Effects of Chlortetracycline on the Growth of Eggplant and Associated Rhizosphere Bacterial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Collection
2.2. Potted Experiment
2.3. Measurement of Soil Properties
2.4. Measurement of Growth and Physiological Indexes
2.5. Soil Microbial Community Analysis
2.6. Statistical Analysis
3. Results
3.1. Changes in Soil Properties
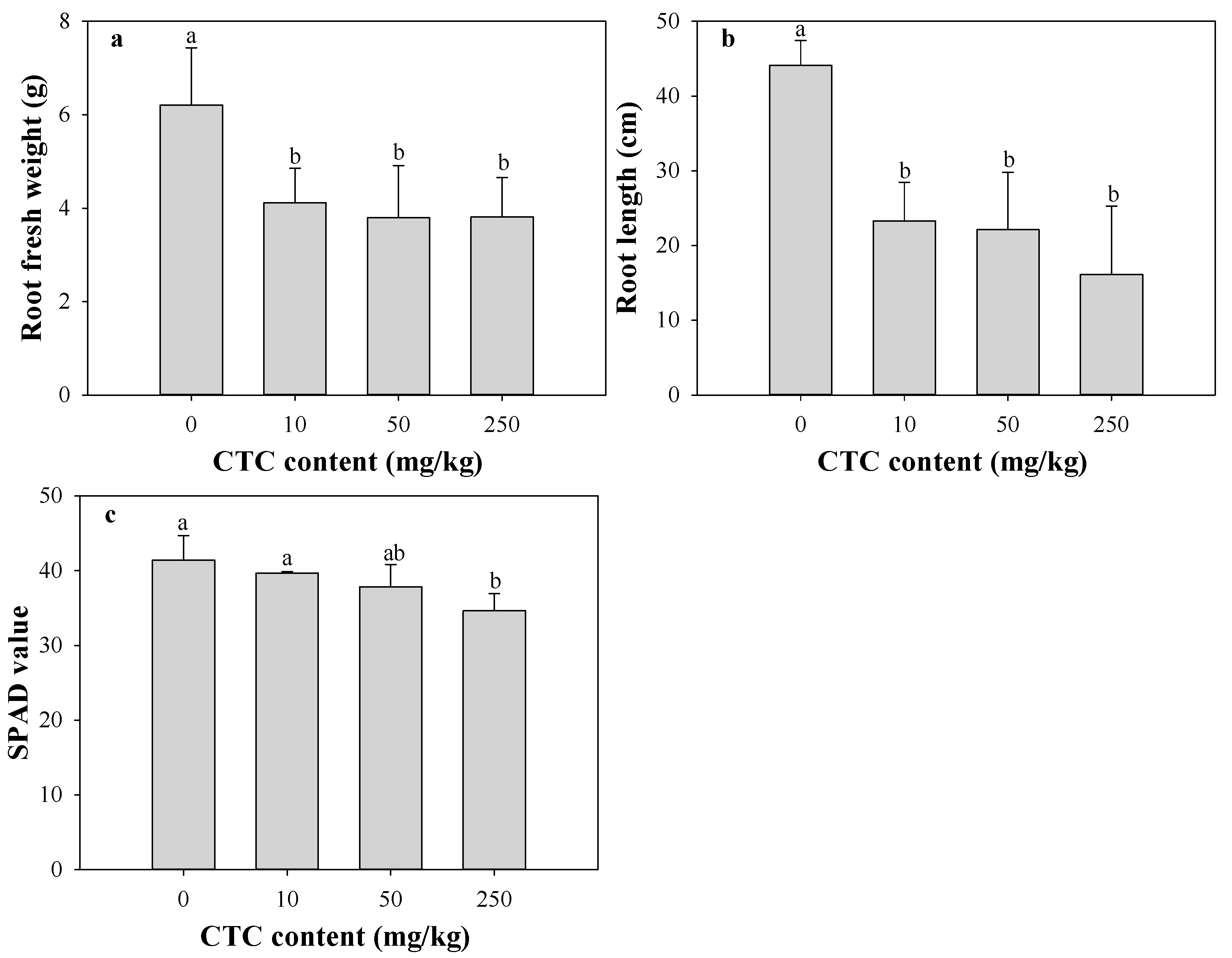
3.2. Effects of CTC on Eggplant Growth and Fluorescence Parameters
3.3. Effects of CTC on Soil Microorganism Communities
3.3.1. CTC Effects on Soil Microbial Diversity
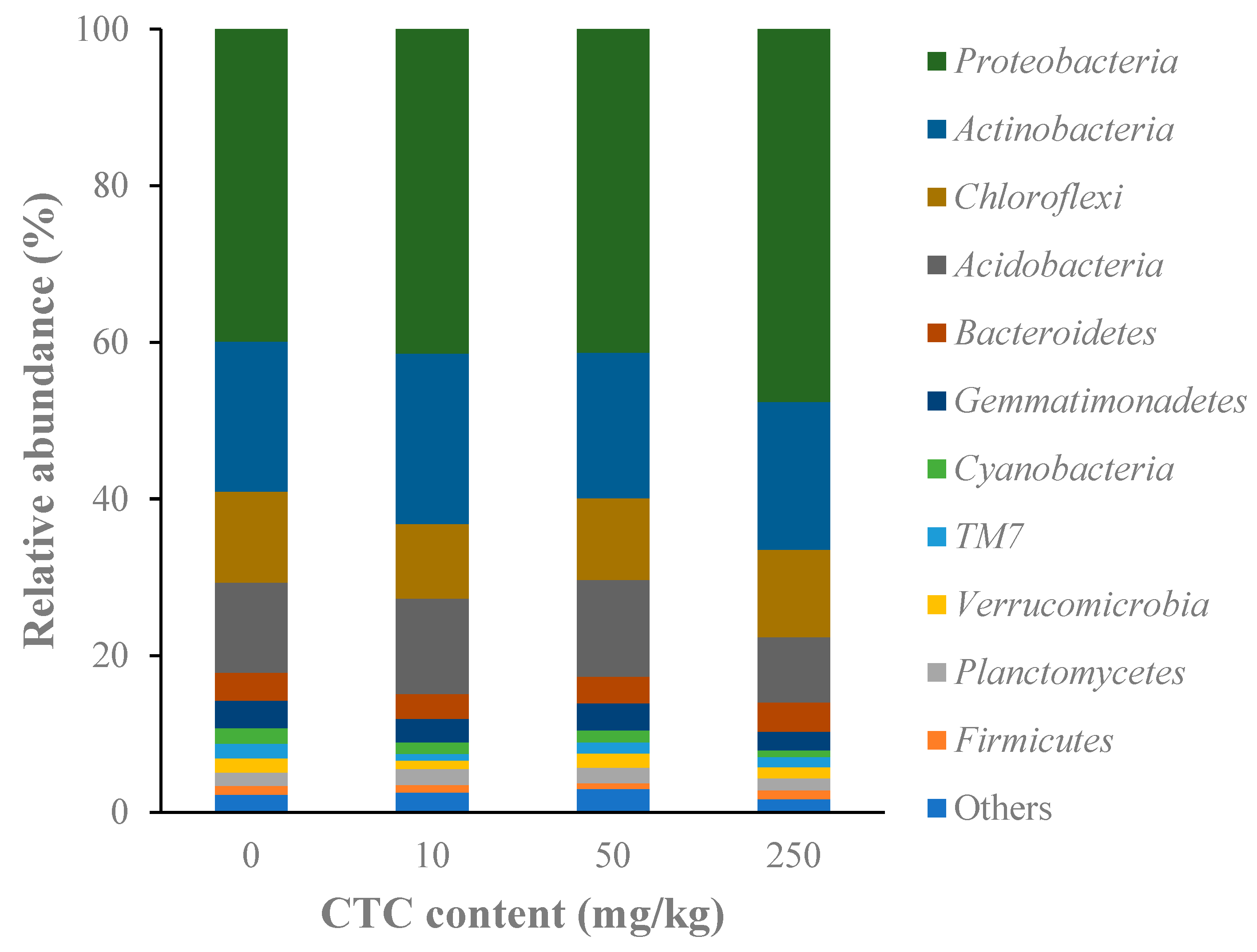
3.3.2. CTC Effects on Soil Microbial Composition
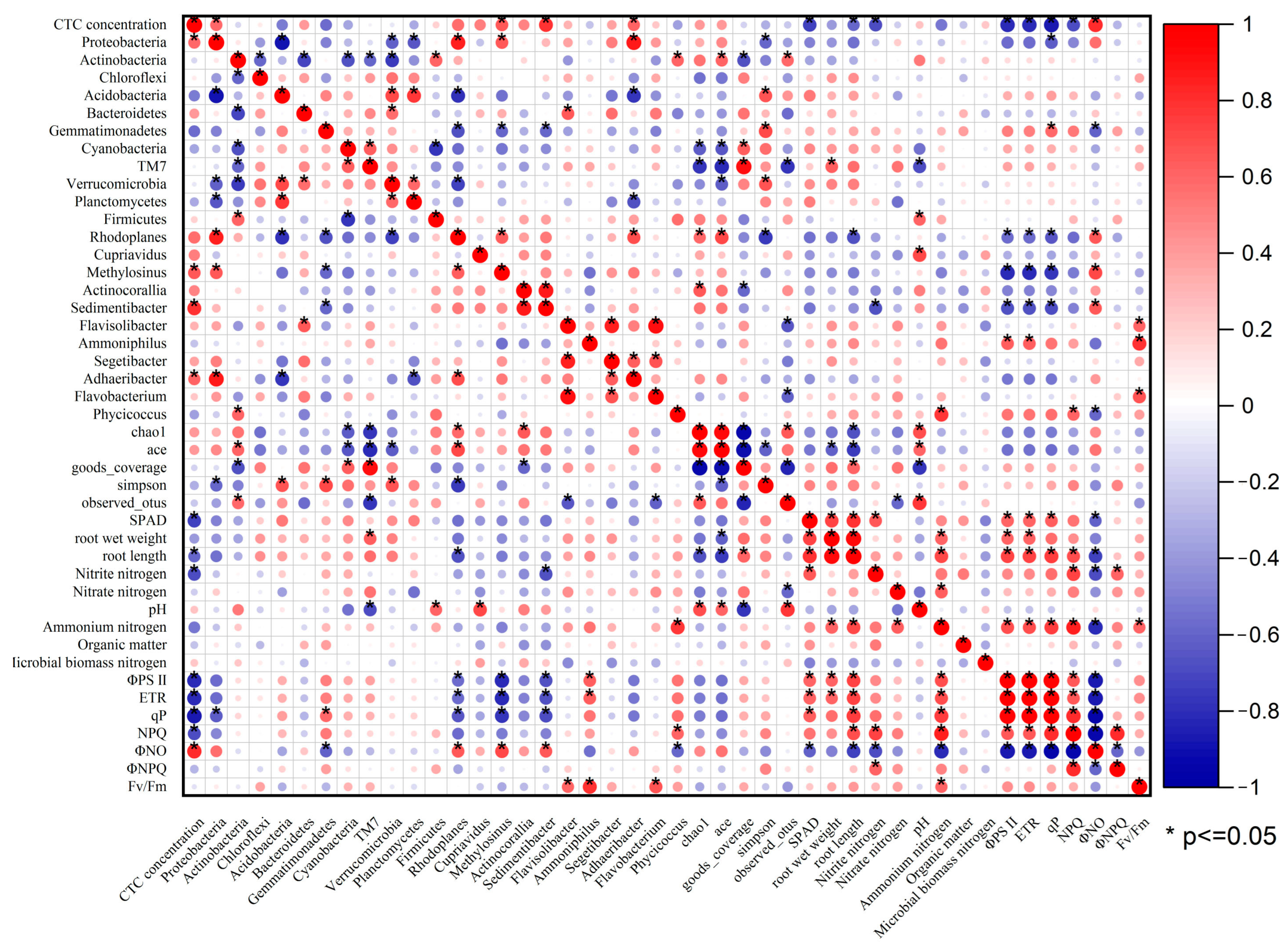
3.4. Correlation between Each Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Guo, A.; Pan, C.; Su, X.; Zhou, X.; Bao, Y. Combined effects of oxytetracycline and microplastic on wheat seedling growth and associated rhizosphere bacterial communities and soil metabolite profiles. Environ. Pollut. 2022, 302, 119046. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Yang, L.; Zhang, L.; Ye, B.; Wang, L. Antibiotics in soil and water in China-a systematic review and source analysis. Environ. Pollut. 2020, 266, 115147. [Google Scholar] [CrossRef] [PubMed]
- Patyra, E.; Kowalczyk, E.; Grelik, A.; Przeniosło-Siwczyńska, M.; Kwiatek, K. Screening method for the determination of tetracyclines and fluoroquinolones in animal drinking water by liquid chromatography with diode array detector. Pol. J. Vet. Sci. 2015, 18, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chen, L.; Yang, L.; Li, S.; Sun, L.; Yu, X. Distribution, dynamics and determinants of antibiotics in soils in a peri-urban area of Yangtze River Delta, Eastern China. Chemosphere 2018, 211, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; He, T.; Zhang, S.; Zhu, L.; Shang, B.; Li, Z.; Wang, R. Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China. Chemosphere 2019, 215, 234–240. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, S.; Zhao, L.; Li, X.; Weng, L.; Sun, Y.; Li, Y. Temporal and spatial variability of antibiotics in agricultural soils from Huang-Huai-Hai Plain, northern China. Chemosphere 2021, 272, 129803. [Google Scholar] [CrossRef]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef]
- Lee, C.; An, J.; Lee, Y.S.; Choi, K.; Kim, J.Y. Uncertainty-based concentration estimation of chlortetracycline antibiotics in swine farms and risk probability assessment for agricultural application of manure. J. Hazard. Mater. 2021, 402, 123763. [Google Scholar] [CrossRef]
- Jechalke, S.; Heuer, H.; Siemens, J.; Amelung, W.; Smalla, K. Fate and effects of veterinary antibiotics in soil. Trends Microbiol. 2014, 22, 536–545. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, D.; Sheng, G.D.; O’Connor, P.; Zhu, Y.G. Soil oxytetracycline exposure alters the microbial community and enhances the abundance of antibiotic resistance genes in the gut of Enchytraeus crypticus. Sci. Total Environ. 2019, 673, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.T.; Pan, X.; Chen, L.K.; Liu, W.X.; Christie, P.; Luo, Y.M.; Wu, L.H. Effects of different concentrations and application frequencies of oxytetracycline on soil enzyme activities and microbial community diversity. Eur. J. Soil Biol. 2016, 76, 53–60. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Grinyer, J.; Reich, P.B.; Singh, B.K. Relative importance of soil properties and microbial community for soil functionality: Insights from a microbial swap experiment. Funct. Ecol. 2016, 30, 1862–1873. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Chessa, L.; Pusino, A.; Garau, G.; Mangia, N.P.; Pinna, M.V. Soil microbial response to tetracycline in two different soils amended with cow manure. Environ. Sci. Pollut. Res. Int. 2016, 23, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, W.; Xu, W. Effects of tetracycline antibiotics in chicken manure on soil microbes and antibiotic resistance genes (ARGs). Environ. Geochem. Health 2022, 44, 273–284. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, J.; Gao, L.; Kong, F.; Shen, G.; Wang, R.; Gao, J.; Zhang, J. The Effects of Tetracycline Residues on the Microbial Community Structure of Tobacco Soil in Pot Experiment. Sci. Rep. 2020, 10, 8804. [Google Scholar] [CrossRef]
- Han, L.; Zhang, H.; Long, Z.; Ge, Q.; Mei, J.; Yu, Y.; Fang, H. Exploring microbial community structure and biological function in manured soil during ten repeated treatments with chlortetracycline and ciprofloxacin. Chemosphere 2019, 228, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, Y.; Xu, K.; Xiao, X.; Xi, B.; Lu, S. Response of ginger growth to a tetracycline-contaminated environment and residues of antibiotic and antibiotic resistance genes. Chemosphere 2018, 201, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liu, X.; Wang, J.; Li, S. Accumulation and risk assessment of antibiotics in edible plants grown in contaminated farmlands: A review. Sci. Total Environ. 2022, 853, 158616. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, L.; Wu, L.; Christie, P.; Luo, Y. Toxicity of OTC to Ipomoea aquatica Forsk. and to microorganisms in a long-term sewage-irrigated farmland soil. Environ. Sci. Pollut. Res. Int. 2016, 23, 15101–15110. [Google Scholar] [CrossRef]
- Ding, D.; Huang, X.Y.; Gu, J.Y.; Chen, C.Y.; Long, X.X.; Zeng, Q.Y. Distribution Characteristics and Risk Assessment of Tetracycline Antibiotics (TCs) in Soil-Vegetable System with Soil Fertilized with Animal Manure. Environ. Sci. 2023, 44, 4440–4447. (In Chinese) [Google Scholar]
- Xiang, L.; Wu, X.L.; Jiang, Y.N.; Yan, Q.Y.; Li, Y.W.; Huang, X.P.; Cai, Q.Y.; Mo, C.H. Occurrence and risk assessment of tetracycline antibiotics in soil from organic vegetable farms in a subtropical city, south China. Environ. Sci. Pollut. Res. Int. 2016, 23, 13984–13995. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Phytotoxicity of veterinary antibiotics to seed germination and root elongation of crops. Ecotoxicol. Environ. Saf. 2016, 126, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Lu, C.; Liao, Q.; Gudda, F.O.; Ling, W. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessment. Chemosphere 2020, 255, 127006. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, Y.; Shi, M.; Qiu, T.; Gao, M.; Tian, S.; Wang, X. Effect of antibiotic type and vegetable species on antibiotic accumulation in soil-vegetable system, soil microbiota, and resistance genes. Chemosphere 2021, 263, 128099. [Google Scholar] [CrossRef]
- Wang, W.Z.; Chi, S.L.; Xu, W.H. Biological Effect of Tetracycline Antibiotics on a Soil-Lettuce System and Its Migration Degradation Characteristics. Environ. Sci. 2021, 42, 1545–1558. (In Chinese) [Google Scholar]
- Cheong, M.S.; Choe, H.; Jeong, M.S.; Yoon, Y.E.; Jung, H.S.; Lee, Y.B. Different Inhibitory Effects of Erythromycin and Chlortetracycline on Early Growth of Brassica campestris Seedlings. Antibiotics 2021, 10, 1273. [Google Scholar] [CrossRef]
- Grayston, S.J.; Wang, S.Q.; Campbell, C.D.; Anthony, C.E. Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol. Biochem. 1998, 30, 369–378. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.; Barber, S.A. Effect of ammonium and nitrate fertilization on phosphorus uptake as related to root-Induced pH changes at the root-soil interface. Soil Sci. Soc. Amer. Proc. 1971, 35, 301–306. [Google Scholar] [CrossRef]
- Liu, F.; Mo, X.; Kong, W.; Song, Y. Soil bacterial diversity, structure, and function of Suaeda salsa in rhizosphere and non-rhizosphere soils in various habitats in the Yellow River Delta, China. Sci. Total Environ. 2020, 740, 140144. [Google Scholar] [CrossRef]
- NY/T 1377-2007; Determination of pH in Soil. Ministry of Agriculture of the People’s Republic of China. Standards Press of China: Beijing, China, 2007.
- NY/T 1121.6-2006; Soil Testing Part 6: Method for Determination of Soil Organic Matter. Ministry of Agriculture of the People’s Republic of China. Standards Press of China: Beijing, China, 2006.
- HJ 634-2012; Soil-Determination of Ammonium, Nitrite and Nitrate by Extraction with Potassium Chloride Solution-Spectrophotometric Methods. Ministry of Environmental Protection of the People’s Republic of China. China Environmental Press: Beijing, China, 2012.
- Wu, J.S.; Lin, Q.M.; Huang, Q.Y.; Xiao, H.A. Method for Calculating Soil Microbial Biomass and Its Application; China Meteorological Press: Beijing, China, 2006; pp. 54–71. [Google Scholar]
- Xue, Y.G.; Liu, F.; Zhou, L.L.; Jin, S.; Jiang, Y.; Wang, Y.C.; Jiang, X.D.; Wang, Q.; Shi, X.L.; Xue, K. Comparison study of bacterial community structure between groundwater and soil in industrial park based on high throughput sequencing. Asian J. Ecotoxicol. 2017, 12, 107–115. (In Chinese) [Google Scholar]
- Mao, B.; Zeng, Y.; Lai, C.T.; Yang, Y.; Zhou, C.H.; Li, Z.T. Effects of nitrogen reduction fertilization on sugarcane biomass, soil nitrate nitrogen and ammonium nitrogen. Chin. J. Ecol. 2023. Available online: https://kns.cnki.net/kcms/detail/21.1148.q.20230303.1019.006.html (accessed on 6 March 2023). (In Chinese).
- Pashaei, R.; Zahedipour-Sheshglani, P.; Dzingelevičienė, R.; Abbasi, S.; Rees, R.M. Effects of pharmaceuticals on the nitrogen cycle in water and soil: A review. Environ. Monit. Assess. 2022, 194, 105. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Kotzerke, A.; Sharma, S.; Schauss, K.; Heuer, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.M.; Schloter, M. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ. Pollut. 2008, 153, 315–322. [Google Scholar] [CrossRef]
- Toth, J.D.; Feng, Y.C.; Dou, Z.X. Veterinary antibiotics at environmentally relevant concentrations inhibit soil iron reduction and nitrification. Soil Biol. Biochem. 2011, 43, 2470–2472. [Google Scholar] [CrossRef]
- Yadava, U.L. A rapid and nondestructive method to determine chlorophyll in intact leaves. Hortscience 1986, 21, 1449–1500. [Google Scholar] [CrossRef]
- Park, S.Y.; Yu, J.W.; Park, J.S.; Li, J.; Yoo, S.C.; Lee, N.Y.; Lee, S.K.; Jeong, S.W.; Seo, H.S.; Koh, H.J.; et al. The senescence-induced staygreen protein regulates chlorophyll degradation. Plant Cell 2007, 19, 1649–1664. [Google Scholar] [CrossRef]
- Shimoda, Y.; Ito, H.; Tanaka, A. Arabidopsis STAY-GREEN, Mendel’s Green Cotyledon Gene, Encodes Magnesium-Dechelatase. Plant Cell 2016, 28, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Rydzyński, D.; Piotrowicz-Cieślak, A.I.; Grajek, H.; Wasilewski, J. Investigation of chlorophyll degradation by tetracycline. Chemosphere 2019, 229, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Liu, Y.; Wang, P.; Wu, T.; Zhang, S.; Shan, X.; Lu, J. Toxic effects of chlortetracycline on maize growth, reactive oxygen species generation and the antioxidant response. J. Environ. Sci. 2012, 24, 1099–1105. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Cheong, M.S.; Yoon, Y.E.; Kim, J.W.; Hong, Y.K.; Kim, S.C.; Lee, Y.B. Chlortetracycline inhibits seed germination and seedling growth in Brassica campestris by disrupting H2O2 signaling. Appl. Biol. Chem. 2020, 63, 1. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Kudo, G. Short-Term Responses in Maximum Quantum Yield of PSII (Fv/Fm) to ex situ Temperature Treatment of Populations of Bryophytes Originating from Different Sites in Hokkaido, Northern Japan. Plants 2016, 5, 22. [Google Scholar] [CrossRef]
- Zhang, S.R. A discussion on Chlorophyll Fluorescence kinetics parameters and their significance. Chin. Bull. Bot. 1999, 17, 444–448. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W.I. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Du, P.Z.; Liao, S.B.; Sun, B.; Chen, Y.; Luo, S.X.; Chen, L.; Huang, Y.F. Photosynthetic pigments and chlorophyll fluorescent characteristics of 17 provenances of Banksia seedlings. J. Central South Univ. Forest. Technol. 2014, 34, 49–54. (In Chinese) [Google Scholar]
- Costa, E.S.; Bressan-Smith, R.; Oliveira, J.G.; Campostrini, E. Chlorophyll a fluorescence analysis in response to excitation irradiance in bean plants (Phaseolus vulgaris L. and unguiculata L. Walp) submitted to high temperature stress. Photosynthetica 2003, 41, 77–82. [Google Scholar] [CrossRef]
- Shi, S.B.; Zhou, D.W.; Li, T.C.; De, K.J.; Gao, X.Z.; Ma, J.L.; Sun, T.; Wang, F.L. Responses of photosynthetic function of Kobresia pygmaea to simulated nocturnal low temperature on the Qingzang Plateau. Chin. J. Plant Ecol. 2023, 47, 361–373. (In Chinese) [Google Scholar] [CrossRef]
- Liu, M.; Cao, J.; Wang, C. Bioremediation by earthworms on soil microbial diversity and partial nitrification processes in oxytetracycline-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109996. [Google Scholar] [CrossRef]
- Zhao, L.; Pan, Z.; Sun, B.; Sun, Y.; Weng, L.; Li, X.; Ye, H.; Ye, J.; Pan, X.; Zhou, B.; et al. Responses of soil microbial communities to concentration gradients of antibiotic residues in typical greenhouse vegetable soils. Sci. Total Environ. 2023, 855, 158587. [Google Scholar] [CrossRef]
- Adams, D.G.; Duggan, P.S. Cyanobacteria-bryophyte symbioses. J. Exp. Bot. 2008, 59, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, F.; Xu, S.; Wu, S.; Zhuang, G.; Deng, Y.; Wu, J.; Zhuang, X. Myriophyllum aquaticum Constructed Wetland Effectively Removes Nitrogen in Swine Wastewater. Front. Microbiol. 2017, 8, 1932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chang, L.; Liu, X.; Wang, J.; Ge, X.; Cheng, J.; Xie, J.; Lin, Y.; Fu, Y.; Jiang, D.; et al. Rapeseed Domestication Affects the Diversity of Rhizosphere Microbiota. Microorganisms 2023, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, T.A.; Daghio, M.; González, M.; Papacchini, M.; Franzetti, A.; Seeger, M. Toluene degradation by Cupriavidus metallidurans CH34 in nitrate-reducing conditions and in Bioelectrochemical Systems. FEMS Microbiol. Lett. 2018, 365, 4996784. [Google Scholar]
- Bravo, G.; Vega-Celedón, P.; Gentina, J.C.; Seeger, M. Bioremediation by Cupriavidus metallidurans Strain MSR33 of Mercury-Polluted Agricultural Soil in a Rotary Drum Bioreactor and Its Effects on Nitrogen Cycle Microorganisms. Microorganisms 2020, 8, 1952. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y.; Klotz, M.G. Nitrifying and denitrifying pathways of methanotrophic bacteria. Biochem. Soc. Trans. 2011, 39, 1826–1831. [Google Scholar] [CrossRef]
- Shinjo, R.; Oe, F.; Nakagawa, K.; Murase, J.; Asakawa, S.; Watanabe, T. Type-specific quantification of particulate methane monooxygenase gene of methane-oxidizing bacteria at the oxic-anoxic interface of a surface paddy soil by digital PCR. Environ. Microbiol. Rep. 2023, 15, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Qiu, T.; Zhu, F.Y.; Xiao, J.L.; Wei, L.; Liang, Z.H. Calcium oxide regulation of fusarium wilt and rhizosphere bacterial community. J. Hunan Agric. Univ. (Nat. Sci.) 2018, 44, 620–624. (In Chinese) [Google Scholar]
- Zhang, T.; Shao, M.F.; Ye, L. 454 pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 2012, 6, 1137–1147. [Google Scholar] [CrossRef]
- Zhu, J.S.; Qin, H.L.; Sun, Q.Y.; Wang, B.Z.; Gao, R.X.; Guo, R.L.; Li, W.B. Microbial Diversity and Influencing Factors in a Small Watershed in winter. Environ. Sci. 2020, 41, 5016–5026. (In Chinese) [Google Scholar]
- Li, H.Y.; Yao, T.; Zhang, J.G.; Gao, Y.M.; Ma, Y.C.; Lu, Y.W.; Zhang, H.R.; Yang, X.L. Relationship between soil bacterial community and environmental factors in the degraded alpine grassland of eastern Qilian Mountains, China. Chin. J. Appl. Ecol. 2018, 29, 3793–3801. (In Chinese) [Google Scholar]
- Guo, H.; Tang, W.P. Enzyme activity and microbial community diversity in rhizosphere and non-rhizosphere of Larix principis-rupprechtii. Ecol. Environ. Sci. 2020, 29, 2163–2170. (In Chinese) [Google Scholar]
- Gilbert, J.A.; Steele, J.A.; Caporaso, J.G.; Steinbrück, L.; Reeder, J.; Temperton, B.; Huse, S.; McHardy, A.C.; Knight, R.; Joint, I.; et al. Defining seasonal marine microbial community dynamics. ISME J. 2012, 6, 298–308. [Google Scholar] [CrossRef]




| CTC Content (mg/kg) | 0 | 10 | 50 | 250 |
|---|---|---|---|---|
| NH4+–N (mg/kg) | 5.09 ± 0.41 a | 3.44 ± 0.21 b | 2.36 ± 0.27 c | 2.63 ± 0.45 c |
| NO2−–N (mg/kg) | 9.32 ± 3.53 a | 9.47 ± 4.25 a | 5.53 ± 1.35 ab | 3.05 ± 0.45 b |
| NO3−–N (mg/kg) | 5.27 ± 1.64 a | 3.80 ± 0.59 a | 3.81 ± 0.23 a | 4.01 ± 1.10 a |
| Microbial biomass N (mg/kg) | 7.88 ± 1.29 a | 8.71 ± 2.85 a | 9.11 ± 3.11 a | 9.53 ± 1.99 a |
| Organic matter (g/kg) | 120.00 ± 15.00 a | 114.33 ± 11.59 a | 119.33 ± 21.94 a | 110.67 ± 7.02 a |
| pH | 6.18 ± 0.08 a | 6.27 ± 0.05 a | 6.16 ± 0.05 a | 6.26 ± 0.13 a |
| CTC Content (mg/kg) | 0 | 10 | 50 | 250 |
|---|---|---|---|---|
| Fv/Fm | 0.807 ± 0.002 a | 0.796 ± 0.005 ab | 0.793 ± 0.010 b | 0.799 ± 0.006 ab |
| ETR | 62.66 ± 3.92 a | 54.49 ± 1.13 b | 54.14 ± 4.22 b | 45.66 ± 2.64 c |
| qP | 0.57 ± 0.01 a | 0.49 ± 0.02 b | 0.48 ± 0.03 b | 0.39 ± 0.02 c |
| NPQ | 1.21 ± 0.17 a | 1.01 ± 0.08 ab | 0.88 ± 0.09 bc | 0.79 ± 0.07 c |
| ΦPS II | 0.36 ± 0.02 a | 0.31 ± 0.01 b | 0.31 ± 0.02 b | 0.26 ± 0.02 c |
| ΦNPQ | 0.37 ± 0.04 a | 0.35 ± 0.02 a | 0.34 ± 0.02 a | 0.34 ± 0.02 a |
| ΦNO | 0.28 ± 0.02 c | 0.34 ± 0.02 b | 0.35 ± 0.01 b | 0.40 ± 0.02 a |
| CTC Content (mg/kg) | 0 | 10 | 50 | 250 |
|---|---|---|---|---|
| Chao | 15,493.03 ± 823.22 a | 16,934.68 ± 730.13 a | 16,057.85 ± 521.00 a | 17,146.30 ± 1462.67 a |
| Ace | 15,826.9 ± 502.09 a | 17,650.74 ± 987.84 a | 16,577.00 ± 503.5 a | 17,576.4 ± 921.40 a |
| Simpson | 0.997 ± 0.000 a | 0.997 ± 0.001 a | 0.997 ± 0.002 a | 0.996 ± 0.001 a |
| Observed_OTUs | 5966.67 ± 136.39 a | 6253.33 ± 112.83 a | 6060.67 ± 75.57 a | 6007.67 ± 253.25 a |
| Goods_coverage (%) | 81.89 ± 0.55 a | 80.43 ± 0.66 b | 81.39 ± 0.59 ab | 80.95 ± 0.90 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Xue, Y.; Wang, H.; Chen, Y. Effects of Chlortetracycline on the Growth of Eggplant and Associated Rhizosphere Bacterial Communities. Sustainability 2023, 15, 14593. https://doi.org/10.3390/su151914593
Li L, Xue Y, Wang H, Chen Y. Effects of Chlortetracycline on the Growth of Eggplant and Associated Rhizosphere Bacterial Communities. Sustainability. 2023; 15(19):14593. https://doi.org/10.3390/su151914593
Chicago/Turabian StyleLi, Lingling, Yuanyuan Xue, Hengsheng Wang, and Yansong Chen. 2023. "Effects of Chlortetracycline on the Growth of Eggplant and Associated Rhizosphere Bacterial Communities" Sustainability 15, no. 19: 14593. https://doi.org/10.3390/su151914593
APA StyleLi, L., Xue, Y., Wang, H., & Chen, Y. (2023). Effects of Chlortetracycline on the Growth of Eggplant and Associated Rhizosphere Bacterial Communities. Sustainability, 15(19), 14593. https://doi.org/10.3390/su151914593














