Granulated Animal Feed and Fuel Based on Sea Buckthorn Agro-Waste Biomass for Sustainable Berry Production
Abstract
1. Introduction
2. Materials and Methods
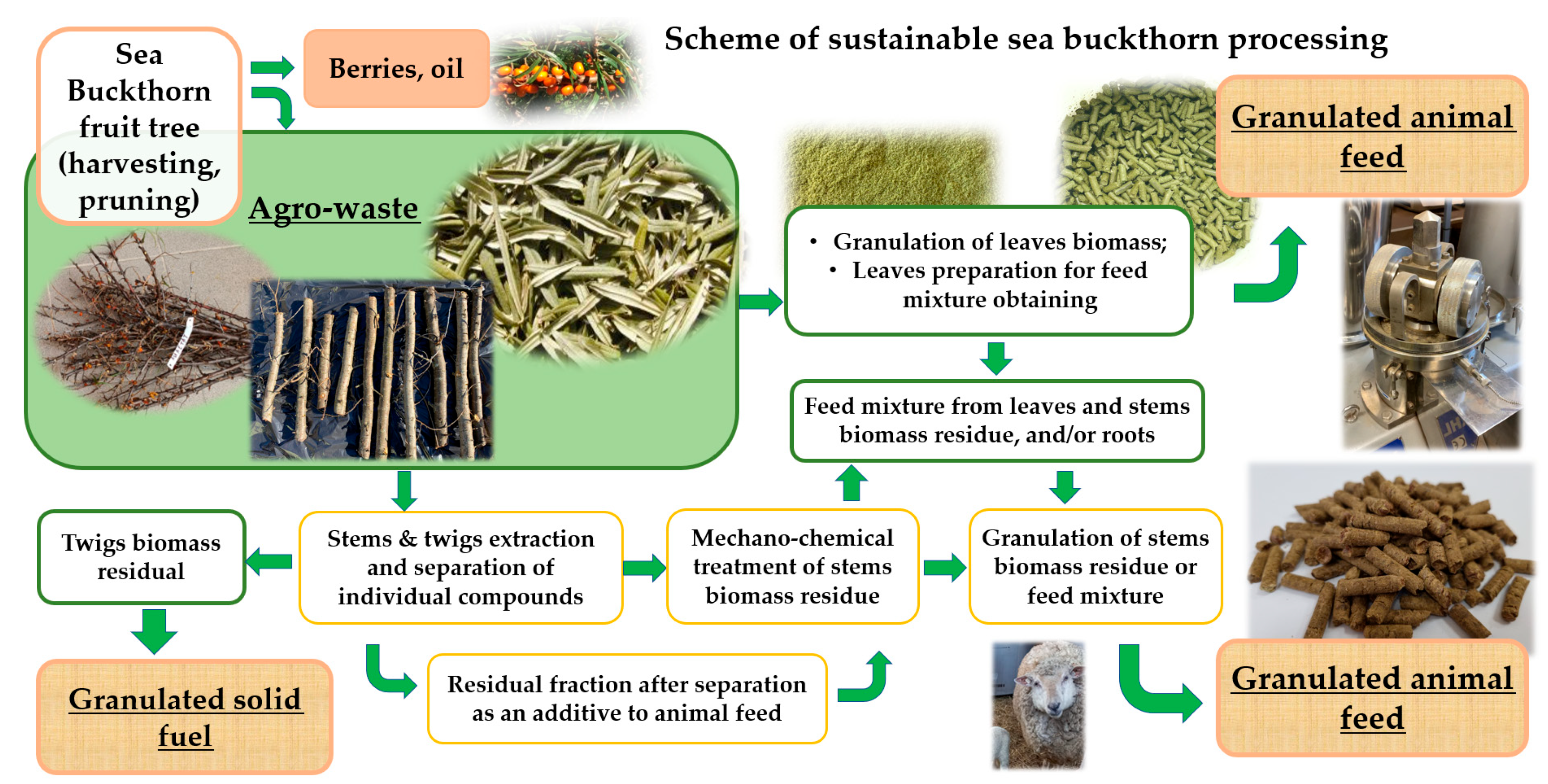
2.1. Collection of Agro-Waste Biomass
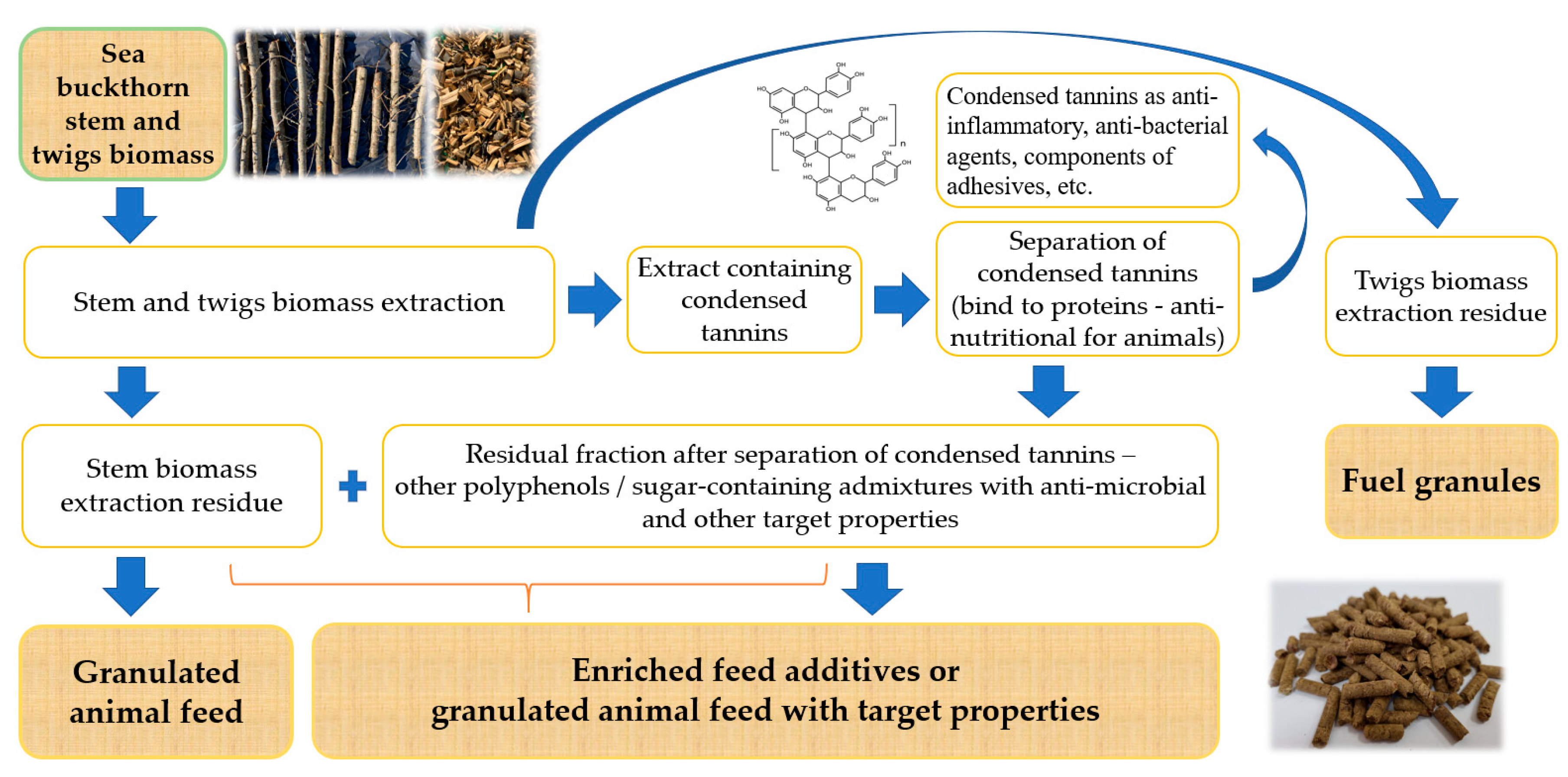
2.2. Isolation of the Condensed Tannins from the ST and TW Biomass
2.3. Mechanochemical Treatment of SBT Biomass
2.4. Initial and Treated Biomass Characterization
2.4.1. Crude Fiber Content Determination
2.4.2. Total Protein Content Determination
2.4.3. Determination of Crude Fat, Crude Ash, Macro-Elements, and Heavy Metal Content
2.4.4. Determination of The Total Amount of Carbohydrates
2.4.5. Determination of Vitamin Content
2.4.6. Analytical Pyrolysis
2.4.7. Elemental Analysis
2.5. Preparation of Animal Feed Compositions
2.6. Determination of Released Gas Emissions, In Vitro Analysis
2.7. Determination of Digestibility of the SBT Biomass Samples
2.8. Antimicrobial Activity of the SBT Fraction
2.9. SBT Biomass Granulation and Characterization of the Pellets
2.9.1. Biomass Granulation
2.9.2. Characterization of the Pellets
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of SBT Biomass
3.1.1. Organic Matter in the Biomass
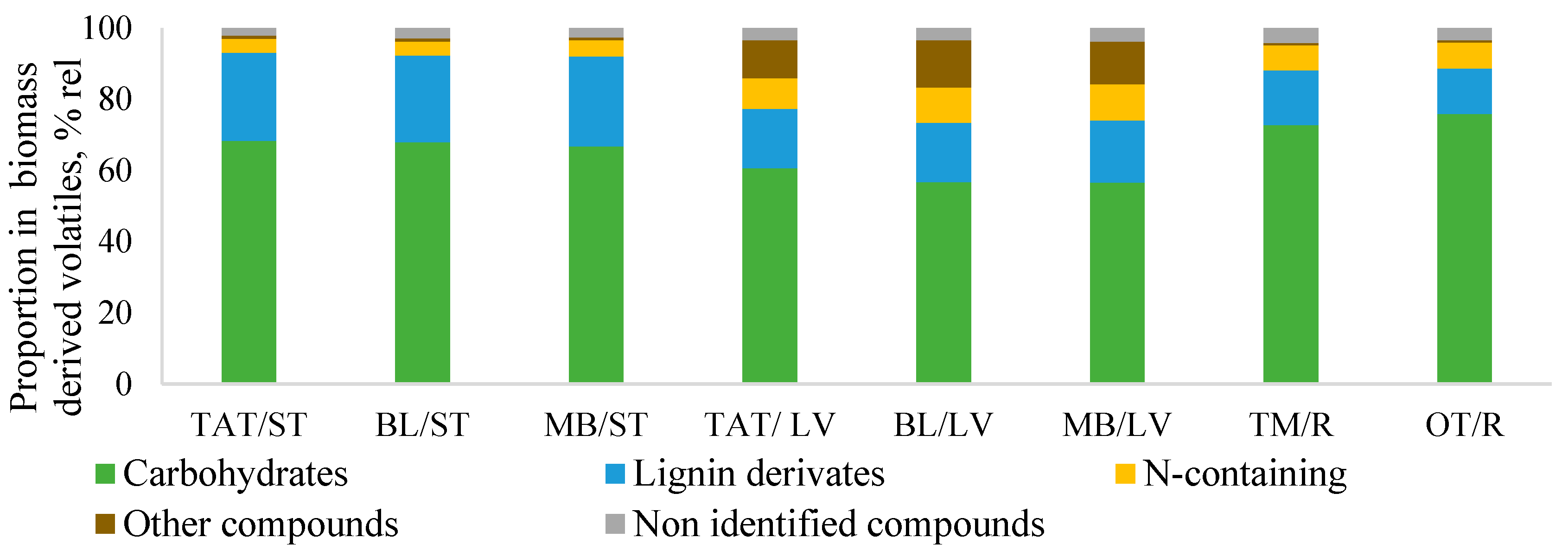
3.1.2. Relative Composition of SBT Biomass by Py-GC/MS/FID
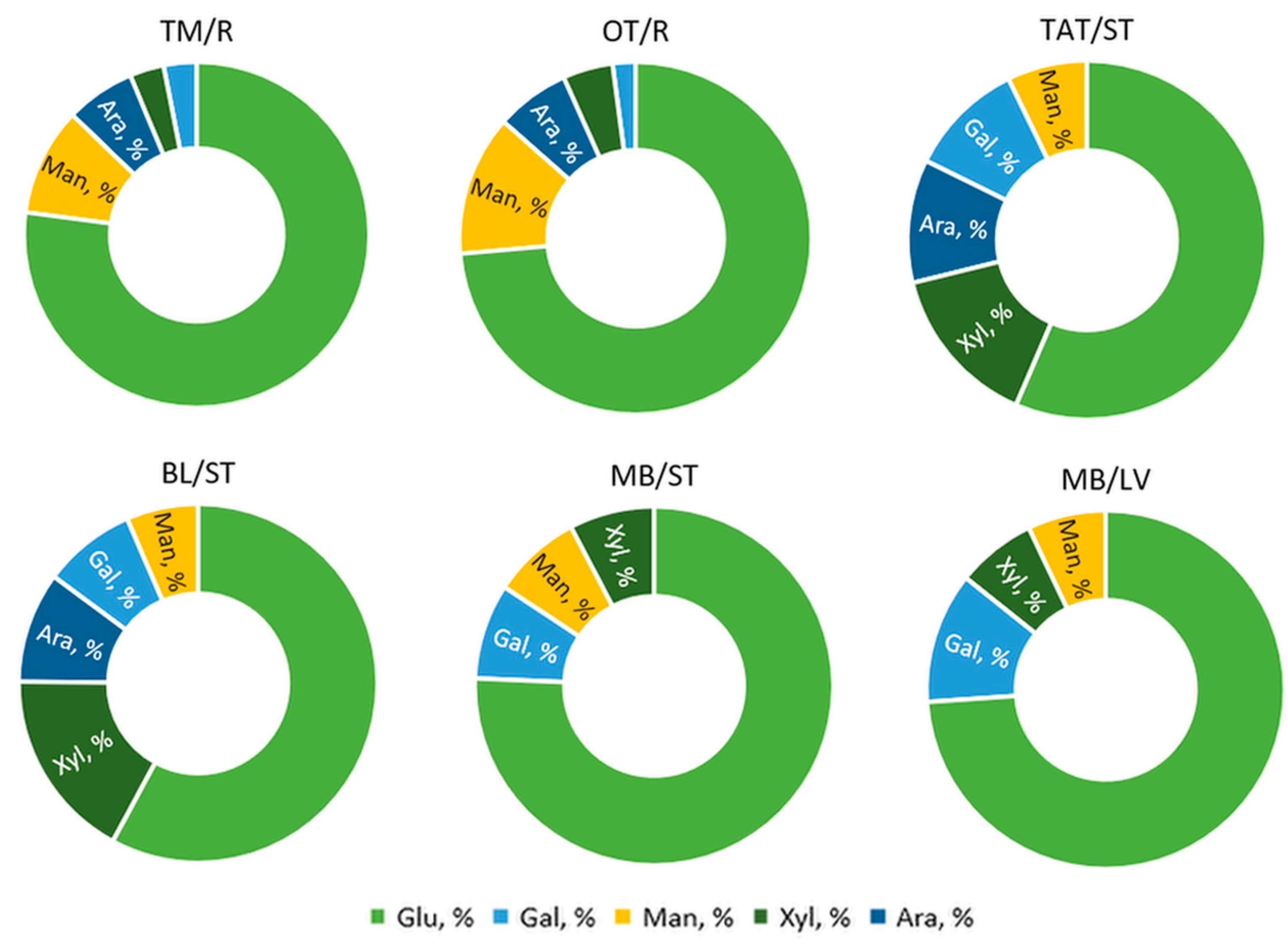
3.1.3. Carbohydrate Composition by GC-MS
3.1.4. Relative Composition of SBT Biomass Phenol/Lignin Part by Py-GC/MS/FID
3.2. CT Separation
3.3. Mechanochemical Pre-Treatment for the Improvement of Digestibility
3.4. The Anti-Microbial Properties of the Residual Fraction after CT Separation
3.5. Macro-Nutrients and Vitamins in SBT Biomass
3.6. Main Compounds in SBT Biomass and Their Role in Rumen Digestion
3.7. Determination of the in vitro Gas Production and Digestibility of SBT Biomass
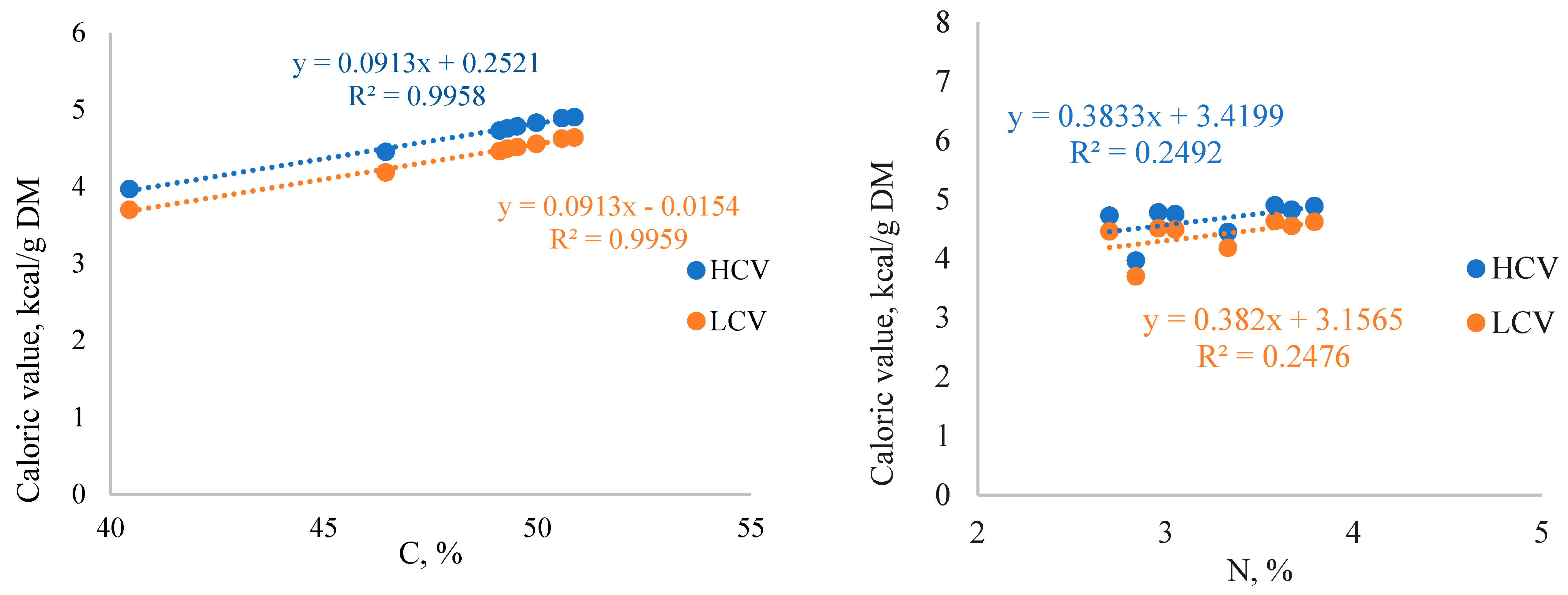
3.8. Caloric Value of SBT Biomass
3.9. Granulation of SBT Biomass Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranganathan, J.; Waite, R.; Searchinger, T. Craig Hanson How to Sustainably Feed 10 Billion People by 2050, in 21 Charts. Available online: https://www.wri.org/insights/how-sustainably-feed-10-billion-people-2050-21-charts (accessed on 15 April 2023).
- Mehmet Tuğrul, K. Soil Management in Sustainable Agriculture. In Sustainable Crop Production; Hasanuzzaman, M., Carvalho, M.T.F.M., Fujita, M., Assis Rodrigues Nogueira, T., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-317-9. [Google Scholar]
- Bonciu, E.; Olaru, A.L.; Rosculete, E.; Rosculete, C.A. Cytogenetic Study on the Biostimulation Potential of the Aqueous Fruit Extract of Hippophae Rhamnoides for a Sustainable Agricultural Ecosystem. Plants 2020, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Anderson, J.D.; Sawyer, J. Impact of the Ethanol Boom on Livestock and Dairy Industries: What Are They Going to Eat? J. Agric. Appl. Econ. 2008, 40, 573–579. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 978-92-5-105571-7. [Google Scholar]
- Veolia. Available online: https://www.veolia.com/en/solutions/bioconversion-sustainable-animal-feed (accessed on 15 April 2023).
- Lawrence, J.D.; Mintert, J.; Anderson, J.D.; Anderson, D.P. Feed Grains and Livestock: Impacts on Meat Supplies and Prices. Choices 2008, 23, 11–15. [Google Scholar]
- Ritchie, H. Vegan Diet Reduces Land Use by 75%. Vegan Sustain. Mag. 2022. [Google Scholar]
- Osborn, J.F. How Many Vegans Are in the World? Exploring the Global Population of Vegans. World Anim. Found. 2023. [Google Scholar]
- Quarta, S.; Massaro, M.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; Jorge, R.; Andrade, V.; Philippou, E.; Zisimou, C.; Maksimova, V.; et al. Persistent Moderate-to-Weak Mediterranean Diet Adherence and Low Scoring for Plant-Based Foods across Several Southern European Countries: Are We Overlooking the Mediterranean Diet Recommendations? Nutrients 2021, 13, 1432. [Google Scholar] [CrossRef]
- Chen, A.; Feng, X.; Dorjsuren, B.; Chimedtseren, C.; Damda, T.-A.; Zhang, C. Traditional Food, Modern Food and Nutritional Value of Sea Buckthorn (Hippophae rhamnoides L.): A Review. J. Future Foods 2023, 3, 191–205. [Google Scholar] [CrossRef]
- Gurčík, Ľ.; Porhajaš, V.; Červený, D.; Bajusová, Z. Economic Evaluation of Cultivation and Finalization of the Products from the Sea Buckthorn. Visegr. J. Bioecon. Sustain. Dev. 2019, 8, 27–30. [Google Scholar] [CrossRef]
- Kumar, M.; Devi, T.; Lata, S. Nutritional Potential of Seabuckthorn Plant for Livestock Feeding. Indian Farmer 2022, 9, 488–492. [Google Scholar]
- Singh, B. Indian Sea Buckthorn. In New Age Herbals; Singh, B., Peter, K.V., Eds.; Springer Singapore: Singapore, 2018; pp. 29–54. ISBN 978-981-10-8290-0. [Google Scholar]
- He, N.; Wang, Q.; Huang, H.; Chen, J.; Wu, G.; Zhu, M.; Shao, F.; Yan, Z.; Sang, Z.; Cao, L.; et al. A Comprehensive Review on Extraction, Structure, Detection, Bioactivity, and Metabolism of Flavonoids from Sea Buckthorn (Hippophae rhamnoides L.). J. Food Biochem. 2023, 2023, 4839124. [Google Scholar] [CrossRef]
- Gâtlan, A.-M.; Gutt, G. Sea Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef]
- Hu, J.; Guo, X. Evaluation of Nutrient Value of Seabuckthorn in North China. For. Stud. China 2006, 8, 50–52. [Google Scholar] [CrossRef]
- Rathore, J.P.; Nagar, P.K.; Kumar, S.; Meena, R. Seabuckthorn: A Powerhouse of Nutrition. Just Agric. 2021, 1–4. [Google Scholar]
- Janceva, S.; Andersone, A.; Lauberte, L.; Bikovens, O.; Nikolajeva, V.; Jashina, L.; Zaharova, N.; Telysheva, G.; Senkovs, M.; Rieksts, G.; et al. Sea Buckthorn (Hippophae rhamnoides L.) Waste Biomass after Harvesting as a Source of Valuable Biologically Active Compounds with Nutraceutical and Antibacterial Potential. Plants 2022, 11, 642. [Google Scholar] [CrossRef]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, L.; Panovská, Z.; et al. Why Is Sea Buckthorn (Hippophae rhamnoides L.) so Exceptional? A Review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Stobdan, T.; Korekar, G.; Srivastava, R.B. Nutritional Attributes and Health Application of Seabuckthorn (Hippophae rhamnoides L.)—A Review. CNF 2013, 9, 151–165. [Google Scholar] [CrossRef]
- Olas, B. Sea Buckthorn as a Source of Important Bioactive Compounds in Cardiovascular Diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Fu, L.; Peng, J.; Nan, Q.; He, D.; Yang, Y.; Cui, Y. Simulation of Vibration Harvesting Mechanism for Sea Buckthorn. Eng. Agric. Environ. Food 2016, 9, 101–108. [Google Scholar] [CrossRef]
- Thomas, S.C. Li Product Development of Sea Buckthorn. In Trends in New Crops and New Uses; Alexandria, V.A., Ed.; ASHS Press: Dallas, TX, USA, 2002; pp. 393–398. [Google Scholar]
- Fu, L.; Su, H.; Li, R.; Cui, Y. Harvesting Technologies for Sea Buckthorn Fruit. Eng. Agric. Environ. Food 2014, 7, 64–69. [Google Scholar] [CrossRef]
- Jamala, G.Y.; Tarimbuka, I.L.; Moris, D.; Mahai, S.; Adamawa, S. The Scope and Potentials of Fodder Trees and Shrubs in Agroforestry. IOSR J. Agric. Vet. Sci. (IOSR-JAVS) 2013, 5, 11–17. [Google Scholar]
- Franzel, S.; Kiptot, E.; Lukuyu, B. Agroforestry: Fodder Trees. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 235–243. ISBN 978-0-08-093139-5. [Google Scholar]
- Rajesh, R. Seabuckthorn (Hippophae salicifolia) Management for the Upliftment of Local Livelihood in Mustang District; Final Report for The Rufford Small Grants for Nature Conservation; Rufford Maurice Laing Foundation: London, UK, 2008. [Google Scholar]
- Burri, S.C.M.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant Capacity and Major Phenol Compounds of Horticultural Plant Materials Not Usually Used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, S. Sea Buckthorn Leaves and the Novel Food Evaluation. Proc. Latv. Acad. Sciences. Sect. B Nat. Exact Appl. Sci. 2017, 71, 111–114. [Google Scholar] [CrossRef]
- Bittová, M.; Krejzová, E.; Roblová, V.; Kubáň, P.; Kubáň, V. Monitoring of HPLC Profiles of Selected Polyphenolic Compounds in Sea Buckthorn (Hippophaë Rhamnoides L.) Plant Parts during Annual Growth Cycle and Estimation of Their Antioxidant Potential. Open Chem. 2014, 12, 1152–1161. [Google Scholar] [CrossRef]
- Singh, B.; Bhat, T.K. Singh Virendra Seabuckthorn Leaves as Cattle Feed-a Comparative Evaluation. Indian J. Anim. Nutr. 2005, 22, 16–20. [Google Scholar]
- Guliyev, V.; Gul, M.; Yildirim, A. Hippophae Rhamnoides L.: Chromatographic Methods to Determine Chemical Composition, Use in Traditional Medicine and Pharmacological Effects. J. Chromatogr. B 2004, 812, 291–307. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A. The Nutritional Value of Leaves of Selected Berry Species. Sci. Agric. (Piracicaba Braz.) 2017, 74, 405–410. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Nowicka, P. Triterpenoids, Phenolic Compounds, Macro- and Microelements in Anatomical Parts of Sea Buckthorn (Hippophaë rhamnoides L.) Berries, Branches and Leaves. J. Food Compos. Anal. 2021, 103, 104107. [Google Scholar] [CrossRef]
- Vilas-Franquesa, A.; Saldo, J.; Juan, B. Potential of Sea Buckthorn-Based Ingredients for the Food and Feed Industry—A Review. Food Prod. Process Nutr. 2020, 2, 17. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Mansbridge, S.; Whiting, I.; Abdulla, J.; Rose, S.; Mihova, T. Metabolisable Energy of Dried Sea Buckthorn (Hippophaes rhamnoides) Berries for Broiler Chickens. J. Cent. Eur. Agric. 2022, 23, 507–512. [Google Scholar] [CrossRef]
- Hao, X.; Diao, X.; Yu, S.; Ding, N.; Mu, C.; Zhao, J.; Zhang, J. Nutrient Digestibility, Rumen Microbial Protein Synthesis, and Growth Performance in Sheep Consuming Rations Containing Sea Buckthorn Pomace. J. Anim. Sci. 2018, 96, 3412–3419. [Google Scholar] [CrossRef]
- Product Seabuckthorn-Horses-Digestive-Supplement. Available online: https://nupafeed.co.uk/product/bsc-seabuckthorn-horses-digestive-supplement/ (accessed on 3 May 2023).
- Poultry, D.V.M. Sea Buckthorn Hippophae rhamnoides. Available online: https://www.Poultrydvm.Com/Supplement/Sea-Buckthorn (accessed on 15 March 2023).
- Mezzomo, R.; Paulino, P.V.R.; Detmann, E.; Valadares Filho, S.C.; Paulino, M.F.; Monnerat, J.P.I.S.; Duarte, M.S.; Silva, L.H.P.; Moura, L.S. Influence of Condensed Tannin on Intake, Digestibility, and Efficiency of Protein Utilization in Beef Steers Fed High Concentrate Diet. Livest. Sci. 2011, 141, 1–11. [Google Scholar] [CrossRef]
- Andersone, A.; Janceva, S.; Lauberte, L.; Ramata-Stunda, A.; Nikolajeva, V.; Zaharova, N.; Rieksts, G.; Telysheva, G. Anti-Inflammatory, Anti-Bacterial, and Anti-Fungal Activity of Oligomeric Proanthocyanidins and Extracts Obtained from Lignocellulosic Agricultural Waste. Molecules 2023, 28, 863. [Google Scholar] [CrossRef]
- Joslyn, M.A. Food Science and Technology. Methods in Food Analysis: Physical, Chemical, and Instrumental Methods of Analysis, 2nd ed.; Joslyn, M.A., Ed.; Academic Press: New York, NY, USA, 1970; ISBN 978-0-12-390156-9. [Google Scholar]
- Evers, J.M.; Hughes, C.G. ANALYSIS | Chemical Analysis. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2002; pp. 34–40. ISBN 978-0-12-227235-6. [Google Scholar]
- Naccarato, A.; Vommaro, M.L.; Amico, D.; Sprovieri, F.; Pirrone, N.; Tagarelli, A.; Giglio, A. Triazine Herbicide and NPK Fertilizer Exposure: Accumulation of Heavy Metals and Rare Earth Elements, Effects on Cuticle Melanization, and Immunocompetence in the Model Species Tenebrio Molitor. Toxics 2023, 11, 499. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A Simple and Rapid Preparation of Alditol Acetates for Monosaccharide Analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Ciulu, M.; Solinas, S.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Spano, N.; Sanna, G. RP-HPLC Determination of Water-Soluble Vitamins in Honey. Talanta 2011, 83, 924–929. [Google Scholar] [CrossRef]
- Konyalιoğlu, S.; Sağlam, H.; Kιvçak, B. α-Tocopherol, Flavonoid, and Phenol Contents and Antioxidant Activity of Ficus Carica Leaves. Pharm. Biol. 2005, 43, 683–686. [Google Scholar] [CrossRef]
- Fortina, R.; Glorio Patrucco, S.; Barbera, S.; Tassone, S. Rumen Fluid from Slaughtered Animals: A Standardized Procedure for Sampling, Storage and Use in Digestibility Trials. MPs 2022, 5, 59. [Google Scholar] [CrossRef]
- Beyihayo, G.; Omaria, R.; Namazzi, C.; Atuhaire, A. Comparison of in Vitro Digestibility Using Slaughtered and Fistulated Cattle as Sources of Inoculum. Uganda J. Agric. Sci. 2016, 16, 93. [Google Scholar] [CrossRef]
- Tassone, S.; Fortina, R.; Peiretti, P.G. In Vitro Techniques Using the DaisyII Incubator for the Assessment of Digestibility: A Review. Animals 2020, 10, 775. [Google Scholar] [CrossRef]
- Videv, E. Monitoring of Cumulative Greenhouse Gas Emissions from “in Vitro” Decomposition of Cereal Grains Forages. J. Mt. Agric. Balk. 2021, 24, 160–174. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A Simple Gas Production Method Using a Pressure Transducer to Determine the Fermentation Kinetics of Ruminant Feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Kiliç, Ü.; EriŞek, A.; GariPoğlu, A.; Ayan, İ.; Önder, H. The Effects of Different Forage Types on Feed Values and Digestibilities in Some Brassica Fodder Crops. Türk Tarım Ve Doğa Bilim. Derg. 2021, 8, 94–102. [Google Scholar] [CrossRef]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. (Eds.) Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-13234-6. [Google Scholar]
- SIST EN ISO 18122; Solid Biofuels—Determination of Ash Content. Available online: https://standards.iteh.ai/catalog/standards/sist/3bd743d6-8afe-4937-83e4-6525f0701e1a/sist-en-iso-18122-2023 (accessed on 7 May 2023).
- ISO 18125; Solid Biofuels—Determination of Calorific Value. Available online: https://standards.iteh.ai/catalog/standards/cen/ba50c859-a368-4cd5-9f04-7cdbdbe0a7cc/en-iso-18125-2017 (accessed on 7 May 2023).
- ISO 3310-1; Test Sieves—Technical Requirements and Testing—Part 1: Test Sieves of Metal Wire Cloth. Available online: https://standards.iteh.ai/catalog/standards/iso/93292afd-3d19-4959-ada2-663fedaf82f9/iso-3310-1-1982 (accessed on 7 May 2023).
- EN ISO 17831-1; Solid Biofuels—Determination of Mechanical Durability of Pellets and Briquettes—Part 1: Pellets (ISO 17831-1:2015). Available online: https://standards.iteh.ai/catalog/standards/cen/b393399d-4743-4c9a-aedc-d00a8c852344/en-iso-17831-1-2015 (accessed on 10 July 2023).
- EN ISO 17828; Solid Biofuels—Determination of Bulk Density. Available online: https://standards.iteh.ai/catalog/standards/cen/8741121a-9d32-440f-ba14-267a0cbd268f/en-iso-17828-2015 (accessed on 7 September 2023).
- Campos, F.P.; Nussio, L.G.; Sarmento, P.; Daniel, J.L.P.; Lima, C.G. Effects of Addition of Different Sources and Doses of Sugars on in Vitro Digestibilities of Dry Matter, Fibre and Cell Wall Monosaccharides of Corn Silage in Ruminants. Animal 2020, 14, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Rigon, F.; Pereira, D.A.B.; Loregian, K.E.; Magnani, E.; Marcondes, M.I.; Branco, R.H.; Benedeti, P.D.B.; Paula, E.M. Use of Heating Methods and Xylose to Increase Rumen Undegradable Protein of Alternative Protein Sources: 1) Peanut Meal. Animals 2022, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Muck, R.E.; Weimer, P.J. Quantitative Analysis of Cellulose Degradation and Growth of Cellulolytic Bacteria in the Rumen: Bacterial QS and Rhizosphere N Cycling. FEMS Microbiol. Ecol. 2009, 67, 183–197. [Google Scholar] [CrossRef]
- Weimer, P.J. Why Don’t Ruminal Bacteria Digest Cellulose Faster? J. Dairy Sci. 1996, 79, 1496–1502. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Martens, S.D.; Wildner, V.; Zeyner, A.; Steinhöfel, O. In Vitro Ruminal Degradability of Wheat Straw Cultivated with White-Rot Fungi Adapted to Mushroom Farming Conditions. Sci. Rep. 2023, 13, 7794. [Google Scholar] [CrossRef]
- Janceva, S.; Andersone, A.; Spulle, U.; Tupciauskas, R.; Papadopoulou, E.; Bikovens, O.; Andzs, M.; Zaharova, N.; Rieksts, G.; Telysheva, G. Eco-Friendly Adhesives Based on the Oligomeric Condensed Tannins-Rich Extract from Alder Bark for Particleboard and Plywood Production. Materials 2022, 15, 3894. [Google Scholar] [CrossRef]
- European Comission. A European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 5 May 2023).
- Bekker, N.P.; Glushenkova, A.I. Components of Certain Species of the Elagnaceae Family. Chem. Nat. Comp. 2001, 37, 97–116. [Google Scholar] [CrossRef]
- Principles of Nutrition. Fats (Lipids). Available online: https://www.dairynz.co.nz/feed/nutrition/principles-of-nutrition/fats/ (accessed on 15 May 2023).
- Garry, F.B. Rumen Indigestion and Putrefaction. In Food Animal Practice; Elsevier: Amsterdam, The Netherlands, 2009; pp. 20–23. ISBN 978-1-4160-3591-6. [Google Scholar]
- Coles, L.T.; Moughan, P.J.; Darragh, A.J. In Vitro Digestion and Fermentation Methods, Including Gas Production Techniques, as Applied to Nutritive Evaluation of Foods in the Hindgut of Humans and Other Simple-Stomached Animals. Anim. Feed Sci. Technol. 2005, 123, 421–444. [Google Scholar] [CrossRef]
- Örün, M.; Erdoğan, S. Determination of In Vitro True Digestibility and Relative Feed Values of Alternative Roughage Sources. Yüzüncü Yıl Üniversitesi Tarım Bilim. Derg. 2022, 32, 576–583. [Google Scholar] [CrossRef]
- Salman, M.; Cetinkaya, N.; Selcuk, Z.; Genc, B.; Acici, M. Effects of Various Inulin Levels on in Vitro Digestibility of Corn Silage, Perennial Ryegrass (Lolium perenne L.) and Common Vetch (Vicia Sativa L.)/Oat (Avena sativa L.) Hay. SA J. An. Sci. 2017, 47, 723. [Google Scholar] [CrossRef]
- Gnaiger, E.; Bitterlich, G. Proximate Biochemical Composition and Caloric Content Calculated from Elemental CHN Analysis: A Stoichiometric Concept. Oecologia 1984, 62, 289–298. [Google Scholar] [CrossRef]
- EN ISO 17225-1:2021; Solid Biofuels—Fuel Specifications and Classes. Available online: https://standards.iteh.ai/e-library/reader/8e670b0a-1de2-11ee-9863-6fe73a1f8994 (accessed on 10 July 2023).



| Macronutrients and Heavy Metals * | MB/LV | MB/ST | BL/LV | BL/ST | TAT/LV | TAT/ST |
|---|---|---|---|---|---|---|
| P, mg/100 g DM | 225 ± 22 | 220 ± 22 | 210 ± 21 | 199 ± 20 | 212 ± 18 | 217 ± 26 |
| K, mg/100 g DM | 1376 ± 113 | 1109 ± 107 | 1209 ± 104 | 1037 ± 56 | 1216 ± 114 | 1119 ± 108 |
| Na, mg/100 g DM | 1.72 ± 0.40 | 22.5 ± 5.2 | 2.25 ± 0.52 | 7.83 ± 1.80 | 1.88 ± 0.36 | 11.4 ± 3.5 |
| Ca, mg/100 g DM | 989 ± 237 | 281 ± 67 | 856 ± 205 | 332 ± 80 | 917 ± 162 | 306 ± 46 |
| Cd **, mg/kg DM | 0.011 ± 0.003 | 0.027 ± 0.006 | 0.011 ± 0.003 | 0.011 ± 0.002 | 0.014 ± 0.002 | 0.018 ± 0.004 |
| Hg **, mg/kg DM | 0.0069 ± 0.0012 | 0.0031 ± 0.0006 | 0.0067 ± 0.0012 | 0.0022 ± 0.0004 | 0.0058 ± 0.0004 | 0.0028 ± 0.0005 |
| Pb **, mg/kg DM | 0.086 ± 0.0022 | 0.086 ± 0.0022 | 0.10 ± 0.030 | 0.037 ± 0.010 | 0.0042 ± 0.0021 | 0.073 ± 0.0016 |
| Samples * | Vitamin C, mg/100 g DM‘ | Vitamin E (α-tocopherol), mg/100 g DM | Vitamin A (Retinol), mg/100 g DM |
|---|---|---|---|
| MB/LV | 15.6 ± 4.4 | 30.9 ± 4.3 | 1.29 ± 0.02 |
| MB/ST | 178.0 ± 50.0 | 17.3 ± 2.4 | n.f. |
| BL/LV | 12.0 ± 3.0 | 44.3 ± 6.2 | 1.14 ± 0.03 |
| BL/ST | 9.2 ± 3.6 | 14.7 ± 2.1 | n.f |
| TAT/LV | 13.3 ± 3.6 | 42.6 ± 2.2 | 0.86 ± 0.07 |
| TAT/ST | 8.0 ± 3.0 | 16.2 ± 2.6 | n.f. |
| TM/R | n.d. | n.d. | n.f. |
| OT/R | n.d. | n.d. | n.f. |
| Sample * | GP24, mL/g DM | GP48, mL/g DM | IVTD, %/DM |
|---|---|---|---|
| MB/LV | 59.97 ± 1.94 | 71.76 ± 1.61 | 82.60 ± 4.80 |
| MB/ST/MT | 72.38 ± 3.46 | 83.18 ± 2.21 | 39.12 ± 6.06 |
| MB/ST | 53.99 ± 8.19 | 65.33 ± 5.56 | 18.11 ± 4.61 |
| MB/LV: MB/ST/MT (w/w; 1:1) | 76.29 ± 5.73 | 84.65 ± 7.18 | 58.11 ± 5.05 |
| MB/LV: MB/ST (w/w; 1:1) | 76.73 ± 5.51 | 81.93 ± 8.97 | 49.83 ± 5.16 |
| MB residual fraction after CT separation | 134.58 ± 3.94 | 141.51 ± 4.10 | 98.69 ± 4.44 |
| SBT Biomass | C | H | N | Organic Matter | Calorific Value, MJ/kg DM | Caloric Value, kcal/g DM | ||
|---|---|---|---|---|---|---|---|---|
| HCV | LCV | HCV | LCV | |||||
| %/DM; CI ≤ 0.2% at α = 0.05 | CI ≤ 0.03% at α = 0.05 | |||||||
| MB/ST | 50.6 | 5.6 | 3.8 | 97.2 | 20.47 | 19.34 | 4.89 | 4.62 |
| BL/ST | 49.9 | 5.6 | 3.7 | 98.1 | 20.21 | 19.07 | 4.83 | 4.55 |
| TAT/ST | 50.9 | 5.5 | 3.6 | 97.6 | 20.52 | 19.41 | 4.90 | 4.64 |
| MB/LV | 49.1 | 5.9 | 2.7 | 94.8 | 19.79 | 18.67 | 4.73 | 4.46 |
| BL/LV | 49.3 | 5.8 | 3.1 | 96.2 | 19.90 | 18.80 | 4.75 | 4.49 |
| TAT/LV | 49.5 | 5.9 | 3.0 | 94.8 | 20.02 | 18.89 | 4.78 | 4.51 |
| TM/R | 46.5 | 4.8 | 3.3 | 95.5 | 18.63 | 17.51 | 4.45 | 4.18 |
| OT/R | 40.5 | 5.0 | 2.8 | 96.0 | 16.60 | 15.48 | 3.96 | 3.70 |
| Samples | Pellets Durability, % | Pellets Moisture, % | Bulk Density, kg/m3 | Average Length, mm |
|---|---|---|---|---|
| MB/ST/MT | 96.9 | 5.8 | 714.8 | 12 |
| MB/LV | 97.7 | 5.4 | 715.7 | 12 |
| MB/LV: MB/ST/MT (1:1, w/w) | 97.2 | 5.5 | 714.2 | 12 |
| MB/LV: MB/ST/MT (1:1, w/w) + 5% TM/R | 98.1 | 5.6 | 714.8 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersone, A.; Janceva, S.; Lauberte, L.; Zaharova, N.; Chervenkov, M.; Jurkjane, V.; Jashina, L.; Rieksts, G.; Telysheva, G. Granulated Animal Feed and Fuel Based on Sea Buckthorn Agro-Waste Biomass for Sustainable Berry Production. Sustainability 2023, 15, 11152. https://doi.org/10.3390/su151411152
Andersone A, Janceva S, Lauberte L, Zaharova N, Chervenkov M, Jurkjane V, Jashina L, Rieksts G, Telysheva G. Granulated Animal Feed and Fuel Based on Sea Buckthorn Agro-Waste Biomass for Sustainable Berry Production. Sustainability. 2023; 15(14):11152. https://doi.org/10.3390/su151411152
Chicago/Turabian StyleAndersone, Anna, Sarmite Janceva, Liga Lauberte, Natalija Zaharova, Mihail Chervenkov, Vilhelmine Jurkjane, Lilija Jashina, Gints Rieksts, and Galina Telysheva. 2023. "Granulated Animal Feed and Fuel Based on Sea Buckthorn Agro-Waste Biomass for Sustainable Berry Production" Sustainability 15, no. 14: 11152. https://doi.org/10.3390/su151411152
APA StyleAndersone, A., Janceva, S., Lauberte, L., Zaharova, N., Chervenkov, M., Jurkjane, V., Jashina, L., Rieksts, G., & Telysheva, G. (2023). Granulated Animal Feed and Fuel Based on Sea Buckthorn Agro-Waste Biomass for Sustainable Berry Production. Sustainability, 15(14), 11152. https://doi.org/10.3390/su151411152







