Treatment of Domestic Wastewater in Small-Scale Sand Filters Fortified with Gypsum, Biotite, and Peat
Abstract
1. Introduction
2. Materials and Methods
2.1. Column Experiments and Wastewater Sampling
2.2. Physico-Chemical Analyses of Influent and Effluent
2.3. Bacteriological and Virus Analyses of Influent and Effluent
2.4. Filter Mass Analyses
2.5. Data Analyses
3. Results
3.1. Phosphate-P
3.2. Total Nitrogen (Ntot)
3.3. Chemical Oxygen Demand (CODCr)
3.4. The Electrical Conductivity and the pH in Effluents
3.5. Microbial Quality
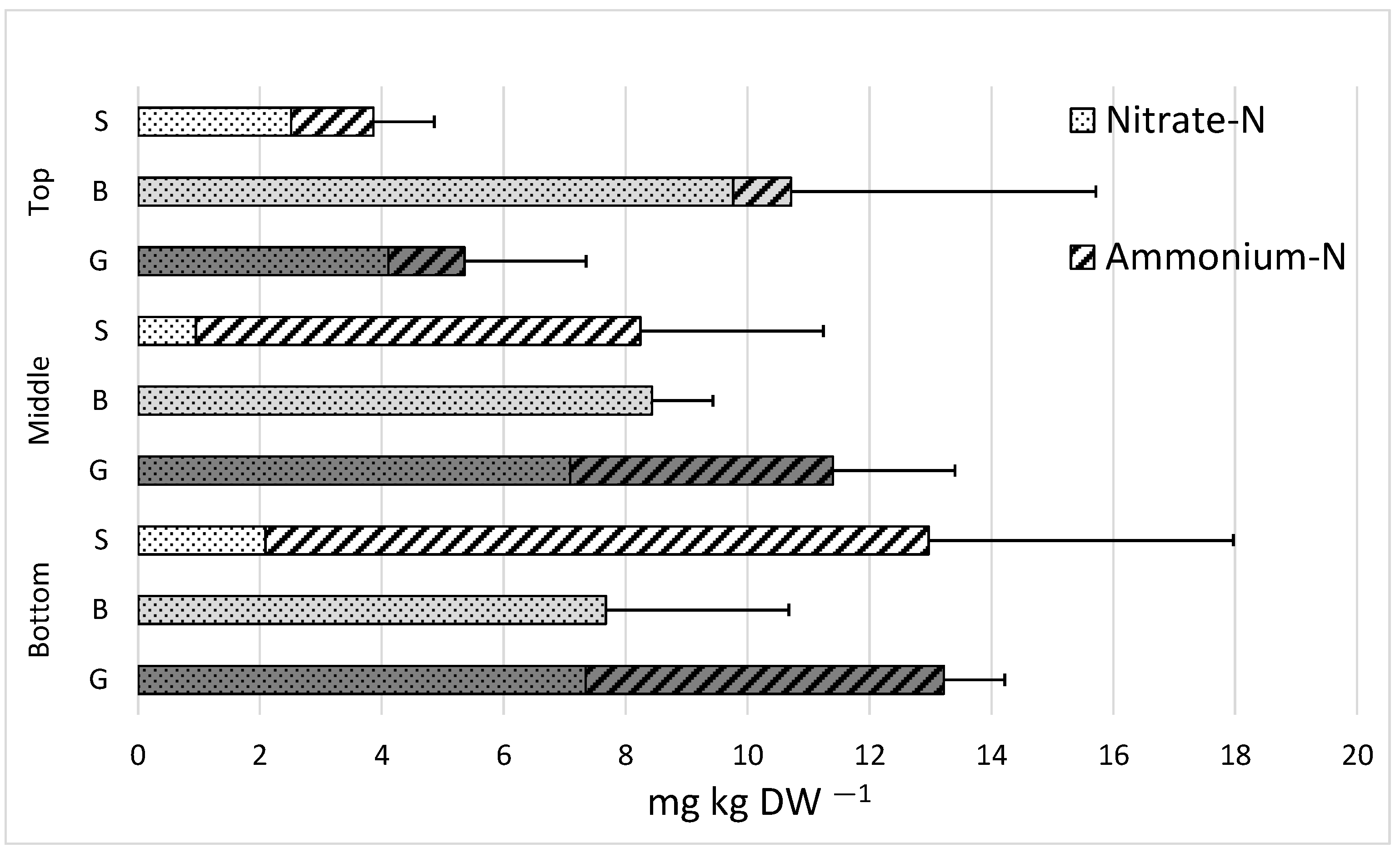
3.6. Filter Masses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, K.C.; Venkitachalam, T.H. Improving phosphate removal of sand infiltration system using alkaline fly ash. Chemosphere 2000, 41, 243–249. [Google Scholar] [CrossRef]
- Robalds, A.; Dreijalte, L.; Bikovens, O.; Kalavins, M. A novel peat-based biosorbent for the removal of phosphate from synthetic and real wastewater and possible utilization of spent sorbent in land application. Desalination Water Treat. 2015, 57, 13285–13294. [Google Scholar] [CrossRef]
- Bahgat, M.; Dewedar, A.; Zayed, A. Sand-filters used for wastewater treatment: Buildup and distribution of microorganisms. Water Res. 1999, 33, 1949–1955. [Google Scholar] [CrossRef]
- Eveborn, D.; Kong, D.; Gustafsson, J.P. Wastewater treatment by soil infiltration: Long-term phosphorus removal. J. Contam. Hydrol. 2012, 140–141, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, S.; Laukka, V.; Vidal, B.; Heiderscheidt, E.; Postila, H.; Nilivaara, R.; Herrmann, I. Circular Economy in Wastewater Management—The Potential of Source-Separating Sanitation in Rural and Peri-Urban Areas of Northern Finland and Sweden. Front. Environ. Sci. 2022, 97. [Google Scholar] [CrossRef]
- Li, Z.; Hu, K.; Zhang, X.; Gong, L.; Jiang, Z.; Xing, Y.; Ding, J.; Tian, J.; Huang, J. Distributed treatment of rural environmental wastewater by artificial ecological geographic information system. J. King Saud Univ. Sci. 2022, 34, 101806. [Google Scholar] [CrossRef]
- Renman, A.; Renman, G. Long-term phosphate removal by the calcium-silicate material polonite in wastewater filtration systems. Chemosphere 2010, 79, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.; Eveborn, D.; Kärrman, E.; Gustafsson, J.P. Environmental systems analysis of four on-site wastewater treatment options. Resour. Conserv. Recycl. 2008, 52, 1153–1161. [Google Scholar] [CrossRef]
- Vidal, B.; Hedström, A.; Herrman, I. Phosphorus reduction in filters for on-site wastewater treatment. J. Water Eng. 2018, 22, 210–217. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of Freshwater and Coastal Marine Ecosystems, a Global Problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Bouraoui, F.; Kabbe, C.; Oenema, O.; van Dijik, K.C. Phosphorus management in Europe in a changing world. Ambio 2015, 44, 180–192. [Google Scholar] [CrossRef]
- Boesch, D.; Hecky, R.; O’Melia, C.; Schindler, C.D.; Seitzinger, S. Eutrophication of Swedish Seas, Final Report; Swedish Environmental Protection Agency: Stockholm, Sweden, 2006; ISBN 91-620-5509-7. [Google Scholar]
- Macintosh, K.A.; Jordan, P.; Cassidy, R.; Arnsheidt, J.; Ward, C. Low flow water quality in rivers; septic tank systems and high-resolution phosphorus signals. Sci. Total Environ. 2011, 412–413, 58–65. [Google Scholar] [CrossRef]
- Kauppinen, A.; Martikainen, K.; Matikka, V.; Veijalainen, A.M.; Pitkänen, T.; Heinonen-Tanski, H.; Miettinen, I.T. Sand filters for removal of microbes and nutrients from wastewater during a one-year pilot study. J. Environ. Manag. 2014, 133, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, K.; Kauppinen, A.; Matikka, V.; Veijalainen, A.M.; Torvinen, E.; Pitkänen, T.; Miettinen, I.T.; Heinonen-Tanski, H. Efficiency of Private Household Sand Filters in Removing Nutrients and Microbes from Wastewater in Finland. Water 2018, 10, 1000. [Google Scholar] [CrossRef]
- Hagedorn, C.; Hansen, D.T.; Simonson, G.H. Survival and Movement of Fecal Indicator Bacteria in Soil under Conditions of Saturated Flow 1. J. Environ. Qual. 1978, 7, 55–59. [Google Scholar] [CrossRef]
- Pitkänen, T.; Juselius, T.; Isomäki, E.; Miettinen, I.T.; Valve, M.; Kivimäki, A.-L.; Lahti, K.; Hänninen, M.-L. Drinking water quality and occurrence of Giardia in Finnish small groundwater supplies. Resources 2015, 4, 637–654. [Google Scholar] [CrossRef]
- Kauppinen, A.; Pitkänen, T.; Miettinen, I.T. Persistent Norovirus Contamination of Groundwater Supplies in Two Waterborne Outbreaks. Food Environ. Virol. 2018, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.; Odumeru, J. Irrigation water as source of foodborne pathogens on fruit and vegetables. J. Food Prot. 2004, 67, 2839–2849. [Google Scholar] [CrossRef]
- Xu, D.; Xu, J.; Wu, J.; Muhammad, A. Studies on the phosphorus sorption capacity of substrates used in constructed wetland systems. Chemosphere 2006, 63, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.P.; Renman, A.; Renman, G.; Poll, K. Phosphate removal by mineral-based sorbents used in filters for small-scale wastewater treatment. Water Res. 2008, 42, 189–197. [Google Scholar] [CrossRef]
- Bellier, N.; Chazarenc, F.; Comeau, Y. Phosphorus removal from wastewater by mineral apatite. Water Res. 2006, 40, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Ádám, K.; Krogstad, T.; Vråle, L.; Søvik, A.K.; Jenssen, P.D. Phosphorus retention in the filter materials shellsand and Filtralite P®—Batch and column experiment with synthetic P solution and secondary wastewater. Ecol. Eng. 2007, 29, 200–208. [Google Scholar] [CrossRef]
- Yin, H.; Yun, Y.; Zhang, Y.; Fan, C. Phosphate removal from wastewaters by a naturally occurring, calcium-rich sepiolite. J. Hazard. Mater. 2011, 198, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Vohla, C.; Alas, R.; Nurk, K.; Baatz, S.; Mander, Ü. Dynamics of phosphorus, nitrogen and carbon removal in a horizontal subsurface flow constructed wetland. Sci. Total Environ. 2007, 380, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Korkusuz, E.A.; Beklioğlu, M.; Demirer, G.N. Comparison of the treatment performances of blast furnace slag-based and gravel-based vertical flow wetlands operated identically for domestic wastewater treatment in Turkey. Ecol. Eng. 2005, 24, 187–200. [Google Scholar] [CrossRef]
- Matikka, V.; Heinonen-Tanski, H. Reduction of phosphorus, nitrogen and microorganisms in pilot scale sand filter bed containing biotite, treating primary wastewater. Environ. Technol. 2016, 37, 46–54. [Google Scholar] [CrossRef]
- Jucherski, A.; Walczowski, A.; Bugajski, P.; Jóźwiakowski, K.; Rodziewicz, J.; Janczukowicz, W.; Rodziewicz, J.; Jaczukowicz, W.; Wu, S.; Kasprzyk, M.; et al. Long-term operating conditions for different sorption materials to capture phosphate from domestic wastewater. Sustain. Mater. Technol. 2022, 31, e00385. [Google Scholar] [CrossRef]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mandera, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Heinonen-Tanski, H.; Matikka, V. Chemical and Microbiological Quality of Effluents from Different On-Site Wastewater Treatment Systems across Finland and Sweden. Water 2017, 9, 47. [Google Scholar] [CrossRef]
- Vilpas, R.; Santala, E. Comparison of the nutrient removal efficiency of one-site wastewater treatments systems: Applications of conventional sand filters and sequencing batch reactors (SBR). Water Sci. Technol. 2007, 55, 109–117. [Google Scholar] [CrossRef]
- Reijnders, L. Phosphorus resources, their depletion and conservation, a review. Resour. Conserv. Recycl. 2014, 93, 32–49. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The potential phosphorus crisis: Resource conservation and possible escape technologies: A review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Spears, B.M.; Brownlie, W.J.; Cordell, D.; Hermann, L.; Mogollón, J.M. Concerns about global phosphorus demand for lithium-iron-phosphate batteries in the light electric vehicle sector. Commun. Mater. 2022, 3, 14. [Google Scholar] [CrossRef]
- Morse, G.K.; Brett, S.W.; Guy, J.A.; Lester, J.N. Review: Phosphorus removal and recovery technologies. Sci. Total Environ. 1998, 212, 69–81. [Google Scholar] [CrossRef]
- Cucarella, V.; Zaleski, T.; Mazurek, R.; Renman, G. Fertilizer potential of calcium-rich substrates used for phosphorus removal from wastewater. Pol. J. Environ. Stud. 2007, 16, 817. [Google Scholar]
- Hylander, L.D.; Kietlińska, A.; Renman, G.; Simán, G. Phosphorus retention in filter materials for wastewater treatment and its subsequent suitability for plant production. Bioresour. Technol. 2006, 97, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Ekholm, P.; Valkama, P.; Jaakkola, E.; Kiirikki, M.; Lahti, K.; Pietola, L. Gypsum amendment of soils reduces phosphorus losses in an agricultural catchment. Agric. Food Sci. 2012, 21, 279–291. [Google Scholar] [CrossRef]
- Airaksinen, S.; Heinonen-Tanski, H.; Heiskanen, M.-L. Quality of different bedding materials and their influence on the compostability of horse manure. J. Equine Vet. Sci. 2001, 21, 125–130. [Google Scholar] [CrossRef]
- Xiong, J.B.; Mahmood, Q. Adsorptive removal of phosphate from aqueous media by peat. Desalination 2010, 259, 59–64. [Google Scholar] [CrossRef]
- Koiv, M.; Vohla, C.; Motlep, R.; Liira, M.; Kirsimäe, K.; Mander, Ü. The performance of peat-filled subsurface flow filters treating landfill leachate and municipal wastewater. Ecol. Eng. 2009, 35, 204–212. [Google Scholar] [CrossRef]
- Gray, N.F.; Coady, J. Evaluation of full-scale vertical peat biofilters for treatment of septic tank effluents. Int. J. Environ. Stud. 2015, 72, 154–165. [Google Scholar] [CrossRef]
- Tao, J.; Mancl, K.M.; Tuovinen, O.H. Treatment of sanitary sewer overflow with fixed media bioreactors. Appl. Eng. Agric. 2009, 25, 39–43. [Google Scholar] [CrossRef]
- Liimatainen, M.; Martikainen, P.J.; Maljanen, M. Why granulated wood ash decreases N2O production in boreal acidic peat soil? Soil Biol. Biochem. 2014, 79, 140–148. [Google Scholar] [CrossRef]
- Vuorinen, J.; Mäkitie, O. The Method of Soil Testing in Use in Finland; Agrogeological Publications: Helsinki, Finland, 1955; Volume 63. [Google Scholar]
- SFS 1189; Water Quality—Determination of Phosphorus–Ammonium Molybdate Spectrometric Method. Suomen Stan-dardisoimisliitto: Helsinki, Finland, 1997; p. 31.
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater. Part 4000 Inorganic Nonmetallic Constituents, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Kutilek, M.; Nielsen, D.R. Soil Hydrology. GeoEcology Textbook; Catena Verlag: Cremlingen-Destedt, Germany, 1994; p. 380. [Google Scholar]
- Ministry of the Environment, Finland. The Finnish Government Decree on Treating Domestic Wastewaters in Areas Outside Sewer Networks, 517/2017. Available online: https://www.finlex.fi/fi/laki/alkup/2017/20170157 (accessed on 1 November 2022).
- Government Decree on Urban Waste Water Treatment, 888/2006. Available online: https://finlex.fi/en/laki/kaannokset/2006/en20060888.pdf (accessed on 1 November 2022).
- FAO. Wastewater Treatment and Use in Agriculture; FAO Irrigation and Drainage Paper 47, M-56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; ISBN 92-5-103135-5. [Google Scholar]
- EU Publications. Directive 2006/7/EC of the European Parliament and of the Council, the EU Bathing Water Directive. Available online: https://publications.europa.eu/en/publication-detail/-/publication/c137dbd0-21c5-492f-81ad-736a1295fc68/language-en (accessed on 1 January 2022).
- Maier, R.M. Chapter 16, Biogeochemical Cycling. In Environmental Microbiology, 3rd ed; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-394626-3. [Google Scholar]
- Makaya, J.M.; Somda, M.K.; Savadogo, A.; Dianou, D.; Barro, N.; Traoré, A.S. Survival of enteric bacteria in source-separated human urine used as fertiliser: Effects of temperature and ammonia. Afr. J. Environ. Sci. Technol. 2014, 8, 511–520. [Google Scholar]
- Hamisi, R.; Renman, A.; Renman, G.; Wörman, A.; Thunvik, R. Long-term phosphorus sorption and leaching in sand filters for onsite treatment systems. Sci. Total Environ. 2022, 833, 155254. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, D.; Kim, Y.; Wan, J.; Jun, Y.S. Effects of phosphate on biotite dissolution and secondary precipitation under conditions relevant to engineered subsurface processes. Phys. Chem. Chem. Phys. 2017, 19, 29895–29904. [Google Scholar] [CrossRef] [PubMed]
- Arocena, J.M.; Velde, B.; Robertson, S.J. Weathering of biotite in the presence of arbuscular mycorrhizae in selected agricultural crops. Appl. Clay Sci. 2012, 64, 12–17. [Google Scholar] [CrossRef]
- Stocker, K.; Ellersdorfer, M. Phosphate Fixation and P Mineralogy on Natural and Ca-Modified Zeolites During Simultaneous Nutrient Removal. Water Air Soil Pollut. 2022, 233, 1–13. [Google Scholar] [CrossRef]
- Uddin, F. Montmorillonite: An Introduction to Properties and Utilization; IntechOpen: London, UK, 2018; pp. 3–23. [Google Scholar]
- Samimi, F.; Ghiyasiyan-Arani, M.; Salavati-Niasari, M. New avenue for preparation of potential hydrogen storage materials based on K10 montmorillonite and Ca2Mn3O8/CaMn3O6 nanocomposites. Fuel 2022, 320, 123933. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M. Effect of Li2CoMn3O8 nanostructures synthesized by a combustion method on montmorillonite K10 as a potential hydrogen storage material. J. Phys. Chem. C. 2018, 122, 16498–16509. [Google Scholar] [CrossRef]
- Hansma, H.G. Potassium at the origins of life: Did biology emerge from biotite in micaceous clay? Life 2022, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Fatehi Pouladi, S. Phosphorus Removal from Domestic Wastewater Using Dual Reactive Materials Polonite® and Absol®; TRITA-LWR Degree Project; Royal Institute of Technology: Stockholm, Sweden, 2011; ISSN 1651-064X. [Google Scholar]
- Shakir, E.; Zahraw, Z.; Al-Obaidy, A.H.M. Environmental and health risks associated with reuse of wastewater for irrigation. Egyp. J. Pet. 2017, 26, 95–102. [Google Scholar] [CrossRef]
- Koponen, H. Maapuhdistamojen Tukkeutuminen ja Käytöstä Poistettujen Suodatinmassojen Koostumus. Master’s Thesis, Tampere University, Tampere, Finland, 2011. [Google Scholar]
- Lehtoranta, S.; Vilpas, R.; Mattila, T.J. Comparison of carbon footprints and eutrophication impacts of rural on-site wastewater treatment plants in Finland. J. Clean. Prod. 2014, 65, 439–446. [Google Scholar] [CrossRef]
- Jenssen, P.D.; Krogstad, T.; Paruch, A.M.; Mæhlum, T.; Adam, K.; Arias, C.A.; Heistad, A.; Jonsson, L.; Hellström, D.; Brix, H. Filter bed systems treating domestic wastewater in the Nordic countries–performance and reuse of filter media. Ecol. Eng. 2010, 36, 1651–1659. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council. Laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 1 January 2022).
- Hellstén, A. Maapuhdistamojen Suodatinmassojen Hygienia ja Hyödyntäminen. Master’s Thesis, University of Eastern Finland, Kuopio, Finland, 2011. [Google Scholar]
- Karim, M.R.; Glenn, E.P.; Gerba, C.P. The effect of wetland vegetation on the survival of Escherichia coli, Salmonella typhimurium, bacteriophage MS-2 and polio virus. J. Water Health 2008, 6, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Nordin, A.; Ottoson, J.R.; Vinnerås, B. Sanitation of faeces from source-separating dry toilets using urea. J. Appl. Microbiol. 2009, 107, 1579–1587. [Google Scholar] [CrossRef]
- Christian, E.; Batista, J.R.; Gerrity, D. Use of COD, TOC, and fluorescence spectroscopy to estimate BOD in wastewater. Water Environ. Res. 2017, 89, 168–177. [Google Scholar] [CrossRef]
- Urban Wastewater Directive 91/271/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31991L0271&from=EN (accessed on 1 January 2022).
- Healy, M.G.; Rodgers, M.; Mulqueen, J. Treatment of dairy wastewater using constructed wetlands and intermittent sand filters. Bioresour. Technol. 2007, 98, 2268–2281. [Google Scholar] [CrossRef]
- Abou-Elela, S.I.; Hellal, M.S.; Aly, O.H.; Abo-Elenin, S.A. Decentralized wastewater treatment using passively aerated biological filter. Environ. Technol. 2019, 40, 250–260. [Google Scholar] [CrossRef]
- Jowett, E.C.; McMaster, M.L. On-site wastewater treatment using unsaturated absorbent biofilters. Am. Soc. Agron. Crop Sci. Soc. Am. Soil Sci. Soc. Am. 1995, 24, 86–95. [Google Scholar] [CrossRef]
- Arias, C.A.; Brix, H.; Johansen, N.H. Phosphorus removal from municipal wastewater in an experimental two-stage vertical flow constructed wetland system equipped with a calcite filter. Water Sci. Technol. 2003, 48, 51–58. [Google Scholar] [CrossRef] [PubMed]




| (a) Exp. 1 | |||||
| Wastewater Loading and Its Duration | Parameter | Influent | Effluents | ||
| Column S | Column B | Column G | |||
| Normal loading 42 L m−2 (3 weeks) | PO4-P mg L−1 | 10 ± 3.1 | 0.05 ± 0.03 | 0.3 ± 0.01 | 0.05 ± 0.02 |
| red% PO4-P | 99.6 (99.3–99.9) | 99.3 (99.6–99.9) | 99.4 (98.8–99.8) | ||
| Ntot mg L−1 | 78 ± 7.6 | 25 ± 21 | 91 ± 13 | 82 ± 0.21 | |
| red% Ntot | 65.3 (43.0–87.6) | no red | no red | ||
| COD mg L−1 | 540 ± 270 | 34 ± 9.3 | 32 ± 5.7 | 77 ± 65 | |
| red% COD | 93.3 (92.5–94.7) | 93.6 (92.1–95.5) | 87 (82.1–90.8) | ||
| High loading 52 L m−2 (3 weeks) | PO4-P mg L−1 | 7 ± 0.1 | 0.01 ± 0.02 | 0.02 ± 0.03 | 0.07 ± 0.03 * |
| red% PO4-P | 99.8 (99.1–99.9) | 99.7 (98.9–99.9) | 99.1 (97.8–99.3) * | ||
| Ntot mg L−1 | 170 ± 156 | 27 ± 23 | 107 ± 11 * | 83 ± 2.2 | |
| red% Ntot | 83.5 (81.5–84.6) | 5.5 (no red-65.7) | 24.5 (no red-75.7) | ||
| COD mg L−1 | 470 ± 27 | 44 ± 7.2 | 44 ± 5.1 | 40 ± 7.8 | |
| red% COD | 90.8 (89.7–91.6) | 90.7 (90.1–91.1) | 94.5 (91.1–92.4) | ||
| Normal loading 42 L m−2 (3 weeks) | PO4-P mg L−1 | 7 ± 0.3 | 0.03 ± 0.03 | 0.02 ± 0.01 | 0.18 ± 0.19 ** |
| red% PO4-P | 99.6 (98.8–99.9) | 99.8 (99.5–99.9) | 97.3 (92.3–99.3) * | ||
| Ntot mg L−1 | 370 ± 27 | 72 ± 20 | 106 ± 11 | 70 ± 14 | |
| red% Ntot | 80.5 (74.3–85.8) | 71.4 (70.3–72.5) | 81.1 (79–83.7) | ||
| COD mg L−1 | 490 ± 9.6 | 50 ± 0.6 | 51 ± 3.0 | 37 ± 2.1 | |
| red% COD | 89.7 (89.4–90) | 89.5 (88.8–90.1) | 92.3 (92.1–92.8) | ||
| Low loading 21 L m−2 (4 weeks) | PO4-P mg L−1 | 6.1 ± 1.7 | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.26 ± 0.18 *** |
| red% PO4-P | 99.6 (98.9–99.9) | 99.7 (99.5–99.9) | 95.8 (88.6–98.2) *** | ||
| Ntot mg L−1 | 260 ± 82 | 70 ± 12 | 88 ± 2.1 | 71 ± 17 | |
| red% Ntot | 70.9 (59.8–82.4) | 69.8 (65.6–74.0) | 70.9 (61.0–82.4) | ||
| COD mg L−1 | 385 ± 217 | 48 ± 15 | 60 ± 27 | 32 ± 12 | |
| red% COD | 80.8 (56.7–89.4) | 51.3 (no red-91.6) | 87.6 (73.3–93.6) | ||
| Normal loading 42 L m−2 (3 weeks) | PO4-P mg L−1 | 7.4 ± 5.1 | 0.02 ± 0.03 | 0.02 ± 0.01 | 0.33 ± 0.16 *** |
| red% PO4-P | 99.7 (99.5–99.9) | 99.6 (98.3–99.9) | 94.6 (86.7–98.2) *** | ||
| Ntot mg L−1 | 35 ± 6.1 | 46 ± 3.3 | 52 ± 1.9 | 34 ± 8.1 | |
| red% Ntot | no red | no red | 4.8 (no red -11) | ||
| COD mg L−1 | 270 ± 260 | 54 ± 8.0 | 12 ± 6.0 | 47.7 ± 8.5 | |
| red% COD | 65.6 (50.8–91.8) | 91.0 (85.7–98.9) | 72.5 (61.9–90) | ||
| PO4-P adding with normal loading 42 L m−2 (3 weeks) | PO4-P mg L−1 | 46 ± 19 | 0.05 ± 0.06 | 0.03 ± 0.03 | 2.51 ± 3.05 *** |
| red% PO4-P | 99.9 (99.2–99.9) | 99.9 (99.4–99.9) | 91.3 (66.7–99.5) *** | ||
| Ntot mg L−1 | 75 ± 11 | 44 ± 1.7 | 46 ± 2.9 | 46 ± 5.1 | |
| red% Ntot | 40.6 (33.3–48.6) | 37.9 (34.7–43.4) | 38.5 (36.4–40.7) | ||
| COD mg L−1 | 390 ± 270 | 87 ± 19 | 52 ± 17 | 83 ± 29 | |
| red% COD | 69.5 (56.1–89.5) | 84.1 (78.3–89.8) | 69.2 (53.3–92.3) | ||
| Normal loading 42 L m−2 (11 weeks) | PO4-P mg L−1 | 10 ± 5.2 | 0.24 ± 0.22 | 1.51 ± 1.07 ** | 6.21 ± 3.42 *** |
| red% PO4-P | 97.4 (92.9–99.9) | 83.6 (64.3–99.5) ** | 31.3 (no red-86.3) *** | ||
| Ntot mg L−1 | 90 ± 5.5 | 51 ± 3.6 | 47 ± 5.9 | 51 ± 3.0 | |
| red% Ntot | 43.4 (40.9–50.4) | 47.7 (40–51.4) | 43.6 (43.2–44.1) | ||
| COD mg L−1 | 350 ± 36 | 130 ± 7.8 | 44 ± 8.6 * | 90 ± 14 | |
| red% COD | 62.2 (57.5–68.6) | 87.7 (84.7–88.8) ** | 73.9 (69.6–83.6) | ||
| (b) Exp. 2 | |||||
| Wastewater Loading and Its Duration | Parameter | Influent | Effluents | ||
| Column S | Column GP | Column GSP | |||
| Normal loading 42 L m−2 (3 weeks) | PO4-P mg L−1 | 6.8 ± 0.6 | 0.07 ± 0.03 | 0.10 ± 0.08 | 0.15 ± 0.09 |
| red% PO4-P | 98.8 (98.3–99.4) | 98.5 (96.6–99.8) | 97.7 (95.4–99.3) | ||
| Ntot mg L−1 | 104 ± 7.2 | 31 ± 2.6 | 28 ± 13 | 27 ± 0 | |
| red% Ntot | 70 (67–75) | 73.9 (61.8–81) | 74.5 (73–76) | ||
| COD mg L−1 | 240 ± 7.6 | 79 ± 3.6 | 110 ± 50 | 110 ± 0 | |
| red% COD | 67.3 (65–70) | 55 (40–77) | 54.2 (52.8–55.6) | ||
| High loading 52 L m−2 (3 weeks) | PO4-P mg L−1 | 8.0 ± 0.3 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 |
| red% PO4-P | 99.5 (99.3–99.7) | 99.5 (99.3–99.7) | 99.6 (96.9–99.2) | ||
| Ntot mg L−1 | 150 ± 16 | 52 ± 19 | 59 ± 24 | 64 ± 6.9 | |
| red% Ntot | 65.3 (58–76.9) | 60.6 (46–74.6) | 56.3 (52–63) | ||
| COD mg L−1 | 160 ± 54 | 91 ± 8.1 | 50 ± 14 * | 69 ± 2.0 | |
| red% COD | 37.9 (14.7–62.6) | 67.1 (52–77.5) | 53.9 (40.4–67.8) | ||
| Normal loading 42 L m−2 (3 weeks) | PO4-P mg L−1 | 7.2 ± 0.8 | 0.06 ± 0.02 | 0.14 ± 0.06 * | 0.05 ± 0.03 |
| red% PO4-P | 99.1 (98.8–99.4) | 98.1 (96.9–99.2) * | 99.3 (98.7–99.7) | ||
| Ntot mg L−1 | 92 ± 38 | 36 ± 15 | 58 ± 26 | 53 ± 23 | |
| red% Ntot | 60.4 (58–64) | 38.1 (35.2–42) * | 42.8 (40–46) | ||
| COD mg L−1 | 700 ± 220 | 59 ± 24 | 97 ± 68 | 83 ± 25 | |
| red% COD | 91.4 (88.4–94.7) | 87.1 (81.3–90.9) | 88.1 (86.6–89.6) | ||
| Low loading 21 L m−2 (3 weeks) | PO4-P mg L−1 | 8.3 ± 1.0 | 0.05 ± 0.02 | 0.08 ± 0.03 | 0.05 ± 0.03 |
| red% PO4-P | 99.4 (99.1–99.7) | 99.0 (98.4–99.7) | 99.4 (98.9–99.8) | ||
| Ntot mg L−1 | 150 ± 1.2 | 62 ± 1.7 | 46 ± 41 | 78 ± 0.0 | |
| red% Ntot | 58.5 (58–59.5) | 36 (0–60) | 48 (48) | ||
| COD mg L−1 | 200 ± 46 | 69 ± 24 | 130 ± 46 | 100 ± 39 | |
| red% COD | 63.4 (53.3–77.2) | 34.1 (29.4–40.4) | 45 (31.7–63.2) | ||
| Normal loading 42 L m−2 (3 weeks) | PO4-P mg L−1 | 8.3 ± 0.9 | 0.12 ± 0.03 | 0.06 ± 0.02 | 0.12 ± 0.09 |
| red% PO4-P | 99.4 (99.1–99.7) | 99.3 (98.9–99.7) * | 98.6 (97.1–99.5) | ||
| Ntot mg L−1 | 160 ± 13 | 67 ± 6.4 | 115 ± 45 | 88 ± 17 | |
| red% Ntot | 58.5 (53.5–64) | 44 (-2.5–84.4) | 44.6 (28–53.7) | ||
| COD mg L−1 | 550 ± 250 | 130 ± 56 | 150 ± 20 | 110 ± 10 | |
| red% COD | 75.8 (53.3–76.5) | 65.5 (29.4–80.6) | 76.7 (31.7–83.5) | ||
| PO4-P adding with normal loading 42 L m−2 (3 weeks) | PO4-P mg L−1 | 16 ± 0.7 | 0.25 ± 0.04 | 0.18 ± 0.08 | 0.20 ± 0.06 |
| red% PO4-P | 98.5 (98.2–99.1) | 98.9 (98.2–99.1) | 98.8 (98.1–99.2) | ||
| Ntot mg L−1 | 120 ± 3.4 | 36 ± 4.9 | 54 ± 10 * | 49 ± 9.2 | |
| red% Ntot | 69.9 (65.8–72.5) | 54.8 (46.1–59.2) | 58.7 (51–63.5) | ||
| COD mg L−1 | 280 ± 5.5 | 115 ± 52 | 170 ± 9.0 | 115 ± 4.4 | |
| red% COD | 58.1 (35.2–70.2) | 38.1 (35.2–43.1) | 58.2 (57.3–59.3) | ||
| Normal loading 42 L m−2 (3 weeks) | PO4-P mg L−1 | 6.9 ± 0.3 | 0.02 ± 0.02 | 0.19 ± 0.02 ** | 0.08 ± 0.03 |
| red% PO4-P | 98.7 (98.2–99.1) | 97.3 (96.9–97.8) ** | 98.8 (98–99.3) | ||
| Ntot mg L−1 | 100 ± 35 | 17 ± 15 | 27 ± 10 | 34 ± 12 | |
| red% Ntot | 85.8 (73.3–96.8) | 72 (64.8–82.5) | 66.7 (65–68) | ||
| COD mg L−1 | 270 ± 18 | 83 ± 31 | 130 ± 61 | 170 ± 58 | |
| red% COD | 69.4 (57.5–77.2) | 53.9 (37.3–77.2) | 53.9 (6.4–57.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martikainen, K.; Veijalainen, A.-M.; Torvinen, E.; Heinonen-Tanski, H. Treatment of Domestic Wastewater in Small-Scale Sand Filters Fortified with Gypsum, Biotite, and Peat. Sustainability 2023, 15, 1351. https://doi.org/10.3390/su15021351
Martikainen K, Veijalainen A-M, Torvinen E, Heinonen-Tanski H. Treatment of Domestic Wastewater in Small-Scale Sand Filters Fortified with Gypsum, Biotite, and Peat. Sustainability. 2023; 15(2):1351. https://doi.org/10.3390/su15021351
Chicago/Turabian StyleMartikainen, Kati, Anna-Maria Veijalainen, Eila Torvinen, and Helvi Heinonen-Tanski. 2023. "Treatment of Domestic Wastewater in Small-Scale Sand Filters Fortified with Gypsum, Biotite, and Peat" Sustainability 15, no. 2: 1351. https://doi.org/10.3390/su15021351
APA StyleMartikainen, K., Veijalainen, A.-M., Torvinen, E., & Heinonen-Tanski, H. (2023). Treatment of Domestic Wastewater in Small-Scale Sand Filters Fortified with Gypsum, Biotite, and Peat. Sustainability, 15(2), 1351. https://doi.org/10.3390/su15021351







