High-Value Utilization of Corn Straw: From Waste to Wealth
Abstract
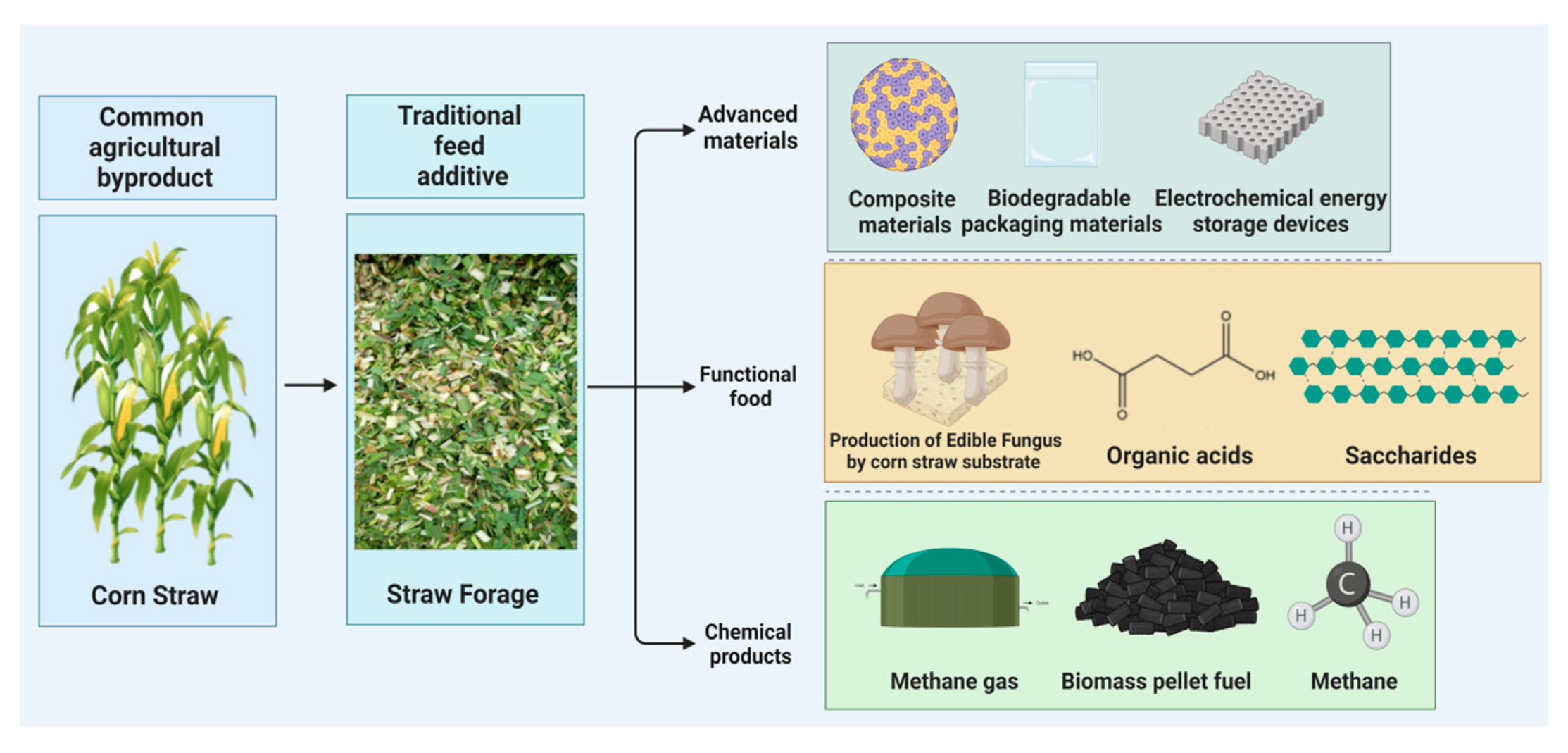
:1. Introduction
2. The Main Active Ingredients of Corn Straw
2.1. Organic Acids
2.1.1. Lactic Acid
2.1.2. Fumaric Acid
2.1.3. Propionic Acid
2.1.4. Succinic Acid
2.1.5. Levulinic Acid
2.1.6. Hydroxycinnamic Acids
2.2. Saccharides
2.2.1. Monosaccharide
2.2.2. Xylo-Oligosaccharides
2.2.3. Xylitol
2.2.4. Furfural
2.2.5. D-allulose
3. Technology Readiness Assessment and Processing Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.I.; Dragone, G. Biomass pretreatment, biorefineries, and potential products for a bioeconomy development. In Biomass Fractionation Technologies for A Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar]
- Asghar, A.; Sairash, S.; Hussain, N.; Baqar, Z.; Sumrin, A.; Bilal, M. Current challenges of biomass refinery and prospects of emerging technologies for sustainable bioproducts and bioeconomy. Biofuels Bioprod. Biorefining 2022, 16, 1478–1494. [Google Scholar] [CrossRef]
- Liu, Z.; de Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of food waste to produce value-added products based on its bioactive compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Venkatkarthick, R.; SenthilKannan, K.; Kuppam, C.; Stephy, G.M.; Kamyab, H.; Chen, W.-H.; Thomas, J.; Ngamcharussrivichai, C. Advanced technologies on the sustainable approaches for conversion of organic waste to valuable bioproducts: Emerging circular bioeconomy perspective. Fuel 2022, 324, 124313. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sindhu, R.; Sirohi, R.; Kumar, V.; Ahluwalia, V.; Binod, P.; Juneja, A.; Kumar, D.; Yan, B.; Sarsaiya, S.; et al. Agricultural waste biorefinery development towards circular bioeconomy. Renew. Sustain. Energy Rev. 2022, 158, 112122. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, S.-E.; Lee, D.-W. Current status and future prospects of biological routes to bio-based products using raw materials, wastes, and residues as renewable resources. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2453–2509. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Biomass-based biorefineries: An important architype towards a circular economy. Fuel 2021, 288, 119622. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Wang, H.; Liu, T.; Zheng, X.; Gao, S.; Lu, J. Recent advances in carbon-based materials for solar-driven interfacial photothermal conversion water evaporation: Assemblies, structures, applications, and prospective. Carbon Energy 2023, e331. [Google Scholar] [CrossRef]
- Danciu, C.-A.; Tulbure, A.; Stanciu, M.-A.; Antonie, I.; Capatana, C. Opportunities in Grain Processing, through the Use of Waste and By-Products: An Overview of Sustainable Valorization in the Food Industry. 2023. Available online: https://www.preprints.org/manuscript/202304.1274/v1 (accessed on 27 September 2023).
- Bentsen, N.S.; Jørgensen, J.R.; Stupak, I.; Jørgensen, U.; Taghizadeh-Toosi, A. Dynamic sustainability assessment of heat and electricity production based on agricultural crop residues in Denmark. J. Clean. Prod. 2019, 213, 491–507. [Google Scholar] [CrossRef]
- Jin, C.; Hu, J.; Wu, J.; Liang, H.; Li, J. Innovative and economically beneficial use of corn and corn products in electrochemical energy storage applications. ACS Sustain. Chem. Eng. 2021, 9, 10678–10703. [Google Scholar] [CrossRef]
- Battaglia, M.; Thomason, W.; Fike, J.H.; Evanylo, G.K.; von Cossel, M.; Babur, E.; Iqbal, Y.; Diatta, A.A. The broad impacts of corn stover and wheat straw removal for biofuel production on crop productivity, soil health and greenhouse gas emissions: A review. Gcb Bioenergy 2021, 13, 45–57. [Google Scholar] [CrossRef]
- Sarkar, S.; Skalicky, M.; Hossain, A.; Brestic, M.; Saha, S.; Garai, S.; Ray, K.; Brahmachari, K. Management of crop residues for improving input use efficiency and agricultural sustainability. Sustainability 2020, 12, 9808. [Google Scholar] [CrossRef]
- Lorenzana-Moreno, A.V.; Leal Lara, H.; Corona, L.; Granados, O.; Márquez-Mota, C.C. Production of 17 strains of edible mushroom grown on corn stover and its effect on the chemical composition and ruminal in vitro digestibility of the residual substrate. PLoS ONE 2023, 18, e0286514. [Google Scholar] [CrossRef]
- Wan, G.; Yan, W.; Wu, C.; Xiao, Y.; Lin, J.; Zhang, S. Research progress and challenges of the gas turbine flowmeter in energy measurement. Front. Energy Res. 2023, 10, 977140. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Saini, A.K.; Saini, V.; Pareek, B.; Gaidukovs, S.; Thakur, V.K. Recovery processes of sustainable energy using different biomass and wastes. Renew. Sustain. Energy Rev. 2021, 150, 111483. [Google Scholar] [CrossRef]
- Poudel, J.; Oh, S.C. Effect of torrefaction on the properties of corn stalk to enhance solid fuel qualities. Energies 2014, 7, 5586–5600. [Google Scholar] [CrossRef]
- Shen, J.; Wyman, C.E. A novel mechanism and kinetic model to explain enhanced xylose yields from dilute sulfuric acid compared to hydrothermal pretreatment of corn stover. Bioresour. Technol. 2011, 102, 9111–9120. [Google Scholar] [CrossRef]
- Kumar, A.; Demirel, Y.; Jones, D.D.; Hanna, M.A. Optimization and economic evaluation of industrial gas production and combined heat and power generation from gasification of corn stover and distillers grains. Bioresour. Technol. 2010, 101, 3696–3701. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Yi, X.; Zhao, Y.; Jin, F.; Chen, L.; Hua, D. Study of two-phase anaerobic digestion of corn stover: Focusing on the conversion of volatile fatty acids and microbial characteristics in UASB reactor. Ind. Crops Prod. 2021, 160, 113097. [Google Scholar] [CrossRef]
- Ma, S.; Wang, H.; Wang, B.; Gu, X.; Zhu, W. Biomethane enhancement from corn straw using anaerobic digestion by-products as pretreatment agents: A highly effective and green strategy. Bioresour. Technol. 2022, 344, 126177. [Google Scholar] [CrossRef]
- Chu, X.; Cheng, Q.; Xu, Y.; Luo, L.; Wang, M.; Zheng, G.; Zhang, H.; Yi, W.; Liu, X.; Sun, Y.; et al. Anaerobic digestion of corn straw pretreated by ultrasonic combined with aerobic hydrolysis. Bioresour. Technol. 2021, 341, 125826. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, T.; Zhao, M.; Wang, Y. Research on hydrogen production and degradation of corn straw by circular electrolysis with polyoxometalate (POM) catalyst. Int. J. Hydrog. Energy 2022, 47, 15357–15369. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M.; Yan, S.; Yong, X.; Zhang, X.; Awasthi, M.K.; Xi, Y.; Zhou, J. Effects of hydrochar and biogas slurry reflux on methane production by mixed anaerobic digestion of cow manure and corn straw. Chemosphere 2023, 310, 136876. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Montini, T.; Zhang, J.; Fornasiero, P.; Fonda, E.; Hou, T.; Nie, W.; Lu, J.; Liu, J.; Heggen, M.; et al. Visible-light-driven coproduction of diesel precursors and hydrogen from lignocellulose-derived methylfurans. Nat. Energy 2019, 4, 575–584. [Google Scholar] [CrossRef]
- Parsimehr, H.; Ehsani, A. Corn-based Electrochemical Energy Storage Devices. Chem. Rec. 2020, 20, 1163–1180. [Google Scholar] [CrossRef]
- Shen, T.; Li, M.; Zhang, B.; Zhong, L.; Lin, X.; Yang, P.; Li, M.; Zhuang, W.; Zhu, C.; Ying, H. Enhanced Mechanical Properties of Polyvinyl Chloride-Based Wood–Plastic Composites With Pretreated Corn Stalk. Front. Bioeng. Biotechnol. 2022, 9, 829821. [Google Scholar] [CrossRef]
- Li, Z.; Guan, J.; Yan, C.; Chen, N.; Wang, C.; Liu, T.; Cheng, F.; Guo, Q.; Zhang, X.; Ye, X.; et al. Corn straw core/cellulose nanofibers composite for food packaging: Improved mechanical, bacteria blocking, ultraviolet and water vapor barrier properties. Food Hydrocoll. 2023, 143, 108884. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A.; Panesar, P.S. Lactic acid and its separation and purification techniques: A review. Rev. Environ. Sci. Bio/Technol. 2019, 18, 823–853. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.-M.; Lebaka, V.R.; Wee, Y.-J. Lactic acid for green chemical industry: Recent advances in and future prospects for production technology, recovery, and applications. Fermentation 2022, 8, 609. [Google Scholar] [CrossRef]
- Panda, S.K.; Sahu, L.; Behera, S.K.; Ray, R.C. Research and production of organic acids and industrial potential. Bioprocess. Biomol. Prod. 2019, 195–209. [Google Scholar]
- Yadav, A.K.; Chaudhari, A.B.; Kothari, R.M. Bioconversion of renewable resources into lactic acid: An industrial view. Crit. Rev. Biotechnol. 2011, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Cho, M.H.; Cho, S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Wei, C.; Qi, W.; Xiu, Z. An aptly industrialized bioprocess for lactic acid production from corn stover using thermotolerant microbial consortia. Bioprocess Biosyst. Eng. 2021, 44, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, J.; Zhang, J.; Tu, Y.; Bao, J. High titer L-lactic acid production from corn stover with minimum wastewater generation and techno-economic evaluation based on Aspen plus modeling. Bioresour. Technol. 2015, 198, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Li, Y.; Wan, C. Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour. Technol. 2011, 102, 1831–1836. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, P.; Sun, J.; Tu, Y.; Gao, Q.; Zhang, J.; Bao, J. Engineering wild-type robust Pediococcus acidilactici strain for high titer L-and D-lactic acid production from corn stover feedstock. J. Biotechnol. 2016, 217, 112–121. [Google Scholar] [CrossRef]
- Xu, Q.; Li, S.; Fu, Y.; Tai, C.; Huang, H. Two-stage utilization of corn straw by Rhizopus oryzae for fumaric acid production. Bioresour. Technol. 2010, 101, 6262–6264. [Google Scholar] [CrossRef]
- Wang, X.; Salvachúa, D.; Sànchez i Nogué, V.; Michener, W.E.; Bratis, A.D.; Dorgan, J.R.; Beckham, G.T. Propionic acid production from corn stover hydrolysate by Propionibacterium acidipropionici. Biotechnol. Biofuels 2017, 10, 200. [Google Scholar] [CrossRef]
- Wang, P.; Shen, C.; Li, L.; Guo, J.; Cong, Q.; Lu, J. Simultaneous production of propionic acid and vitamin B12 from corn stalk hydrolysates by Propionibacterium freudenreichii in an expanded bed adsorption bioreactor. Prep. Biochem. Biotechnol. 2020, 50, 763–767. [Google Scholar] [CrossRef]
- Zheng, P.; Fang, L.; Xu, Y.; Dong, J.-J.; Ni, Y.; Sun, Z.-H. Succinic acid production from corn stover by simultaneous saccharification and fermentation using Actinobacillus succinogenes. Bioresour. Technol. 2010, 101, 7889–7894. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wan, Q.; Liu, R.; Liang, L.; Chen, X.; Wu, M.; Zhang, H.; Chen, K.; Ma, J.; Wei, P. Succinic acid production from corn stalk hydrolysate in an E. coli mutant generated by atmospheric and room-temperature plasmas and metabolic evolution strategies. J. Ind. Microbiol. Biotechnol. 2014, 41, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Omidvarborna, H. High concentration levulinic acid production from corn stover. RSC Adv. 2016, 6, 111616–111621. [Google Scholar] [CrossRef]
- Zhi, Z.; Li, N.; Qiao, Y.; Zheng, X.; Wang, H.; Lu, X. Kinetic study of levulinic acid production from corn stalk at relatively high temperature using FeCl3 as catalyst: A simplified model evaluated. Ind. Crops Prod. 2015, 76, 672–680. [Google Scholar] [CrossRef]
- Zheng, X.; Zhi, Z.; Gu, X.; Li, X.; Zhang, R.; Lu, X. Kinetic study of levulinic acid production from corn stalk at mild temperature using FeCl3 as catalyst. Fuel 2017, 187, 261–267. [Google Scholar] [CrossRef]
- Johnston, P.A.; Zhou, H.; Aui, A.; Wright, M.M.; Wen, Z.; Brown, R.C. A lignin-first strategy to recover hydroxycinnamic acids and improve cellulosic ethanol production from corn stover. Biomass Bioenergy 2020, 138, 105579. [Google Scholar] [CrossRef]
- Yang, M.; Li, W.; Liu, B.; Li, Q.; Xing, J. High-concentration sugars production from corn stover based on combined pretreatments and fed-batch process. Bioresour. Technol. 2010, 101, 4884–4888. [Google Scholar] [CrossRef]
- An, S.; Li, W.; Liu, Q.; Li, M.; Ma, Q.; Ma, L.; Chang, H.-M. A two-stage pretreatment using acidic dioxane followed by dilute hydrochloric acid on sugar production from corn stover. RSC Adv. 2017, 7, 32452–32460. [Google Scholar] [CrossRef]
- Shi, F.; Xiang, H.; Li, Y. Combined pretreatment using ozonolysis and ball milling to improve enzymatic saccharification of corn straw. Bioresour. Technol. 2015, 179, 444–451. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.-H.; Wei, Q.-Y.; Du, X.-J.; Qu, Y.-S. Investigating desorption during ethanol elution to improve the quality and antioxidant activity of xylo-oligosaccharides from corn stalk. Bioresour. Technol. 2018, 249, 342–347. [Google Scholar] [CrossRef]
- Moniz, P.; Ho, A.L.; Duarte, L.C.; Kolida, S.; Rastall, R.A.; Pereira, H.; Carvalheiro, F. Assessment of the bifidogenic effect of substituted xylo-oligosaccharides obtained from corn straw. Carbohydr. Polym. 2016, 136, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ling, H.; Zhao, H. Steam explosion pretreatment of corn straw on xylose recovery and xylitol production using hydrolysate without detoxification. Process Biochem. 2015, 50, 1623–1628. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Lu, Y.; Liu, Q.; Guan, S.; Chang, H.-M.; Jameel, H.; Ma, L. Enhanced furfural production from raw corn stover employing a novel heterogeneous acid catalyst. Bioresour. Technol. 2017, 245, 258–265. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, H.; Zhao, A.; Qu, L.; Xiong, W.; Alam, M.A.; Miao, J.; Wang, W.; Li, F.; Xu, J.; et al. Produce D-allulose from non-food biomass by integrating corn stalk hydrolysis with whole-cell catalysis. Front. Bioeng. Biotechnol. 2023, 11, 1156953. [Google Scholar] [CrossRef] [PubMed]
- Ilica, R.-A.; Kloetzer, L.; Galaction, A.-I.; Caşcaval, D. Fumaric acid: Production and separation. Biotechnol. Lett. 2019, 41, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, S.; Huang, H.; Wen, J. Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol. Adv. 2012, 30, 1685–1696. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Kumar, S.; Babu, B. A Brief Review on Propionic Acid: A Renewal Energy Source. 2006. Available online: https://api.semanticscholar.org/CorpusID:16277408 (accessed on 27 September 2023).
- Raj, M.; Devi, T.; Kumar, V.; Mishra, P.; Upadhyay, S.K.; Yadav, M.; Sharma, A.K.; Sehrawat, N.; Kumar, S.; Singh, M. Succinic acid: Applications and microbial production using organic wastes as low cost substrates. Phys. Sci. Rev. 2023. [Google Scholar] [CrossRef]
- Olajuyin, A.M.; Yang, M.; Liu, Y.; Mu, T.; Tian, J.; Adaramoye, O.A.; Xing, J. Efficient production of succinic acid from Palmaria palmata hydrolysate by metabolically engineered Escherichia coli. Bioresour. Technol. 2016, 214, 653–659. [Google Scholar] [CrossRef]
- Bahú, J.O.; Khouri, N.G.; Concha, V.O.C.; de Jesus Bonon, A.; Schiavon, M.I.R.B.; Maciel Filho, R. Levulinic Acid as a Chemical Platform to Polymers. 2021. Mater. Int. 2021, 3, 7. [Google Scholar]
- Kontovas, S.; Misailidis, N.; Petrides, D. Levulinic Acid Production from Cellulosic Biomass – Process Modeling and Techno–Economic Assessment (TEA) using SuperPro Designer. 2022. Available online: https://www.researchgate.net/publication/364383412_Levulinic_Acid_Production_from_Cellulosic_Biomass_-_Process_Modeling_and_Techno-Economic_Assessment_TEA_using_SuperPro_Designer (accessed on 27 September 2023).
- Mansir, N.; Sidek, H.M.; Teo, S.H.; Mijan, N.-A.; Alsultan, A.G.; Ng, C.H.; Shamsuddin, M.R.; Taufiq-Yap, Y.H. Catalytically active metal oxides studies for the conversion technology of carboxylic acids and bioresource based fatty acids to ketones: A review. Bioresour. Technol. Rep. 2022, 17, 100988. [Google Scholar] [CrossRef]
- Butts-Wilmsmeyer, C.J.; Mumm, R.H.; Bohn, M.O. Quantitative genetic analysis of hydroxycinnamic acids in maize (Zea mays L.) for plant improvement and production of health-promoting compounds. J. Agric. Food Chem. 2020, 68, 9585–9593. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.H.L.; Morais, A.R.C.; da Costa Lopes, A.M.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Pereira Ramos, L. Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef] [PubMed]
- Ferdeș, M.; Dincă, M.N.; Moiceanu, G.; Zăbavă, B.Ș.; Paraschiv, G. Microorganisms and enzymes used in the biological pretreatment of the substrate to enhance biogas production: A review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Terrile, R.J.; Doumani, F.G.; Ho, G.Y.; Jackson, B.L. Calibrating the technology readiness level (TRL) scale using NASA mission data. In Proceedings of the 2015 IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2015; pp. 1–9. [Google Scholar]
- Olechowski, A.L.; Eppinger, S.D.; Joglekar, N.; Tomaschek, K. Technology readiness levels: Shortcomings and improvement opportunities. Syst. Eng. 2020, 23, 395–408. [Google Scholar] [CrossRef]
- Beims, R.; Simonato, C.; Wiggers, V. Technology readiness level assessment of pyrolysis of trygliceride biomass to fuels and chemicals. Renew. Sustain. Energy Rev. 2019, 112, 521–529. [Google Scholar] [CrossRef]
- Sorunmu, Y.; Billen, P.; Spatari, S. A review of thermochemical upgrading of pyrolysis bio-oil: Techno-economic analysis, life cycle assessment, and technology readiness. Gcb Bioenergy 2020, 12, 4–18. [Google Scholar] [CrossRef]
- Dickson, R.; Mancini, E.; Garg, N.; Woodley, J.M.; Gernaey, K.V.; Pinelo, M.; Liu, J.; Mansouri, S.S. Sustainable bio-succinic acid production: Superstructure optimization, techno-economic, and lifecycle assessment. Energy Environ. Sci. 2021, 14, 3542–3558. [Google Scholar] [CrossRef]


| Products | Operating Condition | Output/Yield | Reference |
|---|---|---|---|
| L- and D-lactic acid | Batch SSF with microbial consortium DUT47 at 47 °C and pH 5.5 | 43.73 g‧dm−3 in lactic acid concentration, 0.50 g/g-corn straw in yield, 0.32 g/(L.h) in productivity | [35] |
| Engineered strain P. acidilactici TY112 from dry milling biorefinery processed corn straw | 104.5 g‧dm−3 in L-lactic acid titer, 71.5% in overall yield. | [36] | |
| NaOH-pretreated and washed corn straw with Lb. pentosus FL0421 at 37 °C and pH 6.0 with cellulase activity of 30 FPU/g straw and yeast extract of 10 g‧dm−3 | 92.30 g‧dm−3 in lactic acid titer, 0.66 g/g straw in yield, 1.92 g‧dm−3 h−1 in productivity | [37] | |
| SSF of NaOH-treated corn straw with mixed cultures of Lactobacillus rhamnosus and L. brevis | 0.70 g/g in lactic acid yield | [38] | |
| SSF at 25% (w/w) solid content with dry dilute-acid-pretreated and biodetoxified corn straw | 77.66 g‧dm−3 L-lactic acid from P. acidilactici TY112, 76.76 g‧dm−3 D-lactic acid from P. acidilactici ZP26 | [39] | |
| Fumaric acid | Corn straw pretreated with dilute acid to grow fungal biomass and then digested with enzyme to obtain a glucose-rich liquid for fumaric acid production | Up to 27.79 g‧dm−3 in production, 0.35 g/g in yield, 0.33 g‧dm−3 h−1 in productivity | [40] |
| Propionic acid | Anaerobic production from P. acidipropionici in corn straw via DDAPH fed-batch HCD fermentation | Titer in 64.7 g‧dm−3 with productivity of 2.35 g‧dm−3 h at the batch stage and 0.77 g‧dm−3 h−1 in the overall process | [41] |
| P. freudenreichii CICC 10,019 fermentation combined with expanded bed adsorption bioreactor (EBAB) of liquid hot-water-pretreated corn stalk hydrolysates | 47.6 mg dm−3 vitamin B12 and 91.4 g dm−3 propionic acid at 258 h, with yields of 0.37 mg/g and 0.75 g/g, respectively | [42] | |
| Succinic acid | SSF at 38 °C for 48 h, diluted alkaline-pretreated corn straw as substrate at 70 g dm−3, load of 20 FPU cellulase, and 10 U cellobiase per gram of substrate | 47.4 g dm−3 in maximal concentration, 0.72 g/g-substrate in yield | [43] |
| Anaerobic fermentation of corn stalk hydrolysate with AFP111 | 21.1 g dm−3 in yield with corresponding yield of 76% | [44] | |
| Levulinic acid | SIRE–BE for glucose conversion to fructose (yield > 88%) and transferred fructose to low-pH aqueous medium. Dehydration of fructose to HMF and conversion of HMF to LA at high yield (>60%) | Significant load of 6.4 wt% converted from fructose at high yield (63 mol%) and facile reaction conditions | [45] |
| 230 °C and 10 min with 0.5 mol/L catalyst (FeCl3) with corn stalk as biomass substrate | Highest yield at 48.73% | [46] | |
| 180 °C, 40 min with 0.5 mol/L FeCl3 | Maximum concentration of 16.14 g dm−3, or yield of 48.89% | [47] | |
| Hydroxycinnamic acids | Mild alkaline pretreatment of corn straw with sodium hydroxide, ethanol, and water | Coumaric acid and ferulic acid yields of 20 wt% and 9.5 wt%, respectively, on a lignin basis, total hydroxycinnamic acid yield of 33.5% | [48] |
| Glucose, cellobiose and xylose | Steam explosion and alkaline peroxide treatment to remove hemicellulose and lignin | 220, 175, 22 and 20 g dm−3 reducing sugar, glucose, cellobiose, and xylose, respectively | [49] |
| Glucose and xylose | Removal of lignin at 90 °C, 20 min, 9/1 (v/v) dioxane–water including a 1.0 wt% HCl solution; treatment at 120 °C and 40 min in 1.0 wt% dilute hydrochloric acid | Total yields of glucose and xylose at 91.5% and 79.7%, respectively | [50] |
| Glucose and xylose | Ozonolysis for 90 min followed by planetary ball milling for 8 min with cellulase loading of 15 FPU/g straw | Glucose yield (407.76 mg/g- straw), nearly highest xylose yield (101.87 mg/g- straw) | [51] |
| xylo-oligosaccharides | Extraction of xylan with 10% NaOH, and enzymatic hydrolysis | Maximum yield of 1115 ± 32—1908 ± 26 | [52] |
| purification and separation of oligosaccharides in hydrolysates according to molecular masses using gel filtration chromatography | 4 g dm−3 monosaccharides and acetic acid | [53] | |
| Xylitol | Hydrolysate from the steam explosion pretreated Candida tropicalis CCTCC M2012462 | Maximal xylitol concentration of 35.6 g dm−3, productivity of 0.94 g l−1 h−1, xylose yield of 0.71 g g−1 | [54] |
| Furfural | 45 mg of SC-CaCt-700, 150 mg of corn straw and 7 mL of solvent mixed under magnetic stirring and heated in a preheated oil bath at 200 °C for 100 min | Yield of 93% in γ-valerolactone and 51.5% in water | [55] |
| D-allulose | Escherichia coli whole-cell catalyst-based microfluidic device to produce D-allulose from corn stalk hydrolysate. | Increase in D-allulose titer by 8.61 times to 8.78 g dm−3 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Zhang, J.; Guan, T. High-Value Utilization of Corn Straw: From Waste to Wealth. Sustainability 2023, 15, 14618. https://doi.org/10.3390/su151914618
Fu Y, Zhang J, Guan T. High-Value Utilization of Corn Straw: From Waste to Wealth. Sustainability. 2023; 15(19):14618. https://doi.org/10.3390/su151914618
Chicago/Turabian StyleFu, Yanli, Jie Zhang, and Tianzhu Guan. 2023. "High-Value Utilization of Corn Straw: From Waste to Wealth" Sustainability 15, no. 19: 14618. https://doi.org/10.3390/su151914618
APA StyleFu, Y., Zhang, J., & Guan, T. (2023). High-Value Utilization of Corn Straw: From Waste to Wealth. Sustainability, 15(19), 14618. https://doi.org/10.3390/su151914618








