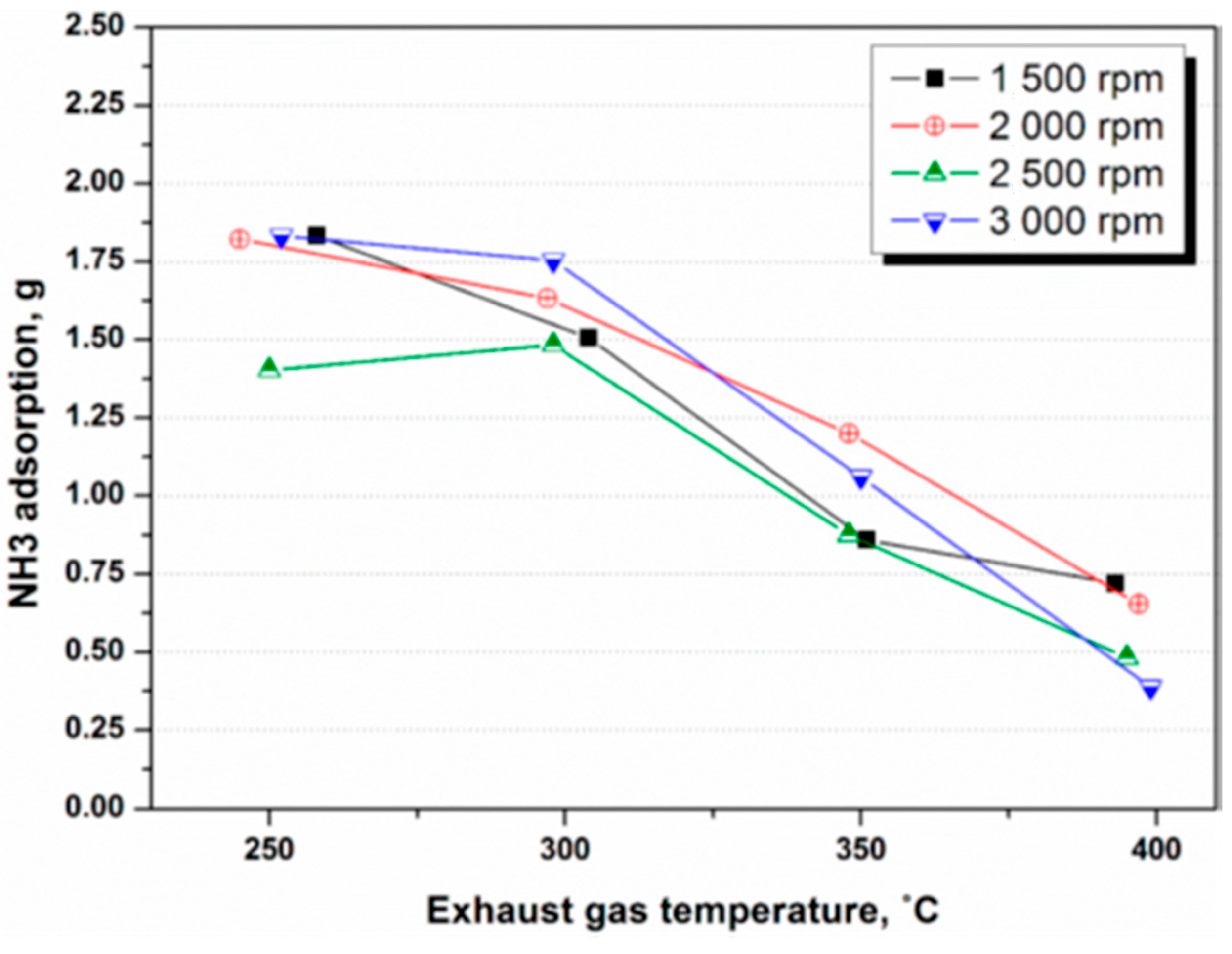
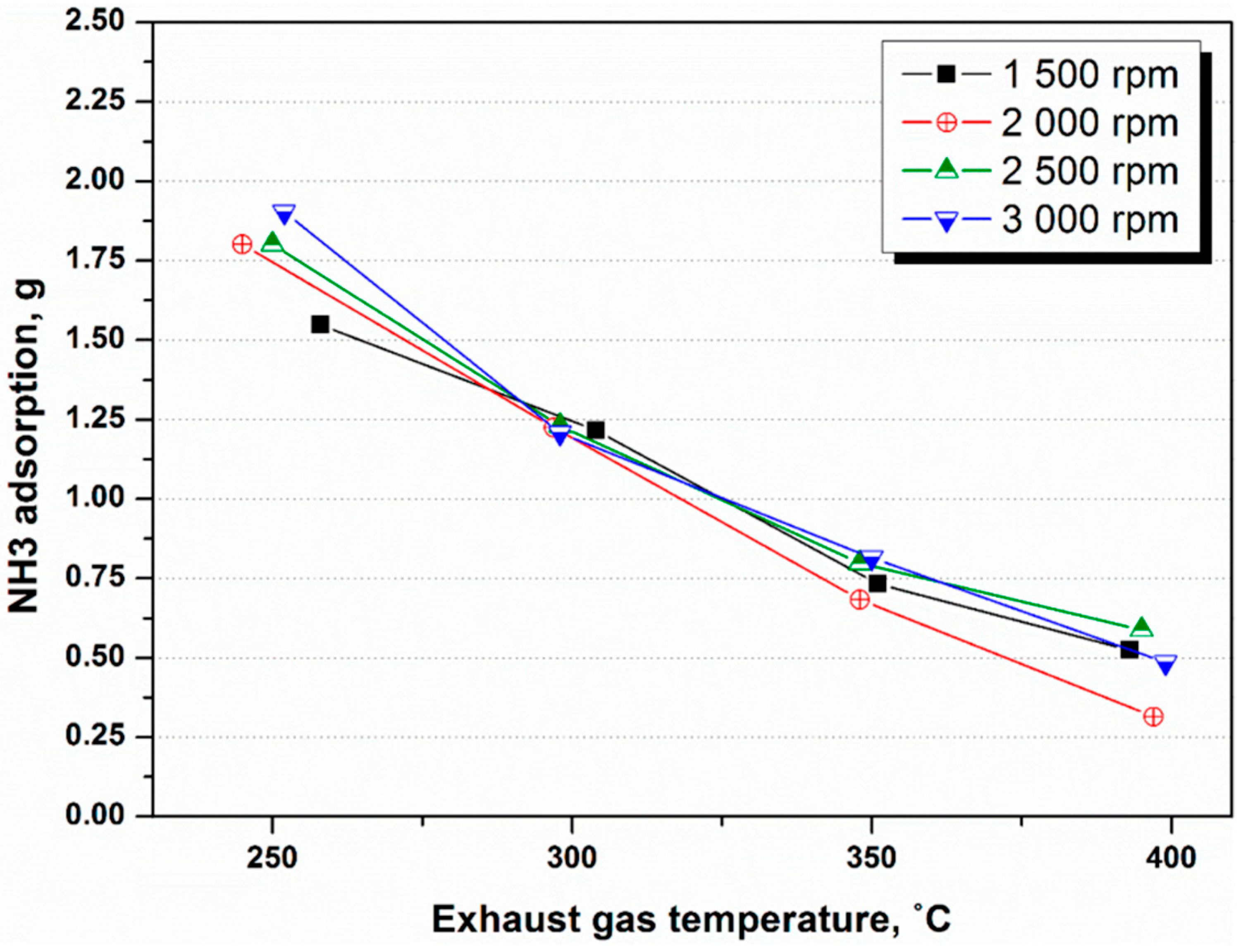
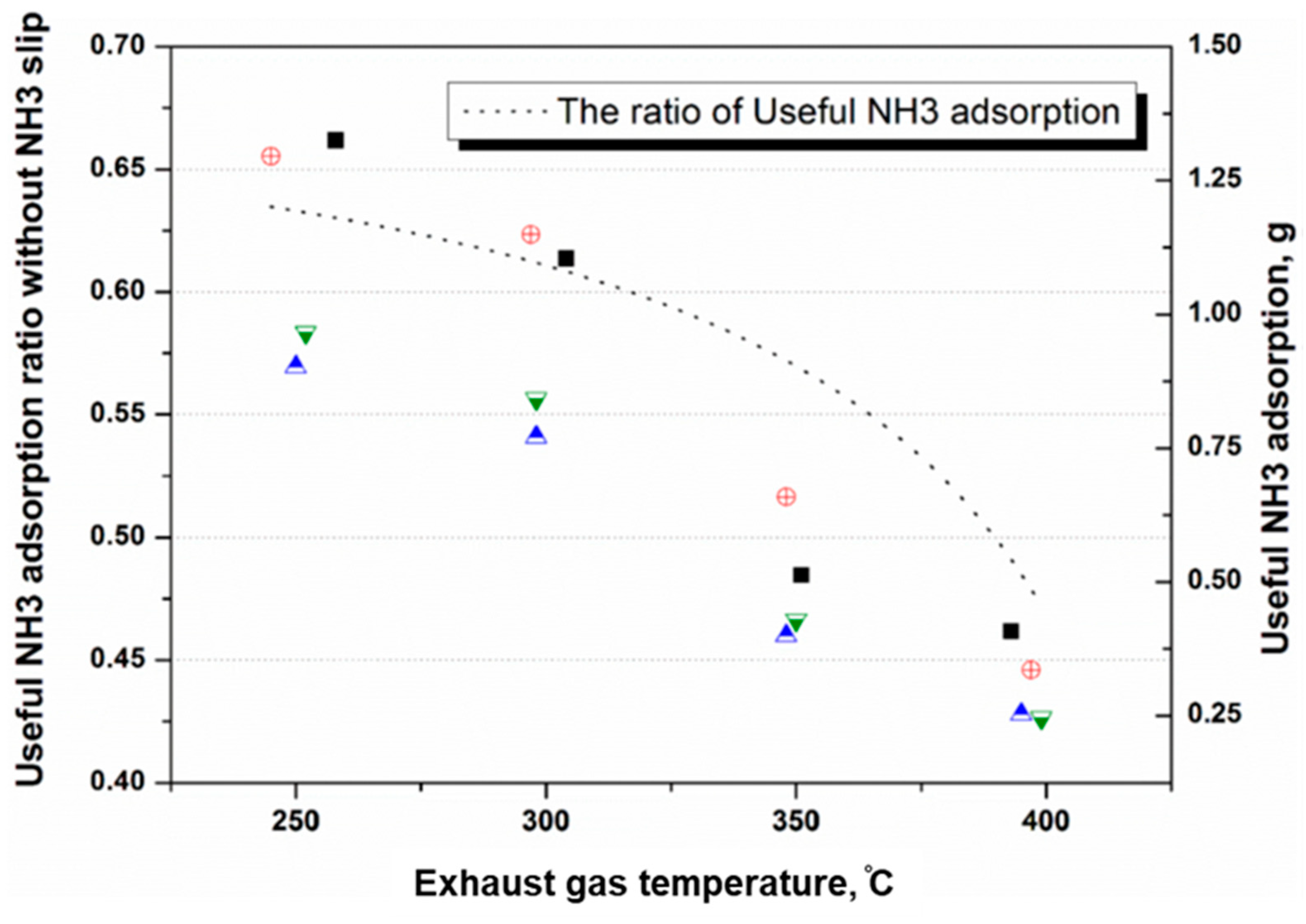
4.1. NH3 Adsorption in SCR in Different Engine Conditions
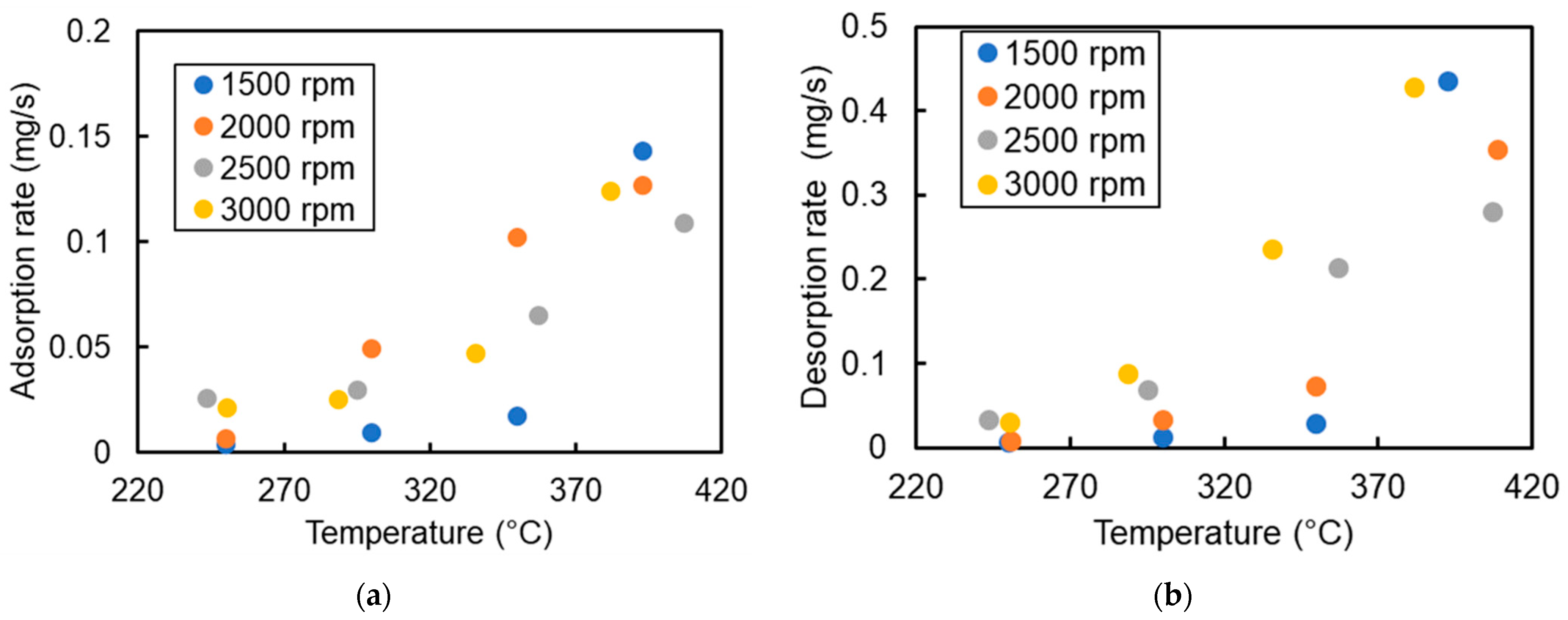
4.2. NH3 Adsorption and Desorption Rate in SCR in Different Engine Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| DOC | Diesel Oxidation Catalyst |
| DPF | Diesel Particulate Filter |
| UWS | Urea Water Solution |
| SCR | Selective Catalyst Reduction |
| NH3 | Ammonia |
| H2O | Water |
| NOx | Nitrogen Oxide |
| N2 | Nitrogen |
| O2 | Oxygen |
| CO | Carbon Oxide |
| THC | Total Hydro Carbon |
| PM | Particle Matter |
| BPT | Balance Point Temperature |
| LNT | Lean NOx Trap |
| DOHC | Double OverHead Camshaft |
| AOC | Ammonia Oxidation Catalyst |
References
- Liang, Y.; Ding, X.; Dai, J.; Zhao, M.; Zhong, L.; Wang, J.; Chen, Y. Active oxygen-promoted NO catalytic on monolithic Pt-based diesel oxidation catalyst modified with Ce. Catal. Today 2019, 327, 64–72. [Google Scholar] [CrossRef]
- Salman, A.; Enger, B.; Auvray, X.; Lødeng, R.; Menon, M.; Waller, D.; Rønning, M. Catalytic oxidation of NO to NO2 for nitric acid production over a Pt/Al2O3 catalyst. Appl. Catal. A Gen. 2018, 564, 142–146. [Google Scholar] [CrossRef]
- Tighe, C.; Twigg, M.; Hayhurst, A.; Dennis, J. The kinetics of oxidation of Diesel soots by NO2. Combust. Flame 2012, 159, 77–90. [Google Scholar] [CrossRef]
- Jiao, P.; Li, Z.; Shen, B.; Zhang, W.; Kong, X.; Jiang, R. Research of DPF regeneration with NOx-PM coupled chemical reaction. Appl. Therm. Eng. 2017, 110, 737–745. [Google Scholar] [CrossRef]
- Radojevic, M. Reduction of nitrogen oxides in flue gases. Environ. Pollut. 1998, 102, 685–689. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yahiro, H.; Tanda, K. Catalytic decomposition of nitrogen-monoxide over copper ionexchanged zeolites. Stud. Surf. Sci. Catal. 1989, 44, 219–226. [Google Scholar]
- Shirahama, N.; Mochida, I.; Korai, Y.; Choi, K.; Enjoji, T.; Shimohara, T.; Yasutake, A. Reaction of NO with urea supported on activated carbons. Appl. Catal. B Environ. 2005, 57, 237–245. [Google Scholar] [CrossRef]
- Traa, Y.; Burger, B.; Weitkamp, J. Zeolite-based materials for the selective catalytic reduction of NOx with hydrocarbons. Microporous Mesoporous Mater. 1999, 30, 3–41. [Google Scholar] [CrossRef]
- Dumesic, J.; Topsøe, N.; Topsøe, H.; Chen, Y.; Slabiak, T. Kinetics of Selective Catalytic Reduction of Nitric Oxide by Ammonia over Vanadia/Titania. J. Catal. 1996, 163, 409–417. [Google Scholar] [CrossRef]
- Madia, G. Measures to Enhance the NOx Conversion in Urea-SCR Systems for Automotive Applications. , ETH Zurich, Zurich Switzerland. Doctoral Dissertation, ETH Zurich, Zurich, Switzerland.
- Gieshoff, J.; Schäfer-Sindlinger, A.; Spurk, P.; van den Tillaart, J.; Garr, G. Improved SCR Systems for Heavy Duty Applications; SAE Technical Paper 2000-01-0189; SAE International: Warrendale, PA, USA, 2000. [Google Scholar]
- Liu, Y.; Liu, Z.; Mnichowicz, B.; Harinath, A.; Li, H.; Bahrami, B. Chemical deactivation of commercial vanadium SCR catalysts in diesel emission control application. Chem. Eng. J. 2016, 287, 680–690. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. A comparative study of the NH3-SCR reactions over a Cu-zeolite and a Fe-zeolite catalyst. Catal. Today 2010, 151, 223–230. [Google Scholar] [CrossRef]
- Lü, L.; Wang, L. Model-based optimization of parameters for a diesel engine SCR system. Int. J. Automot. Technol. 2013, 14, 13–18. [Google Scholar] [CrossRef]
- Fu, M.; Ge, Y.; Wang, X.; Tan, J.; Yu, L.; Liang, B. NOx emissions from Euro IV busses with SCR systems associated with urban, suburban and freeway driving patterns. Sci. Total Environ. 2013, 452–453, 222–226. [Google Scholar] [CrossRef]
- Willems, F.; Cloudt, R.; van den Eijnden, E.; van Genderen, M.; Verbeek, R.; de Jager, B.; Boomsma, W.; den Heuvel, I. Is Closed-Loop SCR Control Required to Meet Future Emission Targets? SAE Technical Paper 2007-01-1574; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Bonfils, A.; Creff, Y.; Lepreux, O.; Petit, N. Closed-Loop Control of a SCR System Using a NOx Sensor Cross-Sensitive to NH3. IFAC Proc. Vol. 2012, 45, 738–743. [Google Scholar]
- Song, Q.; Zhu, G. Model-Based Closed-Loop Control of Urea SCR Exhaust Aftertreatment System for Diesel Engine; SAE Technical Paper 2002-01-0287; SAE International: Warrendale, PA, USA, 2002. [Google Scholar]
- Ham, Y.; Park, S. A Study of NH3 Adsorption/Desorption Characteristics and Model Based Control in the Urea-SCR System. Trans. KSAE 2016, 24, 302–309. [Google Scholar] [CrossRef]
- Schär, C.; Onder, C.; Geering, H. Modeling and control of an SCR system. IFAC Proc. Vol. 2004, 37, 355–360. [Google Scholar] [CrossRef]
- Wang, T.; Baek, S.; Jung, M.; Yeo, G. A Study of NH3 Adsorption/Desorption Characteristics in the Monolithic NH3-SCR Reactor. Trans. KSAE 2006, 14, 125–132. [Google Scholar]
- Chen, P.; Wang, J. Nonlinear and adaptive control of NO/NO2 ratio for improving SCR system performance. J. Frankl. Inst. 2013, 350, 1992–2012. [Google Scholar] [CrossRef]
- Mok, Y.; Yoon, E.; Dors, M.; Mizeraczyk, J. Optimum NO2/NOx ratio for efficient Selective Catalytic Reduction. Acta Phys. Slovaca 2005, 55, 467–478. [Google Scholar]
- Ko, A.; Woo, Y.; Jang, J.; Jung, J.; Pyo, Y.; Jo, H.; Lim, O.; Lee, Y. Availability of NH3 adsorption in vanadium-based SCR for reducing NOx emission and NH3 slip. J. Ind. Eng. Chem. 2019, 78, 433–439. [Google Scholar] [CrossRef]
- Ko, A.; Woo, Y.; Jang, J.; Jung, J.; Pyo, Y.; Jo, H.; Lim, O.; Lee, Y. Complementary effects between NO oxidation of DPF and NO2 decomposition of SCR in light-duty diesel engine. J. Ind. Eng. Chem. 2019, 80, 160–170. [Google Scholar] [CrossRef]


| No. of Cylinders | 5 |
| Valve type | DOHC 20 valve |
| Strokes | 4 |
| Type | Turbo diesel |
| Rated power P (PS) | 186/4000 rpm |
| Rated torque M (Nm) | 418/1600–3000 |
| Experiment engine speed (rpm) | 1500, 2000, 2500, 3000 |
| Exhaust gas temperature (°C) | 250, 300, 350, 400 |
| Manufacture | 2006 |
| Emission standards | Euro 2 |
| Category | DOC | DPF | SCR |
|---|---|---|---|
| Substrate | Cordierite | ||
| Catalyst | Pt + Promoter/TiO2 | V-W/TiO2 | |
| BPT | 285 °C | - | |
| Cell density | 400 cpsi | 300 cpsi | 400 cpsi |
| Diameter | 5.66 inch | 5.66 inch | 6.77 inch |
| Length | 4 inch | 10 inch | 10 inch |
| Category | Engine Operating Conditions |
|---|---|
| Engine speed [rpm] | 1500, 2000, 2500, 3000 |
| Exhaust gas temperature [°C] | 250, 300, 350, 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Ko, A.; Jang, J.; Lim, O. Study on Rates of NH3 Adsorption and Desorption in SCR on Various Engine Operation Conditions. Sustainability 2023, 15, 14468. https://doi.org/10.3390/su151914468
Jo H, Ko A, Jang J, Lim O. Study on Rates of NH3 Adsorption and Desorption in SCR on Various Engine Operation Conditions. Sustainability. 2023; 15(19):14468. https://doi.org/10.3390/su151914468
Chicago/Turabian StyleJo, Hyun, Ahyun Ko, Jinyoung Jang, and Ocktaeck Lim. 2023. "Study on Rates of NH3 Adsorption and Desorption in SCR on Various Engine Operation Conditions" Sustainability 15, no. 19: 14468. https://doi.org/10.3390/su151914468





