Application of All-Ages Lead Model Based on Monte Carlo Simulation of Preschool Children’s Exposure to Lead in Guangdong Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of the ALLM + MC
2.1.1. Improvement of the ALLM
2.1.2. Sensitivity Analysis of Pharmacokinetic Parameters
2.1.3. Construction of the ALLM + MC
2.2. Localization and Application of the Improved Model
2.2.1. Localization of Model Exposure Parameters
2.2.2. Application of the Model
2.3. Statistical Analysis
3. Results
3.1. Construction of ALLM + MC
3.1.1. Results of the Sensitivity Analysis
3.1.2. Construction of ALLM + MC
3.2. Localization and Application of the Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, X.; Wu, S.; Yan, C. Impacts of low-level lead exposure on development of children: Recent studies in China. Clin. Chim. Acta 2001, 313, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, L.S.; Cabañas, E.; Cadet, P.; Zhu, W.; Markowitz, M.E. Cereal and Juice, Lead and Arsenic, Our Chil-dren at Risk: A Call for the FDA to Re-Evaluate the Allowable Limits of Lead and Ar-senic That Children May Ingest. Int. J. Environ. Res. Public Health 2022, 19, 5788. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.; Dietrich, K.N. Low-level lead exposure and cognitive function in children. Pediatr. Ann. 1994, 23, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Shen, X.; Zhang, Y.; Wu, S.; He, J.; Zhou, J.; Zhang, Y.; Ao, L.; Wu, S.; Guo, D. A study on relationship between blood lead level and physical growth and development of babies and young children in Shanghai. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.] 1999, 33, 269–271. [Google Scholar]
- Needleman, H.L.; Gatsonis, C.A. Low-level lead exposure and the IQ of children: A meta-analysis of modern studies. Jama 1990, 263, 673–678. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect 2005, 113, 894–899. [Google Scholar] [CrossRef]
- Abadin, H.; Ashizawa, A.; Stevens, Y.W.; Llados, F.; Diamond, G.; Sage, G.; Citra, M.; Quinones, A.; Bosch, S.J.; Swarts, S.G. Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2007; Volume 4.
- Brondum, J. Attention deficit hyperactivity disorder and blood lead levels in Chinese children. Environ. Health Perspect. 2009, 117, 286. [Google Scholar] [CrossRef]
- Chiodo, L.M.; Covington, C.; Sokol, R.J.; Hannigan, J.H.; Jannise, J.; Ager, J.; Greenwald, M.; Delaney-Black, V. Blood lead levels and specific attention effects in young children. Neurotoxicology Teratol. 2007, 29, 538–546. [Google Scholar] [CrossRef]
- Counter, S.A.; Buchanan, L.H.; Ortega, F. Zinc protoporphyrin levels, blood lead levels and neurocognitive deficits in Andean children with chronic lead exposure. Clin. Biochem. 2008, 41, 41–47. [Google Scholar] [CrossRef]
- Nevin, R. How lead exposure relates to temporal changes in IQ, violent crime, and unwed pregnancy. Environ. Res. 2000, 83, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, C.; Berghmans, P.; Van Sprundel, M.; Van der Auwera, J.-C. Use of the IEUBK model for determination of exposure routes in view of siteremediation. Hum Ecol. Risk Assess 2006, 12, 963–982. [Google Scholar] [CrossRef]
- Khoury, G.A.; Diamond, G.L. Risks to children from exposure to lead in air during remedial or removal activities at superfund sites: A case study of the rsr lead smelter superfund site. J. Expo. Anal. Env. Epidemiol. 2003, 13, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Pierzynski, G.; Erickson, L.; Hester, J.S.R.E.; Harrison, R.M. Remediation Oflead, Zinc-and Cadmium-Contaminated Soils; The Royal Society of Chemistry: London, UK, 1997; pp. 91–102. [Google Scholar]
- Jones, H.M.; Yeo, K.R. Basic Concepts in Physiologically Based Pharmacokinetic Modeling in Drug Discovery and Development. CPT: Pharmacomet. Syst. Pharmacol. 2013, 2, 1–12. [Google Scholar] [CrossRef]
- Lin, Z.; Chou, W.C. Machine learning and artificial intelligence in toxicological sci-ences. Toxicol. Sci. 2022, 189, 7–19. [Google Scholar] [CrossRef]
- Cohen Hubal, E.A.; Wetmore, B.A.; Wambaugh, J.F.; El-Masri, H.; Sobus, J.R.; Bahadori, T. Advancing internal exposure and physiologically-based toxicokinetic modeling for 21st-century risk assess-ments. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 11–20. [Google Scholar] [CrossRef]
- US EPA. Technical Support Document for the All Ages Lead Model (AALM)—Parameters, Equations, and Evaluations. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1012GIX.txt (accessed on 1 January 2023).
- White, P.D.; Van Leeuwen, P.; Davis, B.D.; Maddaloni, M.; Hogan, K.A.; Marcus, A.H.; Elias, R.W. The conceptual structure of the integrated exposure uptake biokinetic model for lead in children. Environ. Health Perspect. 1998, 106 (Suppl. S6), 1513–1530. [Google Scholar] [CrossRef]
- Zaragoza, L.; Hogan, K. The integrated exposure uptake biokinetic model for lead in children: Independent validation and verification. Environ. Health Perspec-Tives 1998, 106 (Suppl. S6), 1551–1556. [Google Scholar] [CrossRef]
- Yinan, Y. Health Risk Assessment and Influencing Factors of Children’s Environmental Lead Exposure in a Mining Area; Lanzhou University: Lanzhou, China, 2021; (In Chinese). [Google Scholar] [CrossRef]
- Wang, X.; Miller, G.; Ding, G.; Lou, X.; Cai, D.; Chen, Z.; Meng, J.; Tang, J.; Chu, C.; Mo, Z.; et al. Health risk assessment of lead for children in tinfoil manufacturing and e-waste recycling areas of Zhejiang Province, China. Sci. Total Environ. 2012, 426, 106–112. [Google Scholar] [CrossRef]
- Cao, S.; Duan, X.; Zhao, X.; Wang, B.; Ma, J.; Fan, D.; Sun, C.; He, B.; Wei, F.; Jiang, G. Health risk assessment of various metal (loid) s via multiple exposure pathways on children living near a typical lead-acid battery plant, China. Environ. Pollut. 2015, 200, 16–23. [Google Scholar] [CrossRef]
- Cao, S.; Duan, X.; Zhao, X.; Ma, J.; Dong, T.; Huang, N.; Sun, C.; He, B.; Wei, F. Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China. Sci. Total Environ. 2014, 472, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Giubilato, E.; Critto, A.; Wang, L.; Marcomini, A.; Zhang, J. Probabilistic modeling of aggregate lead exposure in children of urban China using an adapted IEUBK model. Sci. Total Environ. 2017, 584–585, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Sonawane, B.; Barton, H.A.; DeWoskin, R.S.; Lipscomb, J.C.; Schlosser, P.; Chiu, W.A.; Krishnan, K. Approaches for Applications of Physiologically Based Pharmacokinetic Models in Risk Assessment. J. Toxicol. Environ. Heal. Part B 2008, 11, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Michael, F.; Timothy, B. Guiding Principles for Monte Carlo Analysis. Environ. Prot. Agency 1997, 630, 4–19. [Google Scholar]
- Fakhri, Y.; Khaneghah, A.M.; Conti, G.O.; Ferrante, M.; Khezri, A.; Darvishi, A.; Ahmadi, M.; Hasanzadeh, V.; Rahimizadeh, A.; Keramati, H.; et al. Probabilistic risk assessment (Monte Carlo simulation method) of Pb and Cd in the onion bulb (Allium cepa) and soil of Iran. Environ. Sci. Pollut. Res. 2018, 25, 30894–30906. [Google Scholar] [CrossRef]
- Beck, B.D.; Mattuck, R.L.; Bowers, T.S.; Cohen, J.T.; O’Flaherty, E. The development of a stochastic physiologically-based pharmacokinetic model for lead. Sci. Total Environ. 2001, 274, 15–19. [Google Scholar] [CrossRef]
- Liu, A.; Li, T.; Zhang, S.; Tan, Z.; Dai, Y. A survey on blood lead level and influencing factors of children in 18 cities of China. China Matern. Child Health Res. 2018, 29, 539–542. (In Chinese) [Google Scholar]
- Yaodong, X. Analysis of Blood Lead Level and Influencing Factors of Urban Children in Guangdong Province. Master’s Thesis, Guangzhou Medical University, Guangzhou, China, 2008. (In Chinese). [Google Scholar]
- Keling, Y.; Hongzhi, Z.; Zhigang, Z.; Peisheng, Y. Localization of environmental health risk assessment model for lead exposure. China Popul. Resour. Environ. 2016, 26, 163–169. (In Chinese) [Google Scholar]
- Lin, G.; Yan, C.; Li, K.; Liu, X.; Du, L. Investigation on blood lead level of children in Guangzhou in 2006 and 2009. J. Environ. Health 2011, 28, 821–822. (In Chinese) [Google Scholar]
- Xu, Q.; Wen, D.; Zhang, X.; Xu, J.; Li, M.; Suo, M.; Xiao, J.; Kan, L.; Pang, J. Investigation on blood lead level of children in Zhongshan, Guangdong. Int. J. Lab. Med. 2014, 35, 430–431. (In Chinese) [Google Scholar]
- Zheng, L.; Wu, K.; Li, Y.; Qi, Z.; Han, D.; Zhang, B.; Gu, C.; Chen, G.; Liu, J.; Chen, S.; et al. Blood lead and cadmium levels and relevant factors among children from an e-waste recycling town in China. Environ. Res. 2008, 108, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Fangfang, M.; Xiuhong, Y.; Weijia, W.; Zhiming, B. Development and application of lead pharmacokinetics model. Ind. Hyg. Occup. Dis. 2015, 41, 408–411. (In Chinese) [Google Scholar]
- Bo, W.; Dichu, S.; Hua, X.Z.; Hong, Y.; Weimin, N.; Shuilian, Y.; Cui, W.; Pingjian, L.; Hua, F. Research on the impact of environmental lead on infant blood lead based on IEUBK model. Health Res. 2011, 40, 478–480. (In Chinese) [Google Scholar]
- Jia, H.; Jianwei, C.; Yikai, Z. Application overview of IEUBK model and preliminary discussion on its localization. J. Environ. Health 2013, 30, 655–658. (In Chinese) [Google Scholar]
- US EPA. Technical Support Document: Parameters and Equations Used in the Integrated Exposure Uptake Biokinetic Model for Lead in Children (v. 0.99 d). Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100FKYJ.txt (accessed on 19 November 2022).
- Li, W.B.; Klein, W.; Blanchardon, E.; Puncher, M.; Leggett, R.W.; Oeh, U.; Breustedt, B.; Nosske, D.; Lopez, M.A. Parameter uncertainty analysis of a biokinetic model of caesium. Radiat. Prot. Dosim. 2014, 163, 37–57. [Google Scholar] [CrossRef]
- Yi, C.; Yan, X.; Dan, H. Sensitivity Analysis Summary. J. Beijing Norm. Univ. Nat. Sci. Ed. 2008, 44, 9–16. (In Chinese) [Google Scholar]
- Christopher Frey, H.; Patil, S.R. Identification and review of sensitivity analysis methods. Risk Anal. 2002, 22, 553–578. [Google Scholar] [CrossRef]
- Clewell, R.A.; Merrill, E.A.; Yu, K.O.; Mahle, D.A.; Sterner, T.R.; Mattie, D.R.; Robinson, P.J.; Fisher, J.W.; Gearhart, J.M. Predicting Fetal Perchlorate Dose and Inhibition of Iodide Kinetics during Gestation: A Physiologically-Based Pharmacokinetic Analysis of Perchlorate and Iodide Kinetics in the Rat. Toxicol. Sci. 2003, 73, 235–255. [Google Scholar] [CrossRef]
- Ikeda, M.; Watanabe, T.; Koizumi, A.; Fujita, H.; Nakatsuka, H.; Kasahara, M. Dietary Intake of Lead among Japanese Farmers. Arch. Environ. Heal. Int. J. 1989, 44, 23–29. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Moon, C.S.; Watanabe, T.; Shimbo, S.; He, F.S.; Wu, Y.Q.; Zhou, S.F.; Su, D.M.; Qu, J.B.; Ikeda, M. Background Exposure of Urban Populations to Lead and Cadmium: Comparison between China and Japan. Int. Arch. Occup. Environ. Health 1997, 69, 273–281. [Google Scholar]
- Hsu, S.-C.; Liu, S.C.; Jeng, W.-L.; Chou, C.C.K.; Hsu, R.-T.; Huang, Y.-T.; Chen, Y.-W. Lead isotope ratios in ambient aerosols from Taipei, Taiwan: Identifying long-range transport of airborne Pb from the Yangtze Delta. Atmospheric Environ. 2006, 40, 5393–5404. [Google Scholar] [CrossRef]
- Xia, X.; Chen, X.; Liu, R.; Liu, H. Heavy metals in urban soils with various types of land use in Beijing, China. J. Hazard. Mater. 2011, 186, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Hu, J. Development of lead source-specific exposure standards based on aggregate exposure assessment: Bayesian inversion from biomonitoring information to multipathway exposure. Environ. Sci. Technol. 2012, 46, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- US FDA. FDA Total Diet Study (TDS) FY2018-FY2020 Report Supplement: Summary of Analytical Results. Available online: https://www.fda.gov/media/159751/download (accessed on 19 November 2022).
- Moya, J.; Phillips, L.; Schuda, L.; Wood, P.; Diaz, A.; Lee, R.; Clickner, R.; Birch, R.; Adjei, N.; Blood, P.; et al. Exposure Factors Handbook, 2011 ed.; US Environmental Protection Agency: Washington, DC, USA, 2011.
- Xiaoli, D.; Nan, H.; Beibei, W.; Xiuge, Z.; Jing, N.; Yan, Q.; Xianliang, W.; Jinliang, Z. Comparison of exposure parameters in environmental health risk assessment at home and abroad. J. Environ. Health 2012, 29, 99–104. (In Chinese) [Google Scholar]
- Cao, S.; Duan, X.; Zhao, X.; Wang, B.; Ma, J.; Fan, D.; Sun, C.; He, B.; Wei, F.; Jiang, G. Isotopic ratio based source apportionment of children’s blood lead around coking plant area. Environ. Int. 2014, 73, 158–166. [Google Scholar] [CrossRef]
- Chen, J.; Tong, Y.; Xu, J.; Liu, X.; Li, Y.; Tan, M.; Li, Y. Environmental lead pollution threatens the children living in the Pearl River Delta region, China. Environ. Sci. Pollut. Res. 2012, 19, 3268–3275. [Google Scholar] [CrossRef]
- Lei, Z.; Junquan, G.; Xiaowei, L. 2000 China’s total diet study—dietary lead intake of people of different gender and age groups. Health Res. 2007, 4, 459–467. [Google Scholar]
- Wang, B.; Xiaoli, D.; Xiuge, Z.; Liyun, Z.; Hongguang, C. Exposure Factors Handbook of Chinese Population (Children Volumn); China Environmental Press: Beijing, China, 2016. [Google Scholar]
- Nakanishi, J.; Shigeki, M.; Matsuda, H. Risk Calculation in the Simulated Environment; Iwanami Shoten: Tokyo, Japan, 2003. (In Japanese) [Google Scholar]
- Kobayashi, N.; Naya, M.; Nakanishi, J. Risk Assessment for Lead; Maruzen Bookstores Press: Tokyo, Japan, 2006. (In Japanese) [Google Scholar]
- Schlenker, T.L.; Fritz, C.J.; Mark, D.; Layde, M.; Linke, G.; Murphy, A.; Matte, T. Screening for pediatric lead poisoning: Comparability of simultaneously drawn capillary and venous blood samples. Jama 1994, 271, 1346–1348. [Google Scholar] [CrossRef]
- Huang, J.; Liu, S.; Ma, H. Analysis of the current status of atmospheric heavy metals in the Pearl River Delta region. Liaoning Urban Rural. Environ. Sci. Technol. 2015, 35, 64–66. (In Chinese) [Google Scholar]
- Li, H.-B.; Cui, X.-Y.; Li, K.; Li, J.; Juhasz, A.L.; Ma, L.Q. Assessment of in Vitro Lead Bioaccessibility in House Dust and Its Relationship to in Vivo Lead Relative Bioavailability. Environ. Sci. Technol. 2014, 48, 8548–8555. [Google Scholar] [CrossRef]
- Smith, P.; Smith, J.; Powlson, D.; McGill, W.; Arah, J.; Chertov, O.; Coleman, K.; Franko, U.; Frolking, S.; Jenkinson, D.; et al. A comparison of the performance of nine soil organic matter models using datasets from seven long-term experiments. Geoderma 1997, 81, 153–225. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.; Zhang, W.; Sun, W.; Liu, S.; Jiang, J.; Wu, J.; Yu, W.; Wang, Y.; Yang, Z. Agro-C: A biogeophysical model for simulating the carbon budget of agroecosystems. Agric. For. Meteorol. 2009, 149, 106–129. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Wu, W.; Liu, S.; Li, M.; Yao, N.; Chen, J.; Ye, L.; Wang, Q.; Zhou, Y. Application of IEUBK model in lead risk assessment of children aged 61–84 months old in central China. Sci. Total Environ. 2016, 541, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Morin, R.L. Monte Carlo Simulation: A Ubiquitous Tool. J. Am. Coll. Radiol. 2017, 14, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Shuo, W. Study on Blood Lead Level and Its Change Trend of Chinese Children Based on Monte Carlo Simulation; Qingdao University: Qingdao, China, 2021. [Google Scholar] [CrossRef]
- Hogan, K.; Marcus, A.; Smith, R.; White, P. Integrated exposure uptake biokinetic model for lead in children: Empirical comparisons with epidemiologic data. Environ. Heal. Perspect. 1998, 106, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Bowers, T.S.; Mattuck, R.L. Further comparisons of empirical and epidemiological data with predictions of the integrated exposure uptake biokinetic model for lead in children. Hum. Ecol. Risk Assess. 2001, 7, 1699–1713. [Google Scholar] [CrossRef]
- Jarosińska, D.; Biesiada, M.; Muszyńska-Graca, M. Environmental burden of disease due to lead in urban children from Silesia, Poland. Sci. Total Environ. 2006, 367, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kang, J.; Chen, X.M. Meta analysis of multi subgroup standard deviation combination method. Chin. J. Evid. Based Med. 2016, 16, 851–854. (In Chinese) [Google Scholar]
- Frank, J.J.; Poulakos, A.G.; Tornero-Velez, R.; Xue, J. Systematic review and meta-analyses of lead (Pb) concentrations in environmental media (soil, dust, water, food, and air) reported in the United States from 1996 to 2016. Sci. Total Environ. 2019, 694, 133489. [Google Scholar] [CrossRef]
- Lin, G.Z.; Du, L.; Li, K.; Pan, B.Y.; Liu, X.Y.; Zhou, Q.; Dong, H. 2006 and 2009 Survey of Children’s Blood Lead Levels in Guangzhou. J. Environ. Health 2011, 28, 821–822. (In Chinese) [Google Scholar]
- Luo, X.J.; Cao, K.; Xu, X. Analysis of blood lead level and related factors of children in Shenzhen. Mod. Prev. Med. 2012, 39, 5274–5276. (In Chinese) [Google Scholar]
- Wu, C.E.; Gong, Y.N.; Liu, Z.H.; Yang, H. Investigation on the harm of blood lead level to children’s health and its intervention measures. Chin. Med. Guide 2012, 10, 3–4. (In Chinese) [Google Scholar]
- Deng, K.Y.; Guan, Y.F.; Wang, D.E.; Yuan, B.; Li, X.W. Analysis of children’s blood lead level in Zhongshan, Guangdong. Guangdong Trace Element Science 2007, 12, 46–49. (In Chinese) [Google Scholar]
- Xiang, X.Q.; Yang, H.Y.; Chen, J.P.; Song, G.S. Analysis of blood lead level of preschool children in Qingyuan City. Guangdong Trace Elem. Sci. 2005, 1, 46–49. (In Chinese) [Google Scholar]
- Wang, S.Y.; Zeng, Y.J.; Ju, H.; Wang, X.M. Analysis of Chemical compositon of PM2.5 in the Autumn and winter in Guangzhou. Environ. Monit. Manag. Technol. 2013, 25, 9–12. (In Chinese) [Google Scholar]
- Du, J.H.; Zhang, Y.S.; He, L.Y.; Huang, X.F.; Mei, L.Y.; Lai, M.D.; Luan, S.J. Pollution characteristics and health risk assessment of heavy metals in atmospheric PM2.5 in a certain area of Shenzhen. J. Environ. Health 2012, 29, 838–840. (In Chinese) [Google Scholar]
- Tan, J.H.; Duan, J.C.; Ma, Y.L.; Yang, F.M.; Cheng, Y.; He, K.B.; Yu, Y.C.; Wang, J.W. Source of atmospheric heavy metals in winter in Foshan, China. Sci. Total Environ. 2014, 493, 262–270. [Google Scholar] [CrossRef]
- Shao, R.H.; Su, C.X.; Fan, F.; Qi, J.Y.; Cao, T.H. Ecological risk assessment of heavy metals in ambient air PM2.5 in the Pearl River Delta region. Environ. Sci. Technol. 2019, 42, 273–279. (In Chinese) [Google Scholar]
- Liu, X.; Zhou, M.; Zhao, Y.; Shu, Y.; Chen, H. Comparison of three different methods in blood lead testing. Stud. Trace Elem. Health 2015, 32, 54–56. (In Chinese) [Google Scholar]
- Yu, C.; He, J.Y.; Li, Y.Y.; Lin, X.H.; Xie, C.J.; Zhou, Q.; Chen, K.C. Analysis of food lead pollution in Guangzhou in 2008. Chin. J. Food Hyg. 2009, 21, 460–463. (In Chinese) [Google Scholar]
- Huang, W.; Wang, Z.; Pan, L.B.; Tan, W. Assessment of exposure to lead pollution in food in Shenzhen. Chin. J. Food Hyg. 2008, 5, 405–408. (In Chinese) [Google Scholar]
- Yang, D.M.; Liu, L.B.; Zhou, W.P. Dynamic change analysis of heavy metal pollution of edible agricultural products in Shunde District, Foshan City. Guangdong Anim. Husb. Vet. Sci. Technol. 2016, 41, 13–17+26. [Google Scholar]
- Huang, Y.; Guo, Q.R.; Ren, H.; Yang, G.Y.; Wan, H.F.; Luo, W. Research on the status quo of heavy metal pollution of vegetables in typical areas of the Pearl River Delta—taking Zhongshan City and Dongguan City as examples. Ecol. Environ. 2005, 04, 559–561. (In Chinese) [Google Scholar]
- He, J.F.; Lei, L. Survey of lead and cadmium content in food in Qingyuan City from 2005 to 2006. J. Prev. Med. Inf. 2009, 25, 236–238. (In Chinese) [Google Scholar]
- Jiang, D. Application of Exposure Assessment in Dietary Lead and Cadmium Intake. Doctoral Dissertation, Chinese Center for Disease Control and Prevention, Beijing, China, 2008. (In Chinese). [Google Scholar]
- Zheng, X.J. Determination of Cadmium Content in Rice Sold in the Market and Research on Health Risk Assessment. Doctoral Dissertation, South China University of Technology, Guangzhou, China, 2018. (In Chinese). [Google Scholar]
- Dong, R.D.; Feng, Y.F.; Ou, H. Investigation and analysis of heavy metals in food pollutants in Zhongshan City. China J. Health Insp. 2005, 11, 81–82. (In Chinese) [Google Scholar]
- Jiao, Y.P.; Fu, B.L.; Wen, X.L.; Jiang, F.J.; He, X.Q.; Liu, L. Analysis of the correlation between breast milk lead and maternal blood and cord blood lead content in 500 cases in Guangzhou. China Matern. Child Health Care 2011, 26, 820–821. (In Chinese) [Google Scholar]
- Yan, Y.Y.; Gao, Y.; Luo, X.X.; Gao, X.H.; Hong, Q.; Liu, J.A.; Pan, L.M. Study on lead exposure levels in different stages of pregnancy and neonatal neurodevelopment and audiovisual function in Shenzhen. Chin. J. Child Health 2010, 18, 307–309. (In Chinese) [Google Scholar]
- Su, L.; Wu, Q.; Li, F.Z. Preliminary study on the influence of maternal blood lead level in the second trimester on pregnancy outcome. Guangdong Trace Elem. Sci. 2003, 12, 28–31. (In Chinese) [Google Scholar]
- Zhang, H.Z.; Luo, Y.M.; Zhang, H.B.; Song, J.; Xia, J.Q.; Zhao, Q.G. Reference value of regional soil environmental lead based on human blood lead index. Environ. Sci. 2009, 30, 3036–3042. (In Chinese) [Google Scholar]
- Liu, Y.X.; Long, H.; Tang, M.F.; Chen, R.Q. Investigation and analysis of influencing factors on blood lead content of pregnant women in Qingyuan City. China Med. Sci. 2017, 7, 150–152. (In Chinese) [Google Scholar]
- Zhang, H.Z.; Luo, Y.M.; Zhang, H.B.; Song, J.; Xia, J.Q.; Zhao, Q.G. Development of lead benchmarks for soil based on human blood lead level in China. Environ. Sci. 2009, 30, 3036–3042. (In Chinese) [Google Scholar]

| Parameter | Description | 0~1 | 1~2 | 2~3 | 3~4 | 4~5 | 5~6 | 6~7 |
|---|---|---|---|---|---|---|---|---|
| ARCORT | Rate coefficient for Pb transfer from nonexchangeable cortical bone to diffusible plasma. | 0 | 0.18 | 0.19 | 0.16 | 0.13 | 0.11 | 0.09 |
| ARRBC | Rate coefficient for Pb transfer from RBC to diffusible plasma. | −0.05 | −0.98 | −0.98 | −0.98 | −0.98 | −0.98 | −0.98 |
| ARCS2DF | Rate coefficient for Pb transfer from the cortical bone surface to exchangeable cortical bone volume. | 0 | −0.28 | −0.19 | −0.14 | −0.09 | −0.06 | −0.06 |
| RDIFF | Rate coefficient for Pb transfer from exchangeable bone (cortical or trabecular) volume to surface or non-exchangeable bone volume. | 0 | 0.13 | 0.08 | 0.05 | 0.02 | 0.001 | 0.00 |
| BLDMOT | Maternal blood Pb concentration. | 0.99 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Age Group (Years) | BLLs Predicted by ALLM, μg/dL | BLLs Predicted by ALLM + MC (M ± SD), μg/dL | ||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| 0~1 | 0.61 | 0.62 | 0.61 | 0.58 ± 0.30 | 0.59 ± 0.30 | 0.58 ± 0.30 |
| 1~2 | 1.37 | 1.41 | 1.39 | 0.91 ± 1.09 | 0.93 ± 1.12 | 0.92 ± 1.11 |
| 2~3 | 1.32 | 1.36 | 1.34 | 0.88 ± 1.04 | 0.91 ± 1.07 | 0.89 ± 1.06 |
| 3~4 | 1.21 | 1.25 | 1.23 | 0.82 ± 0.93 | 0.84 ± 0.96 | 0.83 ± 0.95 |
| 4~5 | 1.08 | 1.11 | 1.10 | 0.74 ± 0.82 | 0.76 ± 0.84 | 0.75 ± 0.83 |
| 5~6 | 0.93 | 0.96 | 0.95 | 0.65 ± 0.71 | 0.67 ± 0.73 | 0.66 ± 0.72 |
| 6~7 | 0.84 | 0.85 | 0.85 | 0.59 ± 0.62 | 0.60 ± 0.63 | 0.59 ± 0.63 |
| Age Group | 0~1 | 1~2 | 2~3 | 3~4 | 4~5 | 5~6 | 6~7 | |
|---|---|---|---|---|---|---|---|---|
| Grain (g/day) | Guangdong a | 24.7 | 79.1 | 143.6 | 156.4 | 166.1 | 171.4 | 186.0 |
| U.S. b | 33.0 | 66.0 | 81.0 | 101.0 | 101.0 | 101.0 | 119.0 | |
| Vegetable (g/day) | Guangdong a | 49.5 | 96.4 | 194.5 | 170.6 | 159.7 | 149.0 | 232.2 |
| U.S. b | 91.0 | 120.0 | 145.0 | 170.0 | 170.0 | 170.0 | 210.0 | |
| Dust (g/day) | Guangdong a | 0.03 | 0.06 | 0.06 | 0.04 | 0.04 | 0.04 | 0.10 |
| U.S. b | 0.03 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | |
| Water (L/day) | Guangdong a | 0.59 | 0.91 | 0.81 | 0.86 | 0.85 | 0.86 | 1.19 |
| U.S. b | 0.36 | 0.27 | 0.32 | 0.33 | 0.33 | 0.33 | 0.41 | |
| Ventilation rate (m3/day) | Guangdong a | 5.0 | 5.2 | 5.8 | 7.7 | 8.0 | 8.3 | 9.4 |
| U.S. b | 5.4 | 8.0 | 8.9 | 10.1 | 10.1 | 10.1 | 12.0 |
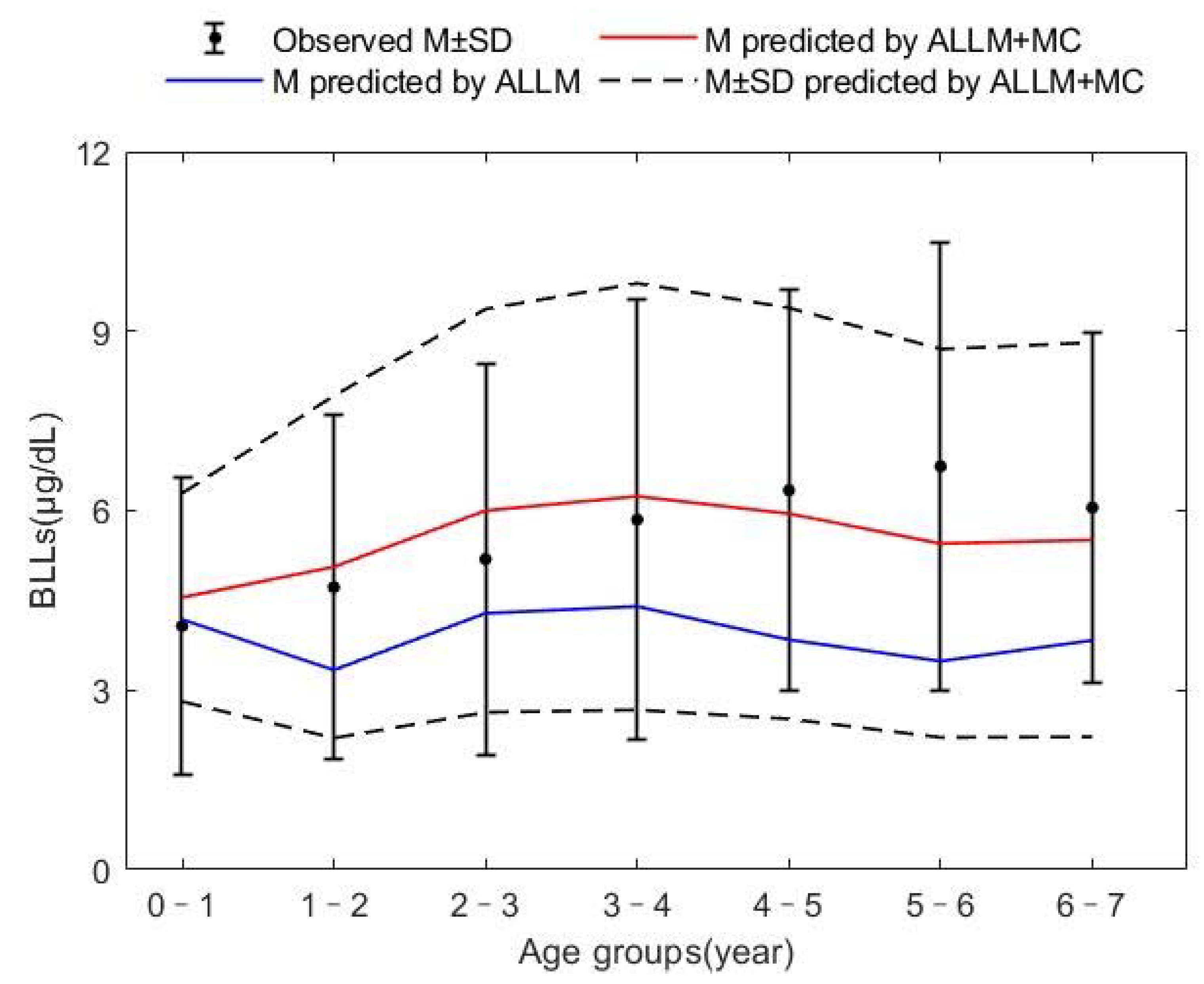
| Age Groups (Year) | BLLs Predicted by ALLM (μg/dL) | BLLs Predicted by ALLM + MC (μg/dL) | Observed BLLs (μg/dL) |
|---|---|---|---|
| 0~1 | 4.18 | 4.55 | 4.07 |
| 1~2 | 3.34 | 5.06 | 4.72 |
| 2~3 | 4.29 | 6.00 | 5.19 |
| 3~4 | 4.40 | 6.24 | 5.85 |
| 4~5 | 3.84 | 5.95 | 6.34 |
| 5~6 | 3.48 | 5.45 | 6.74 |
| 6~7 | 3.83 | 5.51 | 6.05 |
| 0~7 | 3.91 | 5.54 | 5.13 |
| RMSE | 38.03 | 13.30 | |
| RMD | −32.31 | −0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Zhang, Z.; Lin, S.; Zhang, Q.; Du, G.; Zhou, R.; Qu, X.; Xu, G.; Yang, Y.; Cai, Y. Application of All-Ages Lead Model Based on Monte Carlo Simulation of Preschool Children’s Exposure to Lead in Guangdong Province, China. Sustainability 2023, 15, 1068. https://doi.org/10.3390/su15021068
Hu J, Zhang Z, Lin S, Zhang Q, Du G, Zhou R, Qu X, Xu G, Yang Y, Cai Y. Application of All-Ages Lead Model Based on Monte Carlo Simulation of Preschool Children’s Exposure to Lead in Guangdong Province, China. Sustainability. 2023; 15(2):1068. https://doi.org/10.3390/su15021068
Chicago/Turabian StyleHu, Jing, Zhengbao Zhang, Senwei Lin, Qiuhuan Zhang, Guoxia Du, Ruishan Zhou, Xiaohan Qu, Guojiang Xu, Ying Yang, and Yongming Cai. 2023. "Application of All-Ages Lead Model Based on Monte Carlo Simulation of Preschool Children’s Exposure to Lead in Guangdong Province, China" Sustainability 15, no. 2: 1068. https://doi.org/10.3390/su15021068
APA StyleHu, J., Zhang, Z., Lin, S., Zhang, Q., Du, G., Zhou, R., Qu, X., Xu, G., Yang, Y., & Cai, Y. (2023). Application of All-Ages Lead Model Based on Monte Carlo Simulation of Preschool Children’s Exposure to Lead in Guangdong Province, China. Sustainability, 15(2), 1068. https://doi.org/10.3390/su15021068






